Abstract
Stromal support is required during retroviral-mediated transduction of human bone marrow-derived CD34+ cells to maintain the clonogenicity of the primitive progenitors. We hypothesized that the cytokine FLT3 ligand (FL) might be able to replace the maintenance role provided by the stroma. CD34+ progenitors from human bone marrow were transduced by the retroviral vector LN with the cytokines interleukin-3 (IL-3), IL-6, and stem cell factor (SCF ) present in all cultures. Transductions were performed with or without stromal support and with or without the inclusion of 100 U/mL FL. No significant increase in gene transfer into colony-forming cells was obtained by the addition of FL to the cultures. Transduction and survival of more primitive human hematopoietic cells was determined by growth in immune-deficient mice for 7 to 8 months. Human myeloid cells, T lymphocytes, and colony-forming progenitors were recovered from the marrow of mice that had received human cells transduced on stroma or in suspension culture with IL-3, IL-6, SCF, and FL, but not with IL-3, IL-6, and SCF alone. LN provirus was detected by polymerase chain reaction in the marrow recovered from 9 of 10 mice transplanted with human CD34+ cells transduced with stromal support, 5 of 11 mice that received human cells transduced in suspension culture with FL, but none of the 10 mice that received human cells transduced in suspension culture without FL. We conclude that FLT3 ligand, in conjunction with IL-3, IL-6, and SCF, preserves the generative capacity of primitive human hematopoietic cells during in vitro transductions in suspension culture.
A RESEARCH GOAL vital to the field of human gene therapy is to optimize transduction of pluripotent human hematopoietic stem cells (HSCs). Corrected stem cells should contribute to hematopoiesis throughout the lifetime of the recipient. Retroviral vectors are currently the most effective vehicle to stably integrate new genes into the host cell chromosomes. The major limitation of retroviral-mediated gene transfer is the requirement for DNA replication in the target cell.1,2 HSCs are largely quiescent, so exogenous cytokines and/or stromal cells must be used to prompt cell division. One effective transduction strategy involves cocultivation of the hematopoietic cells directly on irradiated vector-producing fibroblasts (VPF ). Cocultivation results in contamination of the marrow sample with VPF, which could lead to infusion of components of the murine cells into patients in clinical trials. Transduction with cell-free retroviral supernatant in suspension culture is a more clinically relevant method of gene transfer, but is less effective.3,4 The addition of cell-free supernatant to marrow progenitors on an irradiated stromal support layer allows efficient transduction.5-8 In addition, our group previously showed that the presence of a human stromal support layer was essential to preserve the ability of human hematopoietic progenitors to sustain durable, long-term engraftment in immune-deficient mice.9 However, obtaining sufficient quantities of autologous stromal cells for clinical trials can be problematic. Therefore, alternate methods are sought.
Human CD34+ progenitors transduced for 72 hours in suspension culture with interleukin-3 (IL-3), IL-6, and stem cell factor (SCF ), in the absence of stromal support, lost the ability to contribute to hematopoiesis in immune-deficient (bnx) mice for greater than 4 months.9 The novel cytokine FLT3 ligand (FL) has been shown to stimulate the proliferation of human CD34+ cells and might be an essential viability factor provided by the stroma.10-14 FL could potentially promote cell division and subsequent retroviral integration during 72-hour in vitro transduction periods. In the current studies, we performed a series of experiments to examine the effects of inclusion of FL in transductions performed in the absence of stromal support. Transduction of CD34+ cells from human bone marrow was performed in the presence of the cytokines IL-3, IL-6, and SCF, comparing suspension culture with or without inclusion of FL to transduction on stromal support. The cells were then assessed for the extent of gene transfer into 14-day colony-forming progenitors and for the survival and transduction of more primitive cells able to sustain human hematopoiesis in bnx mice for 7 to 8 months.
As we had previously observed, human cells cultured for 72 hours in suspension culture with IL-3, IL-6, and SCF had a long-term survival defect and could not be recovered from the mice. In contrast, cells cultured under the same conditions, but with inclusion of stromal support or 100 ng/mL FL, sustained long-term hematopoiesis. Human cells, including colony-forming progenitors, marked by the LN vector were recovered from the marrow of some mice transplanted with CD34+ progenitors transduced in suspension culture, only if FL had been present in vitro. However, FL did not completely replace the beneficial effects of stromal support on transduction or maintenance of primitive cells, suggesting that additional factors are necessary to achieve the full effect. Clonal analysis of marrow samples from each mouse by inverse polymerase chain reaction (invPCR) detected one or two marked progenitors contributing to human hematopoiesis in mice that had received CD34+ cells exposed to IL-3, IL-6, SCF, FL, and the LN vector for 72 hours and 1 to 6 marked human progenitors in mice that had been transplanted with human cells transduced on stroma. The results of the marking studies, coupled with the fact that inclusion of FL was able to maintain the clonogenic capacity of primitive human cells in suspension culture, indicate that there may be a role for the use of FL in retroviral-mediated gene transfer.
