Abstract
The effect of thrombopoietin (TPO) on the functional activity of surface αIIbβ3 (GPIIbIIIa) was investigated in both primary human megakaryocytic cells, derived from peripheral blood CD34+ cells, and HEL hematopoietic cell line. TPO (100 ng/mL) induced a sixfold to ninefold enhancement of adhesion of both primary megakaryocytic and HEL cells to plates coated with either fibrinogen or fibronectin and a parallel increase of immunoreactivity to the PAC1 monoclonal antibody (MoAb) and fluorescein isothiocyanate-fibrinogen, both of which recognize an activated state of αIIbβ3 . The enhanced adhesion to fibrinogen or fibronectin was mediated by the Arg-Gly-Asp (RGD) recognition sequence of αIIbβ3 , as it was abolished by pretreatment of cells with saturating concentrations of RGDS peptide. A MoAb specific for the αIIb subunit of αIIbβ3 also inhibited cell attachment to fibrinogen or fibronectin, while MoAb to anti-αvβ3 or anti-α5 integrins were completely ineffective, clearly indicating that αIIbβ3 participates in this association. A role for PI 3 kinase (PI 3-K) in the TPO-mediated increase in αIIbβ3 function in megakaryocytic cells was suggested by the ability of the PI 3-K inhibitor wortmannin (100 nmol/L) and antisense oligonucleotides directed against the p85 regulatory subunit of PI 3-K to completely block the TPO-induced increase in αIIbβ3 integrin activity upon TPO stimulation. The modulation of adhesiveness to extracellular matrix proteins containing the RGD motif mediated by TPO likely plays a physiologic role in megakaryocytopoiesis, as pretreatment of CD34+ cells with RGDS or anti-αIIb MoAb significantly reduced the number of megakaryocytic colonies obtained in a fibrinclot semisolid assay.
POLYPLOID MEGAKARYOCYTES represent a small fraction (0.1% to 0.5%) of total human bone marrow (BM) cells. Regulation of megakaryocytopoiesis and platelet production involves the interplay of soluble peptide growth factors as well as cell-cell and cell-matrix interactions among megakaryocyte progenitors, BM stromal cells, and extracellular matrix proteins.1
Thrombopoietin (TPO), the ligand for c-Mpl receptor,2 has been recently cloned by five different groups of investigators.3-7 The encoded polypeptide has a predicted molecular mass of 35,000 kD and a novel two-domain structure. The amino-terminal domain shows homology with erythropoietin (Epo), the lineage-specific growth factor for red blood cell production, whereas the carboxyl-terminal domain is rich in serine, threonine, and proline residues and contains seven potential N-linked glycosylation sites.8 Several groups of investigators have shown that TPO acts on both the proliferative and maturative pools of megakaryocytopoiesis, stimulating the growth and differentiation of megakaryocyte progenitors as well as the release of functional platelets in vitro.9-13 The central role of TPO in thrombopoiesis is also provided by c-Mpl–null mice, which present a severe although nonlethal thrombocytopenia.14 Moreover, TPO is able to functionally activate mature platelets in vitro.15
It has been well established that the appearance of surface markers, including platelet glycoproteins, in cells of the megakaryocytic lineage varies with the degree of differentiation.1 In particular, αIIbβ3 integrin, also known as GPIIb-IIIa,16 starts to be expressed very early during the megakaryocyte commitment at the level of CD34+ megakaryocyte progenitor cells.17,18 αIIbβ3 represents the major protein expressed on the platelet surface, being the receptor for fibrinogen, fibronectin, and von Willebrand factor (vWF). While in mature platelets a functional modulation of αIIbβ3 is required to allow the induction of fibrinogen binding and subsequent platelet aggregation after vascular injury,19,20 the only known function of αIIbβ3 in mature megakaryocytes is the receptor-mediated endocytosis of fibrinogen into α granules.21
Once activated, αIIbβ3 shows the ability to specifically interact with fibrinogen and other RGD-containing extracellular matrix proteins in mature platelets.16 The purpose of this study is to investigate whether intracellular signals generated by TPO/c-Mpl interactions are able to modulate αIIbβ3 expression, activation state, and function in megakaryocytic cells. To address this issue, we used primary CD34+ hematopoietic progenitor cells, induced to differentiate along the megakaryocytic lineage in liquid suspension cultures by 100 ng/mL TPO. Parallel experiments were performed on the HEL pluripotent hematopoietic progenitor cell line,22 which was chosen as a model system for the following reasons: HEL cells show a constitutive high expression of αIIbβ3 , they can be induced to differentiate along the megakaryocytic lineage by phorbol esters,23-25 and they express significant levels of c-Mpl receptor.26
MATERIALS AND METHODS
Growth factors, chemicals, oligonucleotides, peptides, and antibodies.Human recombinant TPO and human recombinant Epo were purchased from Genzyme (Cambridge, MA) and Cilag (Milan, Italy), respectively.
The fungal metabolite wortmannin (WT), which represents a potent inhibitor of phosphatidylinositol 3 kinase (PI 3-K),27 was purchased from Sigma Chemicals (St Louis, MO). It was initially dissolved in dimethyl sulfoxide (DMSO) and further diluted in Iscove's Modified Dulbecco's Medium (IMDM). Apyrase and adenosine triphosphate (ATP) were purchased from Sigma.
The sequences of antisense (5′-GTACTGGTACCCCTCAGCACTCAT-3′) and sense (5′-ATGAGTGCTGAGGGGTACCAGTAC-3′) phosphorotioate oligonucleotides specific for the regulatory p85 subunit of PI 3-K were prepared according to Skorski et al.28 The p85 antisense oligonucleotides used in this study are not complementary to any other known gene sequence.
LGGAKQAGDV(γ402-411) fibrinogen γ-chain and RGDS and RGES peptides were synthesized and purified by high-performance liquid chromatography at the Biotechnology Department of the University of Ferrara (Italy). They were added in culture at the final concentrations of 1 mmol/L.
Monoclonal antibodies (MoAbs) to CD34 and CD41a directly conjugated with fluorescein were purchased from Becton Dickinson (San Jose, CA) and Serotec (Oxford, UK), respectively. MoAb to CD41b (SZ22, mouse IgG1), which reacts against the αIIb subunit independently of the presence of β3 , was purchased from Immunotech Inc (Westbrook, ME). The activation-dependent anti-αIIbβ3 PAC1 (mouse IgM) and the LM609 specific for αvβ3 (mouse IgG1) MoAb were purchased from the University of Pennsylvania (Philadelphia) and from Chemicon International Inc (Temecula, CA), respectively. Fibrinogen directly conjugated with fluorescein (FITC-fibrinogen) was a generous gift of Dr Pascal Goldshmidt-Clermont (Division of Cardiology, Department of Medicine, Johns Hopkins University, School of Medicine, Baltimore, MD). MoAb to CD49e, recognizing the α5 chain of one of the high-affinity receptors for fibronectin, was purchased from British Bio-Technology Products (Oxon, UK). Anti-mouse IgM rabbit antibody directly conjugated with fluorescein was from Immunotech.
Cells and liquid suspension cultures.Informed consent to the study was obtained according to the Helsinki declaration of 1975 from 18 normal blood donors. Mononuclear cells were isolated from leukapheresis units by Ficoll-Paque (density = 1.077 g/mL; Pharmacia, Uppsala, Sweden), rinsed, and adherence-depleted overnight. After removal of adherent cells, CD34+ cells were isolated using a magnetic cell sorting program Mini-MACS (Miltenyi Biotec, Auburn, CA) and the CD34 isolation kit in accordance with the manufacturer's recommendations.
The purity of CD34-selected cells was determined for each isolation by FACScan using an MoAb that recognizes a separate epitope of the CD34 molecule (HPCA-2; Becton Dickinson) directly conjugated to fluorescein. CD34+ cells averaged about 85% to 98%.
HEL cells,22 obtained from American Type Culture Collection (ATCC; Rockville, MD), were maintained at an optimal cell density of 5 × 105 to 1.5 × 106 cells/mL. To avoid the interference of serum, both HEL and purified peripheral blood (PB) CD34+ cells were resuspended in a chemically defined serum-free medium: IMDM (GIBCO, Grand Island, NY) containing 10−4 mol/L bovine serum albumin (BSA)-adsorbed cholesterol, nucleosides, 10 μg/mL each, 0.5% BSA (fraction V Chon), 10 μg/mL insulin, 200 μg/mL iron-saturated transferrin, 5 × 10−5 mol/L 2-β-mercaptoethanol (all purchased from Sigma). In some experiments, 2 × 105 primary megakaryocytic or HEL cells were seeded into 48-multiwell culture plates (Costar Corp, Cambridge, MA) in 1 mL of serum-free medium supplemented with 100 ng/mL of TPO. Sense or antisense p85 oligonucleotides were added at a concentration of 80 μg/mL at the beginning of the culture, and readded every day for 3 days at a concentration of 40 μg/mL. The PI 3 kinase (PI 3-K) inhibitor WT was added to the cultures at various concentrations for 2 hours. Control cells were incubated with the same concentrations of DMSO.
Semisolid cultures.Colony-forming units-megakaryocyte (CFUmeg) were grown using the same serum-free ingredients as in liquid culture and the fibrinclot technique as described previously.29 Briefly, 5 × 103 CD34+ cells were seeded in IMDM, supplemented with 200 μg/mL iron-saturated transferrin, 0.5% BSA, 280 μg/mL CaCl2 , 10−4 mol/L BSA-adsorbed cholesterol, 20 μg of L-asparagine, 10 μg/mL insulin, nucleosides (10 μg/mL each), 5 × 10−5 mol/L 2-β-mercaptoethanol, 0.1 mL of 0.2% (wt/vol) purified fibrinogen resuspended in phosphate-buffered saline (PBS), and 0.1 mL of 0.2 U/mL purified human thrombin (95%) in PBS. All reagents, except fibrinogen provided by Kabi (Stockholm, Sweden), were purchased from Sigma.
RGDS or RGES peptides (1 mmol/L), anti-CD41b, or αvβ3-specific MoAb (20 μg/mL) were added to cell suspension (5 × 103 CD34+ cells/plate) immediately before performing the semisolid assays. Colonies were quantified after 12 days of culture by an immunofluorescence labeling technique using CD41a MoAb directly conjugated with fluorescein. Dishes were scanned under an inverted microscope. CFU-meg were always unifocal and composed of 3 to 50 megakaryocytic cells/colony.
Platelet preparation.Blood drawn from four healthy volunteers who had not ingested aspirin during the preceding 10 days was collected into 1/10 vol of 3.8% sodium citrate and then centrifuged at 400g for 20 minutes at room temperature. Platelet-rich plasma was removed and centrifuged in the presence of 1 μmol/L prostaglandin E1 (PGE1 ; Sigma) at 800g for 15 minutes to form a platelet pellet. The pellet was then resuspended in a modified HEPES-Tyrode's buffer, pH 7.4, with 2 U/mL apyrase (Sigma) added. The platelet suspension was layered onto a Sepharose 2B (Pharmacia Biotech, Piscataway, NJ) column preequilibred with HEPES-Tyrode's buffer, and platelets were then collected at a concentration of 2 to 3 × 108 mL.
Evaluation of phenotype and nuclear ploidy.The membrane phenotype of CD34-derived primary cells, HEL, and platelets was analyzed by FACScan (Lysis II program; Becton Dickinson). Staining was performed on 5 × 104 cells or 106 platelets in 200 μL of PBS containing 1% BSA, 0.1% azide, 10 μg of CD41a MoAb, or 5 μg of PAC1 or 50 μg FITC-fibrinogen at 4°C for 30 minutes. In the case of PAC1, a second incubation step at 4°C for 30 minutes was performed using 5 μg of anti-mouse IgM rabbit antibody directly conjugated with fluorescein. Autofluorescence was assessed using cells treated only with 20% normal mouse serum for 10 minutes to block potentially unoccupied binding sites. Nonspecific fluorescence was assessed by using isotype-matched controls: anti-CD8 directly conjugated to fluorescein for anti-CD41a, and mouse IgM myeloma (Calbiochem, La Jolla, CA) followed by anti-mouse IgM rabbit antibody for PAC1. In some experiments, cells were treated with RGDS or RGES peptides (1 mmol/L) for 1 hour at 37°C before performing the phenotypic analysis. To quantify cell-associated immunofluorescence, the FACScan was calibrated before each phenotype analysis by quantitative fluorescein microbeads standards (Flow Cytometry Standards Corp, Research Triangle Park, NC). A standard curve was constructed by plotting the mean fluorescence intensity against the log of fluorescein molecules per bead. Samples were run in duplicate (10,000 events recorded) and the mean fluorescence intensity was expressed in arbitrary units.
Total αIIbβ3 expression and the activated form in platelets were determined essentially as described by Kovacsovics et al,20 using the anti-CD41a MoAb and FITC-fibrinogen, respectively. Platelets (2 × 107/mL) were treated with either human thrombin (10 mU/mL) or TPO (100 ng/mL) for 60 minutes at 37°C. In some experiments, platelets were pretreated with RGDS (1 mmol/L), RGES (1 mmol/L), WT (100 nmol/L), ATP (25 μmol/L), or apyrase (2 U/mL) for 45 minutes before adding TPO (100 ng/mL). Anti-CD41a (10 μg) or FITC-fibrinogen (100 μg/mL) was added to 200 μL of gel-filtered platelets for 30 minutes at 4°C and then platelets were fixed with 2% paraformaldehyde. The samples were gated for platelets, and aggregates were excluded based on forward and side scatter profiles.
To evaluate nuclear ploidy, 5 × 104 primary megakaryocytic or HEL cells were fixed in 1 mL cold 70% ethanol at 4°C for 1 hour. The cells were centrifuged, washed in PBS, resuspended in 0.4 mL PBS, and treated with 0.5 μg RNAse (Type I-A; Sigma) for 1 hour at 37°C. Propidium iodide (PI; Sigma in PBS), 40 μg/mL, was next added to each sample, and after gentle mixing samples were incubated in the dark at 4°C for 10 minutes and then analyzed. The PI fluorescence of individual nuclei was measured by FACScan equipped with an Argon ion laser (Spectra Physics 2020) tuned at 514-nm wavelength, 300-mW output.
Adhesion assays.Ninety-six flat-bottomed multi-wells were coated overnight at 4°C with one of the following proteins diluted in 100 μL of PBS: 5 mg/mL BSA, 10 μg purified human fibrinogen, 10 μg human fibronectin, 10 μg/mL murine myeloma protein IgG1 (MOPC-21), 10 μg/mL poly-L-lysine (all from Sigma except fibrinogen that was from Kabi). The plates were then washed twice with PBS, blocked for 2 hours at 37°C with 5 mg/mL BSA, and then washed twice with HEPES-Tyrode's buffer.
Primary CD34-derived megakaryocytic (2 × 105) or HEL (2 × 105) cells or platelets (2 × 107) were loaded with 1 μCi of (51Cr)carrier-free (DuPont NEN, Boston, MA) for 2 hours at 37°C. The cells and platelets were then washed twice and resuspended in 100 μL of a binding buffer: modified HEPES-Tyrode's buffer supplemented with 1% BSA, 2 mmol/L CaCl2 , and 1 mmol/L MgCl2 and then added to each protein-coated plate. After 90 minutes at 37°C, nonadherent cells were washed off. Quantitation of adherent cells or platelets per well was performed by liquid scintillation counting.
In some experiments, RGDS or RGES (1 mmol/L) or SZ22 (CD41b) MoAb or αvβ3-specific MoAb or α5 (CD49e)-specific MoAb (20 μg/mL) were added to cell or platelet suspension immediately before adding the cells or platelets to protein-coated plates.
Assay of PI 3-K activity.HEL cells (107 cells per experimental point) were treated with 100 ng/mL TPO for the indicated times at 37°C, washed twice with ice-cold buffer A (137 mmol/L NaCl, 20 mmol/L Tris, 1 mmol/L MgCl2 , 1 mmol/L Vanadate [Sigma], pH 7.5), and lysed in lysis buffer (buffer A plus 10% glycerol, 1% NP-40, and 1 mmol/L phenylmethylsulfonyl fluoride [PMSF]). The lysates were incubated for 2 hours at 4°C with 20 μg of antiphosphotyrosine (PY 20; Transduction Laboratories, Lexington, KY) MoAb or 2.5 μg of a rabbit polyclonal anti-p85 serum (UBI, Lake Placid, NY; dilution 1:2,000), directed against the p85 regulatory subunit of PI 3-K. Protein A-Sepharose (4 mg/mL lysate, precoupled to rabbit–anti-mouse IgG when using anti-PY MoAb) was then added for 1 hour at 4°C to the lysates. After precipitation, the lysates were washed twice in lysis buffer, twice in buffer containing 0.5 mol/L LiCl and 0.2% NP-40, and, finally, in 10 mmol/L phenyl phosphate.