MATERIALS AND METHODS
Human marrow and stromal cells.Normal human bone marrow cells were obtained from screens used to filter marrow during the harvest of normal, allogeneic donors. Isolation of CD34+ cells and preparation of stromal monolayers were performed as described.9 Stroma was used as a supporting layer for transduction between passages 3 and 5, after hematopoietic cells and macrophages had been eradicated. Stromal cells were irradiated (20 Gy) and plated at 2 × 105 cells per T-25 vent-cap flask (Costar, Cambridge, MA) in the appropriate transduction medium 1 day before use.
Transduction of human CD34+ progenitors.Human progenitors from bone marrow were transduced by addition of cell-free supernatant from the PG13/LN Gibbon ape leukemia virus (GALV) pseudotype packaging cell line15 with and without stromal support as described.9 The PG13/LN supernatant had a titer of 5 × 106 infectious virions/mL, as assayed on the human colon carcinoma cell line HT29 (American Type Culture Collections [ATCC], Rockville, MD), and was determined to be free of ecotropic, amphotropic, and GALV recombinant helper virus by PCR for env and marker rescue assay on 3T3 and HT29 cells (ATCC). Transductions were performed as described, with the inclusion of 100 ng/mL FLT3 Ligand (generously donated by DNAX Corp, Palo Alto, CA), in designated samples.9
Mice.All studies used 6-week-old beige/nude/xid homozygous mice (bg.bg/nu.nu/xid.xid ) bred at Childrens Hospital Los Angeles (Los Angeles, CA). Breeding stocks were originally obtained from Dr Carl Hansen (National Institutes of Health, Bethesda, MD). Cotransplantation of transduced human progenitors and stromal cells producing IL-3 was performed as previously published.16 Mice were killed by 90% CO2 /10% O2 narcosis 7 to 8 months after transplantation with human cells. Bone marrow was flushed from the tibiae and femurs of each mouse into 1× phosphate-buffered saline (PBS), dispersed with a fine needle, and used for the assays described below. The remainder of each bone marrow sample was viably cryopreserved for later analyses.
Fluorescence-activated cell sorting (FACS) analysis.Single-cell suspensions from the marrow and spleen of cotransplanted mice were preincubated for 15 minutes on ice with unconjugated mouse Ig (MsIgG; Coulter, Hialeah, FL). Directly conjugated antibodies used to identify human-specific cell surface antigens were HLE-1 (anti-CD45; Becton Dickinson [BD], San Jose, CA), My9-RD1 (anti-CD33; Coulter), Leu-12 (anti-CD19; BD), Leu-3a (anti-CD4; BD), and Leu-2a (anti-CD8; BD). The antimouse CD45-R-phycoerythrin (PE) antibody (Pharmingen, San Diego, CA) was used to identify murine leukocytes. After a 15-minute antibody binding period on ice, cells were depleted of red blood cells by resuspension in Ortho Lysis Buffer (BD), washed, and fixed in 1% paraformaldehyde. Samples were acquired on a Becton Dickinson FACScan and analyzed using the Cellquest software package (BD). Ten thousand events were acquired for each sample. In all experiments, parallel staining and FACS analyses were performed on normal human and nontransplanted bnx mouse bone marrow controls to confirm that none of the human-specific antibodies cross-reacted with murine cells.
PCR.For amplification of LN vector sequences from 1 ng of DNA from tissue samples, neo primers were used as described.16 Samples were subjected to electrophoresis in 2% agarose gels with ethidium bromide and photographed with UV illumination. To confirm the identity of the amplified products, gels were transferred to Biotrace B nylon membrane (Pall Biodyne, East Hills, NY) and hybridized with an oligonucleotide probe as described.16
Human-specific colony-forming unit (CFU) plating.To determine the number of human hematopoietic progenitors engrafted within the murine bone marrow, cells harvested