The PI 3-K assay on the immunoprecipitates was then performed by adding sonicated phosphatidylinositol (PI, in 10 mmol/L HEPES, 1 mmol/L EDTA, pH 7.5; 0.5 mg/mL final concentration) and 10 μCi of (γ-32P)ATP to the immunoprecipitates for 15 minutes at room temperature. The reaction was stopped by adding 100 μL 1N HCl and 160 μL of methanol:chloroform (1:1 mixture). The lipid-containing organic phase was resolved on oxalate-coated thin-layer chromatography plates (Silica Gel 60; JT Baker Inc, Phillipsburg, NJ) developed in chlorophorm/methanol/H2O/NH4OH (43:38:7:5 vol/vol). Radiolabeled spots, identified by autoradiography on Kodak X-Omat S films (Eastman Kodak, Rochester, NY), were excised and quantified by scintillation counting.
In some experiments, high-performance liquid chromatography (HPLC) analysis of the lipid products was performed. Separation of deacylated phosphoinositides was performed on a Parthisphere SAX, 120 × 4.6-mm column, equipped with a Sax precolumn insert following a previously described procedure.30
Western blot analysis.For Western blot experiments, extracts obtained from equal numbers (2 × 106) of HEL cells were diluted 1:1 with loading buffer (250 mmol/L Tris-HCl, pH 6.8, 10−4 mol/L 2-β-mercaptoethanol, 40% glycerol, 8% sodium dodecyl sulfate (SDS), 0.003% bromophenol blue) and subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) on 7.5% gel. Gels were then transferred to nitrocellulose membrane by Trans-Blot (Bio-Rad, Richmond, CA). SDS-PAGE and transfer were done according to the manufacturer's recommendations. The membranes were incubated in PBS (GIBCO), supplemented with 3% (wt/vol) nonfat dry milk for 30 minutes at room temperature, then incubated overnight at 4°C with anti-p85 serum or anti–β-tubulin MoAb (Sigma; dilution 1:1,000). After four washings with PBS/0.1% Tween 20/0.1% BSA, peroxidase labeled goat anti-rabbit (for p85) or anti-mouse (for β-tubulin) IgG (both dilutions 1:1,500; Cappel, Durham, NC) was applied to the membrane for 60 minutes at room temperature. Bound antibody was visualized by use of the ECL Western blotting detection reagents and Hyperfilm-ECL luminescence detection film (Amersham Corp, Arlington Heights, IL).
Actin polymerization.To assess changes in actin polymerization upon TPO stimulation, duplicate samples of primary megakaryocytic or HEL cells (2 × 105 cells/sample in a volume of 1 mL PBS plus 0.1% BSA) were either left unstimulated or stimulated with 100 ng/mL TPO for 2 hours at 37°C in the absence or presence of 100 nmol/L WT. After stimulation, cells were fixed and permeabilized with cold 70% ethanol. After permeabilization, 20 μL of a 6.6 μmol/L stock of BODIPY-fluorescein phallacidin (Molecular Probes, Eugene, OR) was added and the samples incubated for an additional 10 minutes at 37°C. Samples were then centrifuged, resuspended in ice-cold Hanks' Balanced Salt Solution (HBSS) and analyzed on a FACScan. The mean channel fluorescence from each of the two duplicate samples was averaged and then normalized in arbitrary units to the average mean channel fluorescence of unstimulated cells in the absence of WT.
Statistical analysis.Statistical analysis was performed using the two-tailed Student's t-test for unpaired data.
RESULTS
TPO upregulates the expression of surface αIIbβ3 in primary CD34-derived megakaryocytic cells.Initially, total αIIbβ3 expression was examined with a CD41a MoAb in both CD34+ primary cells and HEL cell line, seeded in serum-free liquid cultures in the absence or presence of 100 ng/mL TPO or 4 IU/mL of Epo. Both cytokines were added in culture at day 0 and then every 3 days and CD41a analysis was performed at various time points. These preliminary experiments allowed us to establish that a 6-day treatment with TPO represented the optimal condition to investigate the role of αIIbβ3 during the process of megakaryocyte development before platelet formation.
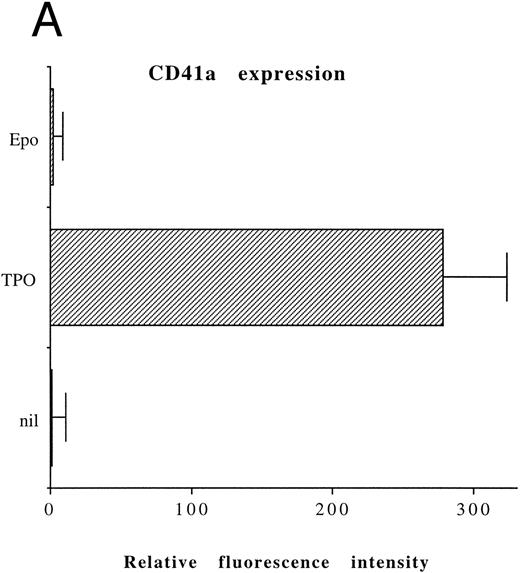
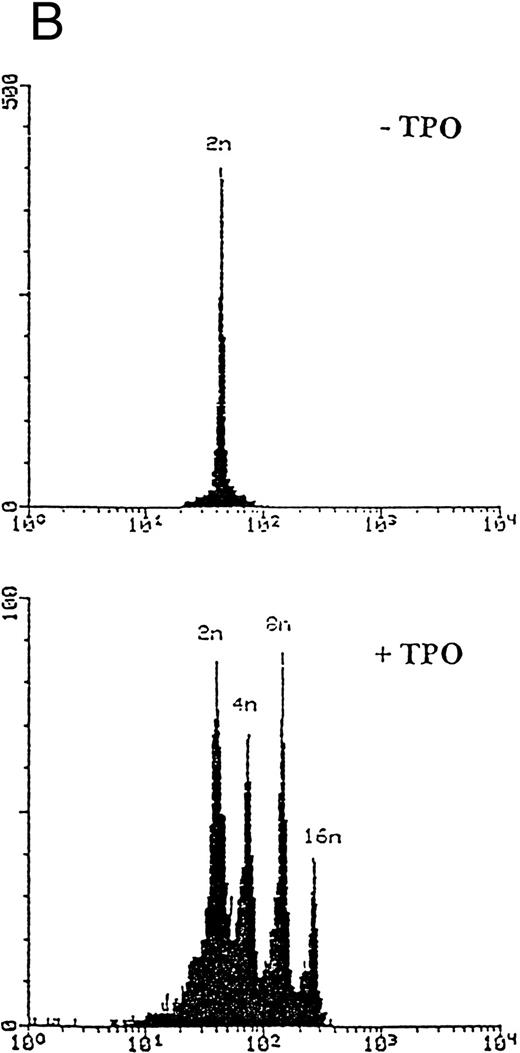
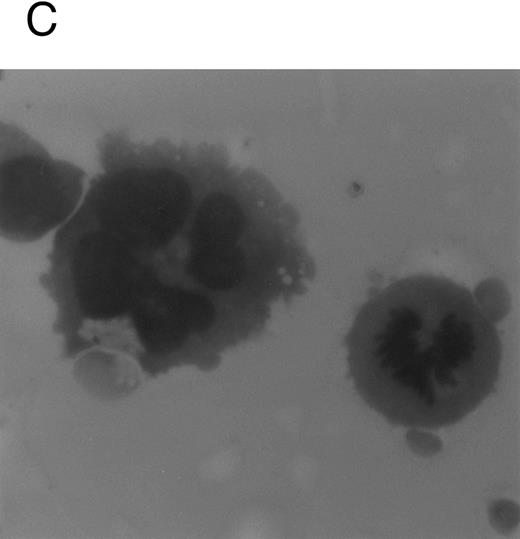
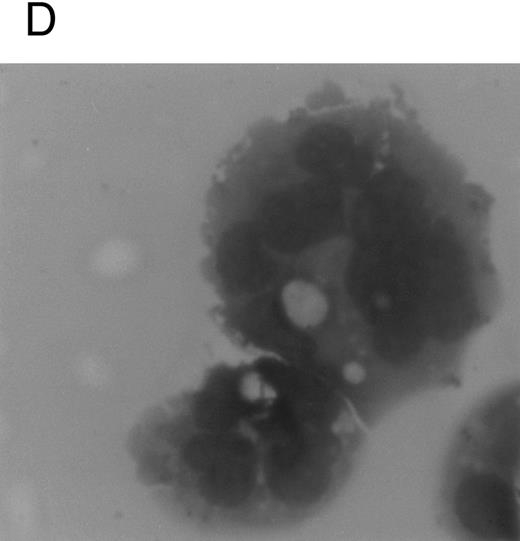
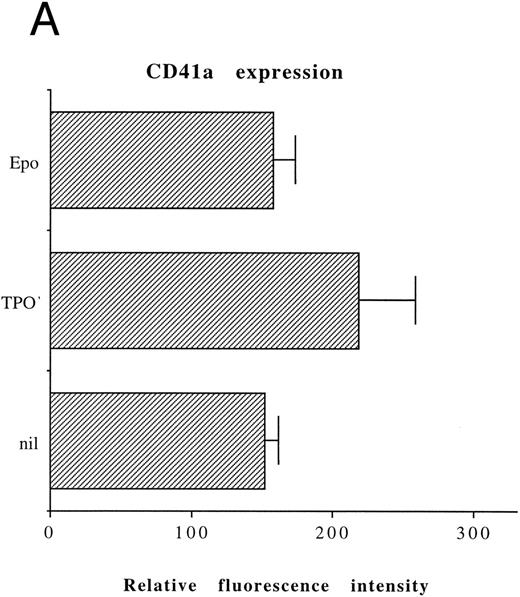
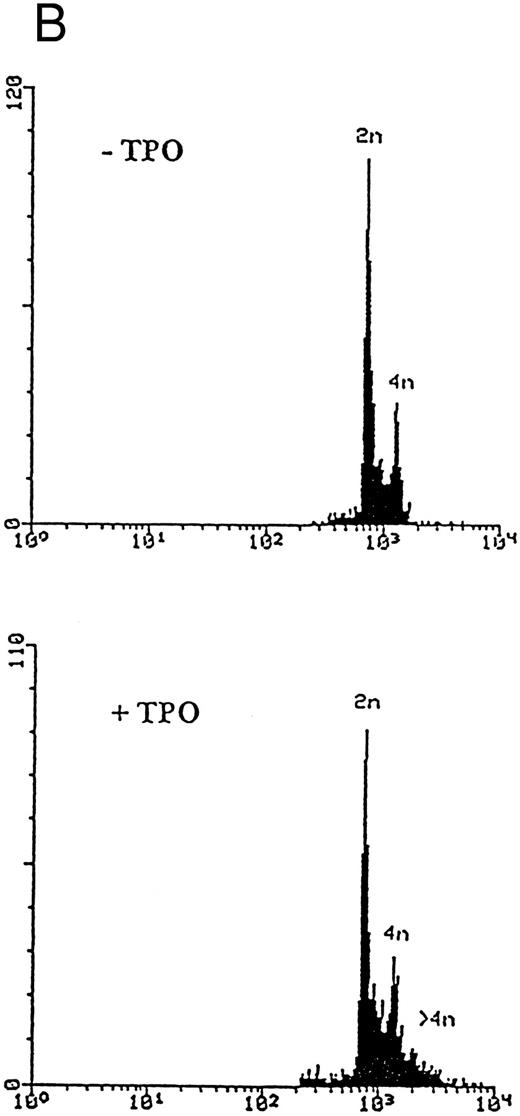
Although freshly isolated primary CD34+ cells showed negligible levels of CD41a (<5%), approximately 70% of primary cells expressed high levels of αIIbβ3 after 6 days of exposure to TPO (Fig 1A). At this time point, TPO induced a significant (P < .01) increase of the DNA content in primary CD34-derived cells with the appearance of 4-8-16N polyploid cells (Fig 1B). Moreover, at morphological analysis, a fraction of TPO-treated cells already showed the characteristic features of megakaryocytes (Fig 1C and D), although proplatelet formation was still absent or very rare.
Analysis of αIIbβ3 expression (A), DNA content (B), and morphology (C and D) of CD34+ cells, performed before or after 6 days of liquid culture with 100 ng/mL TPO. αIIbβ3 expression was examined with anti-CD41a MoAb directly conjugated to fluorescein and fluorescence was analyzed by FACScan. Negative controls are represented by cells treated with an isotype-matched irrelevant MoAb (anti-CD8) directly conjugated to fluorescein. The DNA content was examined by FACScan after PI staining. Cytospins were observed at light microscopy after May-Grünwald Giemsa staining (original magnification × 400). Flow cytometry results are expressed in arbitrary fluorescence units and represent means ± SD of four separate experiments.
Analysis of αIIbβ3 expression (A), DNA content (B), and morphology (C and D) of CD34+ cells, performed before or after 6 days of liquid culture with 100 ng/mL TPO. αIIbβ3 expression was examined with anti-CD41a MoAb directly conjugated to fluorescein and fluorescence was analyzed by FACScan. Negative controls are represented by cells treated with an isotype-matched irrelevant MoAb (anti-CD8) directly conjugated to fluorescein. The DNA content was examined by FACScan after PI staining. Cytospins were observed at light microscopy after May-Grünwald Giemsa staining (original magnification × 400). Flow cytometry results are expressed in arbitrary fluorescence units and represent means ± SD of four separate experiments.
On the other hand, the majority of untreated HEL cells (approximately 90%) displayed a high level expression of αIIbβ3, whose mean fluorescence intensity did not significantly increase upon TPO treatment (Fig 2A). Moreover, TPO-treated HEL cells only showed a modest increase in the DNA content with respect to untreated controls (Fig 2B). On the basis of these data, all the following experiments were performed on CD34+ cells, precultured for 6 days with 100 ng/mL TPO (primary megakaryocytic cells) and untreated HEL cells.
Analysis of αIIbβ3 expression (A) and DNA content (B) of HEL cells, performed before or after 6 days of liquid culture with 100 ng/mL TPO. The αIIbβ3 expression and DNA content were examined as described in the legend to Fig 1. Flow cytometry results are expressed in arbitrary fluorescence units and represent means ± SD of four separate experiments.
Analysis of αIIbβ3 expression (A) and DNA content (B) of HEL cells, performed before or after 6 days of liquid culture with 100 ng/mL TPO. The αIIbβ3 expression and DNA content were examined as described in the legend to Fig 1. Flow cytometry results are expressed in arbitrary fluorescence units and represent means ± SD of four separate experiments.
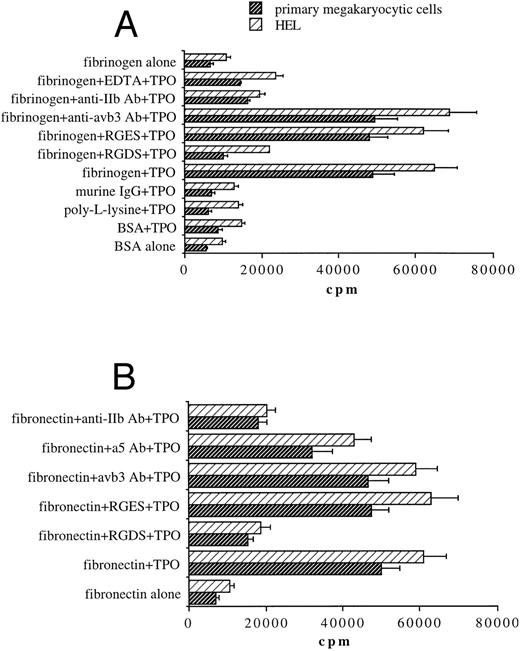
TPO induces the binding of both primary megakaryocytic cells and HEL to fibrinogen- or fibronectin-coated plates through αIIbβ3 .We next investigated whether TPO treatment was able to modulate megakaryocyte cell adhesion to fibrinogen, the extracellular ligand of αIIbβ3 , or other immobilized proteins. A 2-hour exposure to 100 ng/mL TPO before performing the adhesion assay induced a sixfold to ninefold increase of binding to fibrinogen in both 51Cr-labeled primary megakaryocytic cells and HEL cell line with respect to untreated cells (Fig 3A). In contrast, adhesion to wells coated with BSA, poly-L-lisine, or IgG proteins was not affected by TPO stimulation. Moreover, chelation of divalent cations by EDTA abolished adhesion on immobilized fibrinogen. These results suggested that increased adhesion of both primary megakaryocytic and HEL cells to fibrinogen in response to TPO may be mediated by the high-affinity state of the calcium-dependent integrin αIIbβ3 . To further establish that adhesion to immobilized fibrinogen was αIIbβ3-specific, we tested the effects of some αIIbβ3-specific antagonists. The RGDS peptide but not RGES at 1 mmol/L abolished primary megakaryocyte and HEL cell adhesion to fibrinogen. Moreover, adhesion was also significantly inhibited (P < .05) by an anti-αIIb (CD41b) but not by an anti-αvβ3 MoAb.
Stimulation with TPO (100 ng/mL) results in the upregulation of αIIbβ3-mediated adhesion of 51Cr-labeled primary megakaryocytic cells and HEL cells to immobilized fibrinogen (A) or fibronectin (B). Binding of cells stimulated with TPO to various immobilized substrates was assessed as described in Materials and Methods. Results are expressed in cpm and represent means ± SD of five separate experiments.
Stimulation with TPO (100 ng/mL) results in the upregulation of αIIbβ3-mediated adhesion of 51Cr-labeled primary megakaryocytic cells and HEL cells to immobilized fibrinogen (A) or fibronectin (B). Binding of cells stimulated with TPO to various immobilized substrates was assessed as described in Materials and Methods. Results are expressed in cpm and represent means ± SD of five separate experiments.
Because αIIbβ3 also serves as a receptor for other RGD-containing matrix proteins, including fibronectin,16,31 binding experiments were next performed on fibronectin-coated plates (Fig 3B). TPO was also able to increase adhesion of megakaryocytic cells on fibronectin in an RGD-dependent manner. αIIbβ3 integrin also appears to play a primary role in TPO-mediated binding to fibronectin matrix as anti-αIIb (CD41b) MoAb significantly (P < .01) inhibited adhesion to fibronectin, while an anti-α5 (CD49e) MoAb directed against one of the high-affinity integrin receptors (α5β1) for fibronectin32 reduced somewhat megakaryocytic cell adherence, but not to a significant extent.
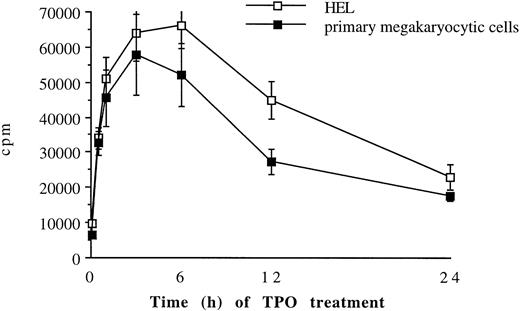
To determine a time course of TPO stimulation, 51Cr-labeled primary megakaryocytic and HEL cells were treated with 100 ng/mL TPO for various time points before plating on immobilized ligands (Fig 4). The effect of TPO was rapid, showing a peak after 0.5 to 3 hours, but it was also reversible with a progressive decline toward the basal condition after 24 hours.
Time course of TPO treatment on megakaryocytic cell adhesion to fibrinogen. 51Cr-labeled primary megakaryocytic (A) and HEL (B) cells were incubated with TPO (100 ng/mL) for increasing periods before plating onto fibrinogen-coated wells. Results are expressed in cpm and represent mean ± SD of three separate experiments.
Time course of TPO treatment on megakaryocytic cell adhesion to fibrinogen. 51Cr-labeled primary megakaryocytic (A) and HEL (B) cells were incubated with TPO (100 ng/mL) for increasing periods before plating onto fibrinogen-coated wells. Results are expressed in cpm and represent mean ± SD of three separate experiments.
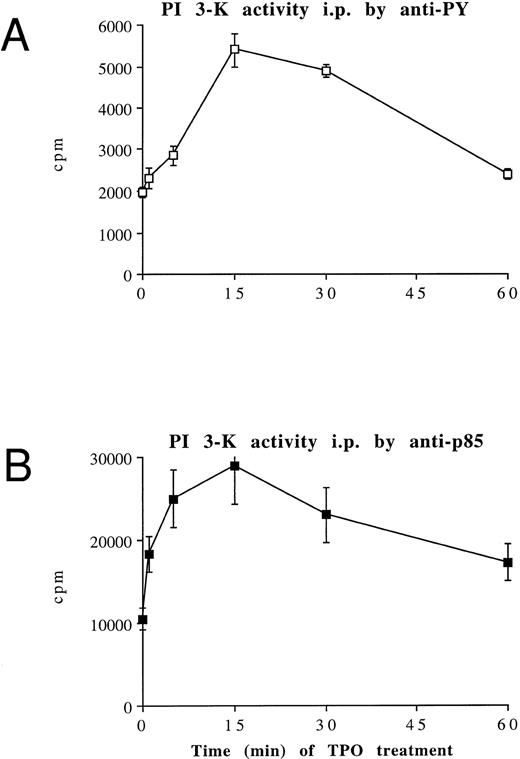
TPO stimulates PI 3-K activity in HEL cells.Because it was previously shown that the exposure of platelets to TPO induces an increase in anti–PY-immunoprecipitable PI 3-K,33 which plays a central role in the process of platelet aggregation,20 we investigated the ability of TPO to stimulate PI 3-K activity in HEL cells. Figure 5A shows PI 3-K activity immunoprecipitated by HEL cell homogenates using an anti-PY MoAb at different time points after addition of TPO. A rapid and significant (P < .05) activation of PI 3-K associated to anti-PY was observed after 15 minutes from the addition of TPO in culture. Activation of PI 3-K was further investigated using a polyclonal serum directed against the 85-kD subunit of the p85/p110 type PI 3-K. As shown in Fig 5B, TPO (100 ng/mL) stimulated the anti-p85 precipitable PI 3-K activity, and maximal stimulation was observed at 15 minutes after addition of TPO. The product of lipid kinase activity recovered in either anti-PY and anti-p85 immunoprecipitates was analyzed by deacylation and HPLC separation and shown to be PI3P (Fig 5C).
TPO stimulation of HEL cells results in activation of PI 3-K. HEL cells were stimulated with 100 ng/mL TPO for 0 to 60 minutes at 37°C, lysed in PI 3-K lysis buffer, and then immunoprecipitated with anti-PY MoAb (A) or anti-p85 serum (B). Samples were then tested for PI 3-K activity as described in Materials and Methods. Data are expressed as means ± SD of five separate experiments. (C) HPLC analysis of deacylated (32P) PIP produced by an anti-p85 immunoprecipitate of HEL cells stimulated by TPO for 15 minutes (○, left peak) or by a total HEL cell lysate used as a standard source of PI-4-P ( — , right peak). Equivalent counts of (32P) PIP, obtained from an anti-p85 immunoprecipitate or total HEL cell lysates, were separated by thin-layer chromatography, deacyled, and subjected to HPLC separation, as described in Materials and Methods.
TPO stimulation of HEL cells results in activation of PI 3-K. HEL cells were stimulated with 100 ng/mL TPO for 0 to 60 minutes at 37°C, lysed in PI 3-K lysis buffer, and then immunoprecipitated with anti-PY MoAb (A) or anti-p85 serum (B). Samples were then tested for PI 3-K activity as described in Materials and Methods. Data are expressed as means ± SD of five separate experiments. (C) HPLC analysis of deacylated (32P) PIP produced by an anti-p85 immunoprecipitate of HEL cells stimulated by TPO for 15 minutes (○, left peak) or by a total HEL cell lysate used as a standard source of PI-4-P ( — , right peak). Equivalent counts of (32P) PIP, obtained from an anti-p85 immunoprecipitate or total HEL cell lysates, were separated by thin-layer chromatography, deacyled, and subjected to HPLC separation, as described in Materials and Methods.
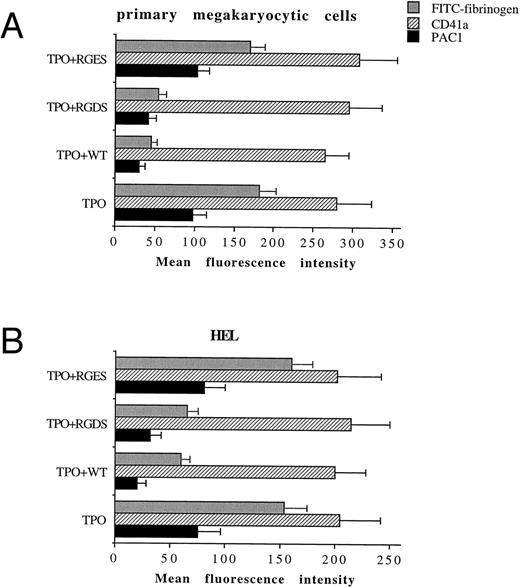
TPO promotes the functional activity of αIIbβ3 in both primary megakaryocytic cells and HEL through PI 3-K.We next tested by FACScan analysis whether the fungal metabolite WT was able to inhibit the conformational change in αIIbβ3 . WT, 100 nmol/L, was added to primary megakaryocytic and HEL cells for 2 hours together with 100 ng/mL TPO before performing the phenotypic analysis of either CD41a or PAC1 MoAb, which specifically reacts against an activated state of αIIbβ3 .19 The addition in culture of 100 nmol/L WT specifically inhibited the catalytic activity of PI 3-K but not that of PI 4-K, as shown by the decreased production of PI3P in WT-treated HEL cells with normal amounts of PI4P detected by HPLC (data not shown). At 100 nmol/L, WT did not induce any significant variation in the total αIIbβ3 surface expression, while it almost completely abolished the TPO-induced immunoreactivity to PAC1 in both primary megakaryocytic (Fig 6A) and HEL (Fig 6B) cells. The specificity of PAC1 immunoreactivity was confirmed by the significant (P < .01) inhibition of PAC1 binding observed also in the presence of RGDS but not RGES peptides. This result was confirmed by analyzing the binding of FITC-fibrinogen (100 μg/mL) to TPO-stimulated cells in the presence of WT.
WT does not affect the total levels of αIIbβ3 , but inhibits the activation state of αIIbβ3 . Primary megakaryocytic (A) and HEL (B) cells were preincubated with 100 nmol/L WT, 1 mmol/L RGDS or RGES peptides, or vehicle buffer (TPO alone) for 2 hours at 37°C and stained with anti-CD41a MoAb, PAC1 MoAb, or FITC-fibrinogen. Staining was assessed by FACScan, as described in Materials and Methods. Data are expressed as means ± SD of three to six separate experiments.
WT does not affect the total levels of αIIbβ3 , but inhibits the activation state of αIIbβ3 . Primary megakaryocytic (A) and HEL (B) cells were preincubated with 100 nmol/L WT, 1 mmol/L RGDS or RGES peptides, or vehicle buffer (TPO alone) for 2 hours at 37°C and stained with anti-CD41a MoAb, PAC1 MoAb, or FITC-fibrinogen. Staining was assessed by FACScan, as described in Materials and Methods. Data are expressed as means ± SD of three to six separate experiments.
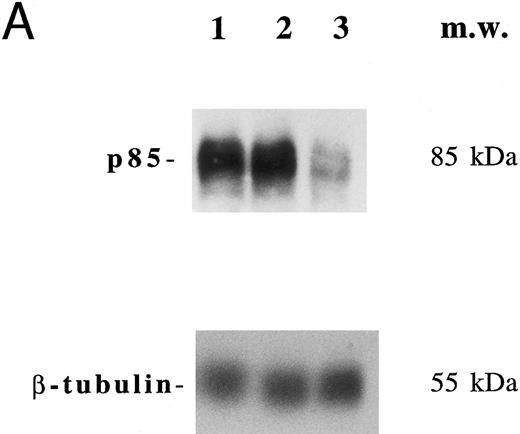
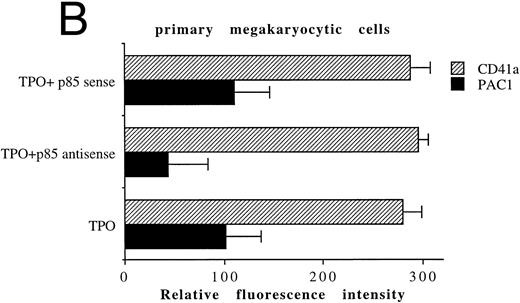
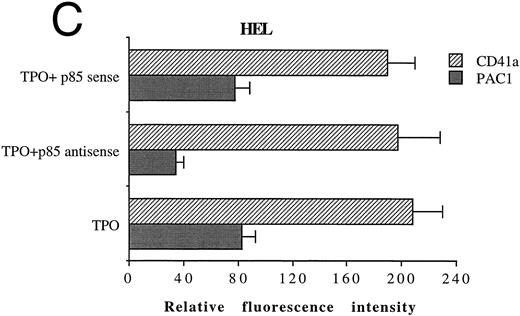
In another set of experiments, primary megakaryocytic and HEL cells were incubated with sense and antisense p85 oligonucleotides specific for the regulatory p85 subunit of PI 3-K for 3 days. The treatment of HEL cells with antisense oligonucleotides induced a significant reduction of the amount of p85 protein, as determined by Western blot analysis performed with anti-p85 rabbit serum or anti–β-tubulin MoAb (Fig 7A) with a 75% inhibition of the PI 3-K catalytic activity (data not shown). Although p85 antisense did not induce any significant variation in the CD41a surface expression, a significant (P < .01) inhibition of the TPO-induced PAC1 expression was noticed in both primary megakaryocytic (Fig 7B) and HEL (Fig 7C) cells.
Inhibition of p85 and PAC1 but not total αIIbβ3 expression upon p85 antisense treatment. HEL and primary megakaryocytic cells were treated with p85 sense or antisense oligonucleotides for 3 days, while control cells were left untreated. (A) p85 protein and β-tubulin were sequentially detected on the same filter after SDS-PAGE and Western blotting with specific antibodies. Lanes: 1, untreated HEL cells; 2, HEL cells treated with p85 sense; 3, HEL cells treated with p85 antisense. m.w., molecular weights. The expression of activated and total αIIbβ3 was analyzed by FACScan with PAC1 or anti-CD41a MoAb, respectively, in primary megakaryocytic (B) and HEL (C) cells as described in Materials and Methods. Data are expressed as means ± SD of three separate experiments.
Inhibition of p85 and PAC1 but not total αIIbβ3 expression upon p85 antisense treatment. HEL and primary megakaryocytic cells were treated with p85 sense or antisense oligonucleotides for 3 days, while control cells were left untreated. (A) p85 protein and β-tubulin were sequentially detected on the same filter after SDS-PAGE and Western blotting with specific antibodies. Lanes: 1, untreated HEL cells; 2, HEL cells treated with p85 sense; 3, HEL cells treated with p85 antisense. m.w., molecular weights. The expression of activated and total αIIbβ3 was analyzed by FACScan with PAC1 or anti-CD41a MoAb, respectively, in primary megakaryocytic (B) and HEL (C) cells as described in Materials and Methods. Data are expressed as means ± SD of three separate experiments.
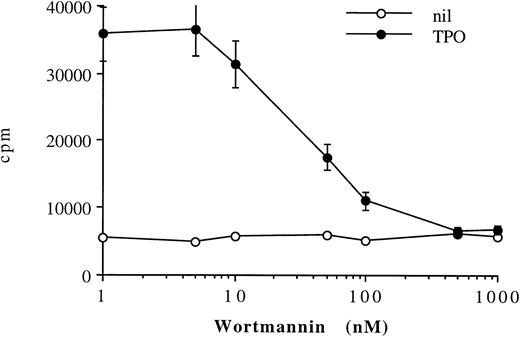
The PI 3-K inhibitor WT inhibits TPO adhesion regulation.Figure 8 shows that treatment of HEL cells with 100 nmol/L of WT completely inhibited the ability of TPO to upregulate αIIbβ3-mediated adhesion to immobilized fibrinogen. The half-maximal concentration (IC50) of WT that inhibits TPO-mediated increases in HEL adhesion to fibrinogen is in the range of 30 to 40 nmol/L. This IC50 value is consistent with concentrations of WT found to specifically inhibit PI 3-K–dependent cellular responses and PI 3-K activity in other systems.27 Therefore, the signal transduced by TPO stimulation, which results in upregulation of αIIbβ3 integrin function in megakaryocytic cells, appears to be dependent on PI 3-K.
TPO-mediated upregulation of adhesion of HEL cells to fibrinogen is completely inhibited by the PI 3-K inhibitor WT. Binding of 51Cr-labeled HEL cells to fibrinogen-coated plates was assessed in the presence of no stimulus (nil) or 100 ng/mL TPO in the absence or presence of various doses of WT. Results are expressed in cpm, and represent means ± SD of three separate experiments.
TPO-mediated upregulation of adhesion of HEL cells to fibrinogen is completely inhibited by the PI 3-K inhibitor WT. Binding of 51Cr-labeled HEL cells to fibrinogen-coated plates was assessed in the presence of no stimulus (nil) or 100 ng/mL TPO in the absence or presence of various doses of WT. Results are expressed in cpm, and represent means ± SD of three separate experiments.
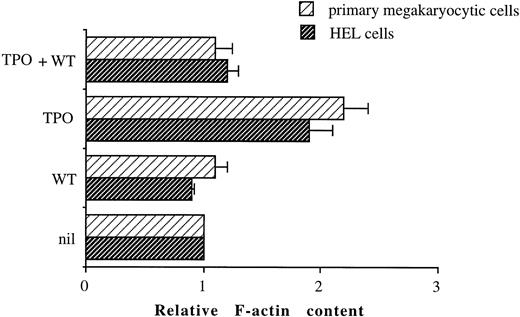
TPO induces a WT-sensitive increase in F-actin content.Activation of PI 3-K has also been implicated in alterations of the cytoskeleton.33 Because such cytoskeletal rearrangements could have an effect on cell adhesion, we investigated whether TPO stimulation of primary megakaryocytic or HEL cells resulted in actin polymerization, as measured by increased staining of the F-actin. Figure 9 shows that stimulation of both primary megakaryocytic cells and HEL cells with 100 ng/mL TPO for 2 hours results in increased F-actin content when compared with unstimulated cells. A role for PI 3-K in TPO-mediated actin polymerization is also suggested by the ability of 100 nmol/L WT to inhibit the increase in F-actin content observed in primary megakaryocytic and HEL cells upon stimulation with TPO.
TPO-induced increases in actin assembly in primary megakaryocytic and HEL cells can be inhibited by WT. Cells were either unstimulated or stimulated for 2 hours with 100 ng/mL TPO in the absence or presence of 100 nmol/L WT. After stimulation, cells were permeabilized, labeled with BODIPY fluorescein-conjugated phallacidin, and fluorescence assessed by FACScan as described in Materials and Methods. All results in a given experiment are normalized to the mean channel fluorescence of unstimulated HEL or primary CD34-derived cells, which was given an arbitrary value of 1. Relative F-actin contents greater than 1 indicate an increase in actin polymerization relative to unstimulated cells.
TPO-induced increases in actin assembly in primary megakaryocytic and HEL cells can be inhibited by WT. Cells were either unstimulated or stimulated for 2 hours with 100 ng/mL TPO in the absence or presence of 100 nmol/L WT. After stimulation, cells were permeabilized, labeled with BODIPY fluorescein-conjugated phallacidin, and fluorescence assessed by FACScan as described in Materials and Methods. All results in a given experiment are normalized to the mean channel fluorescence of unstimulated HEL or primary CD34-derived cells, which was given an arbitrary value of 1. Relative F-actin contents greater than 1 indicate an increase in actin polymerization relative to unstimulated cells.
αIIbβ3-Fibrinogen interactions enhance the yield of CD34-derived megakaryocytic colonies in semisolid fibrinclot cultures.We next investigated whether the increased binding to immobilized fibrinogen or fibronectin mediated by TPO through αIIbβ3 might play a physiologic role in the growth of megakaryocyte progenitors. To address this issue, CD34+ cells were resuspended with RGDS, RGES, γ-chain synthetic peptides, anti-αIIb (CD41b) MoAb, or anti-αvβ3 MoAb for 1 hour at 37°C before performing semisolid fibrinclot cultures supplemented with 100 ng/mL TPO. The presence of anti-αIIb MoAb, RGDS, or γ-chain synthetic peptides but not anti-αvβ3 MoAb or RGES peptides resulted in a variable but significant (P < .01) reduction in the number of megakaryocyte colonies (Table 1), suggesting that adhesive interaction of RGD-containing extracellular matrix protein with αIIbβ3 might play a primary role also in the process of megakaryocyte development and maturation.
Evaluation of the Number of Megakaryocytic Colonies in a Fibrinclot Serum-Free Fibrinclot Assay
| . | No. of CFU-meg/5 × 103 CD34+ Cells . |
|---|---|
| Medium alone | 1.5 ± 1 |
| TPO (100 ng/mL) | 36 ± 6.5 |
| TPO (100 ng/mL) + RGDS | 20 ± 4* |
| TPO (100 ng/mL) + RGES | 41 ± 8 |
| TPO (100 ng/mL) + 402-411 γ peptide | 18.6 ± 5* |
| TPO (100 ng/mL) + anti-αIIb | 21 ± 6.4* |
| TPO (100 ng/mL) + anti-αvβ3 | 33 ± 8.8 |
| . | No. of CFU-meg/5 × 103 CD34+ Cells . |
|---|---|
| Medium alone | 1.5 ± 1 |
| TPO (100 ng/mL) | 36 ± 6.5 |
| TPO (100 ng/mL) + RGDS | 20 ± 4* |
| TPO (100 ng/mL) + RGES | 41 ± 8 |
| TPO (100 ng/mL) + 402-411 γ peptide | 18.6 ± 5* |
| TPO (100 ng/mL) + anti-αIIb | 21 ± 6.4* |
| TPO (100 ng/mL) + anti-αvβ3 | 33 ± 8.8 |
Data represent the means ± SD of five separate experiments performed in triplicate.
Statistically significant (P < .05) differences between cultures stimulated with TPO alone or TPO plus various peptides.
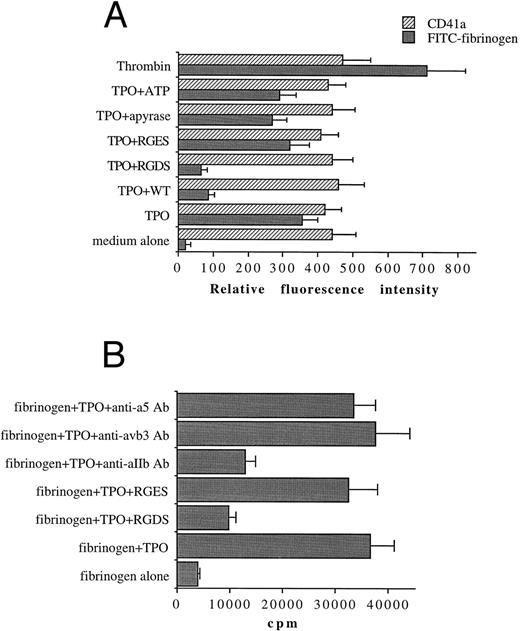
TPO also promotes the functional activity of αIIbβ3 in a PI 3-K–dependent manner in platelets.In the last group of experiments, we investigated the ability of TPO to modulate αIIbβ3 function in peripheral platelets. The addition of 100 ng/mL of TPO to platelets increased the immunoreactivity to FITC-fibrinogen to approximately 50% of the levels observed in the presence of thrombin (10 mU/mL), a major platelet agonist (Fig 10A). To investigate the role of adenosine diphosphate (ADP) release in the action of TPO on αIIbβ3 function, experiments were performed in the presence of the ADP scavanger apyrase (2 U/mL) or ATP (25 μmol/L), an ADP receptor antagonist. The TPO-mediated binding of FITC-fibrinogen to platelets was somewhat decreased under these conditions, but not to a significant extent. These data, together with the ability of WT to significantly (P < .01) suppress FITC-fibrinogen binding to platelet, suggest that TPO can directly modulate αIIbβ3 function through PI-3K signaling events also in platelets.
TPO (100 ng/mL) upregulates the activation state of αIIbβ3 (A) and the αIIbβ3-binding adhesion of 51Cr-labeled platelets to immobilized fibrinogen (B). FACScan analysis and binding experiments were performed as described in Materials and Methods. In (A), data are expressed as means ± SD of three to six separate experiments. In (B), data are expressed in cpm and represent means ± SD of three separate experiments.
TPO (100 ng/mL) upregulates the activation state of αIIbβ3 (A) and the αIIbβ3-binding adhesion of 51Cr-labeled platelets to immobilized fibrinogen (B). FACScan analysis and binding experiments were performed as described in Materials and Methods. In (A), data are expressed as means ± SD of three to six separate experiments. In (B), data are expressed in cpm and represent means ± SD of three separate experiments.
We next evaluated the effect of TPO treatment of platelets on their interaction with immobilized fibrinogen. Platelets labeled with 51Cr were stimulated with TPO or a buffer control for 10 minutes at 37°C and then added onto fibrinogen-coated plates (Fig 10B). These platelet-fibrinogen interactions were almost completely abolished (P < .01) by 1 mmol/L RGDS but not RGES. As previously shown for megakaryocytic cells, treatment with anti-αIIb antibody also significantly (P < .01) inhibited these interactions.
DISCUSSION
Activation-dependent upregulation of integrin activity is characterized by qualitative alterations in the integrin receptor itself as measured by adhesion to relevant ligands or counter-receptors, without changes in the level of cell-surface integrin expression.35 This implies the existence of conformational changes that alter the affinity of an integrin for a ligand, and/or cellular responses, such as receptor microclustering that alter the avidity of integrin-dependent cellular interactions and mediate inside-out transduction through the integrin cytoplasmic domains.36
It has been shown that multiple different receptors on hematopoietic progenitors are capable of delivering a signal that upregulates integrin activity.35 In particular, a specific binding of erythroid progenitors to fibronectin has been shown and likely occurs through the α5β1 integrin receptors in response to Epo.37 Moreover, the α4β1 and α5β1 have been reported to be differentially expressed during myeloid differentiation induced by the addition of granulocyte colony-stimulating factor (G-CSF) to CD34+ hematopoietic progenitors.38 Thus, the rapid and reversible upregulation of the functional activity of integrin receptors on hematopoietic progenitors is a vital step in the adhesive interactions that occur during recognition of hematopoietic progenitors with BM extracellular matrix proteins. Although the ligation of several different cell surface receptors has been shown to rapidly upregulate integrin function in peripheral platelets,20 little is known about the role of integrin activation during the process of megakaryocyte differentiation from CD34+ pluripotent hematopoietic progenitor cells.
In this study we showed that, when cultured in the presence of TPO, primary megakaryocytic cells displayed a marked enhancement of αIIbβ3 integrin function with respect to cells cultured in the absence of TPO. This was expressed by the reactivity to the anti-PAC1 MoAb and by an enhancement of adherence to fibrinogen- or fibronectin-coated wells. The αIIbβ3 specificity of this enhancement was demonstrated by its marked reduction in the presence of RGDS peptide or an anti-αIIb (CD41b) MoAb.
The number of αIIbβ3 complexes only slightly increased in TPO-treated cells, whereas anti-PAC1 MoAb showed a substantial change in the conformational state of the complex. Therefore, the enhancement of cell adhesion induced by exposure to TPO likely represents a manifestation of differences in the αIIbβ3 receptor function and activation to either a high-affinity state or an intermediate state, and not in the number of αIIbβ3 complexes in megakaryocytic cells. An alternative potential mechanism of activation is represented by an increased exposure or recruitment of resting αIIbβ3 on the basal cell surface.
Activation of αIIbβ3 with adhesion to fibrinogen substratum was likely caused by “inside-out” transmembrane signaling with cytoskeletal rearrangements mediated by PI 3-K. In fact, (1) TPO significantly enhanced PI 3-K catalytic activity in HEL cells, and (2) inhibition of PI 3-K by either WT or p85 antisense oligonucleotides reduced the whole content of F-actin and resulted in the almost complete reversal of the TPO-mediated activation of αIIbβ3 in both primary megakaryocytic and HEL cells. These findings underline the role of cytoskeletal rearrangement and focal contact formation in mediating the αIIbβ3 adhesion to fibrinogen or fibronectin.39
PI 3-K phosphorylates the D-3 position of the inositol ring of phosphoinositols and produces novel D-3 phosphoinositides (PI-3P, PI-3P2 , and PI-3,4P3).40 A body of experimental evidence indicates that 3′-phosphorylated inositides represent a class of second messenger molecules whose function and interaction with other proteins remains to be fully elucidated, although recent studies have suggested that the lipid products generated by PI 3-K can activate some isoforms of protein kinase C (PKC)41 and p70 S6 kinase.42 In this respect, we and others23-25,43 have previously shown that differentiation of HEL cells along the megakaryocytic lineage is accompanied by a selective activation of specific PKC isoforms, including PKC-ζ, one of the main targets of the PI 3-K lipid products. A role for PKC in αIIbβ3 activation is also consistent with the observations of other investigators showing that a brief exposure of both myeloid and lymphoid cell lines to phorbol esters greatly enhanced cell adhesion to plates coated with fibrinogen or PAC1 MoAb.44 45
PI 3-K found in cellular complexes with ligand-activated growth factor receptors and oncogene protein tyrosine kinases (PTKs) is a heterodimer composed of a p85 (regulatory) and a p110 (catalytic) subunits.46 The former acts as an adaptor protein allowing the p110 catalytic subunit to interact with receptor and nonreceptor PTKs and tyrosine-phosphorylated proteins, resulting in the activation of PI 3-K enzymatic activity. The best-characterized mode of association of PI 3-K with upstream signaling receptors occurs via tyrosine phosphorylation-dependent association of PI 3-K mediated by src-homology 2 (SH2) domains found on the p85 subunit.47,48 The p85 subunit also contains proline-rich sequences capable of mediating the binding of proteins containing SH3 domains.49 The SH3 domains of the intracellular tyrosine kinases abl, lck, fyn, and lyn have been shown to associate with this sequence in the p85 subunit. Finally, the p85 subunit itself contains an SH3 domain.50
A wide variety of cellular functions appears to be dependent on PI 3-K, including growth factor–dependent mitogenesis, cytoskeletal rearrangements, glucose uptake, prevention of apoptosis, cytokine production, and endocytic trafficking.51,52 Moreover, the ligand stimulation of c-kit, Epo, interleukin-3 (IL-3), granulocyte-macrophage CSF (GM-CSF), or M-CSF receptors53-56 causes association of the receptor and activation of PI 3-K, strongly suggesting that PI 3-K might also play a role for the development of normal hematopoietic cells.
We have shown that TPO is also able to stimulate PI 3-K catalytic activity. It remains to be determined whether TPO-mediated activation of PI 3-K requires tyrosine phosphorylation, as demonstrated in peripheral platelets,15 or can take place through alternative (SH3-mediated) mechanisms as proposed in a different model system.57 Consistently with this latter hypothesis, Drachman et al58 have shown that the BaF3 hematopoietic cell line engineered to express the c-mpl receptor did not show any tyrosine phosphorylation of p85 in the presence of TPO and rather showed a constant association of p85 to the receptor also in untreated cells.
The ability of either RGDS peptides or anti-αIIb antibody to significantly reduce the number of CFU-meg when added to CD34+ cells before plating in a semisolid fibrinclot assay supplemented with 100 ng/mL of TPO suggests that the PI 3-K–mediated activation of αIIbβ3 might play a physiologic role during the process of megakaryocyte development. If this hypothesis is correct, the early expression of αIIbβ3 in megakaryocytic progenitors might allow the expansion of megakaryocytic cells in the presence of specific growth factors and BM matrix proteins containing the RGD sequence, such as fibronectin, which represents a major component of the BM microenvironment.59 60
Supported by the “Blood and AIDS Projects” of the Italian Ministry of Health, by PF ACRO, and by AIRC.
Address reprint requests to Giorgio Zauli, MD, PhD, Institute of Human Anatomy, Via Fossato di Mortara 66, 44100 Ferrara, Italy.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal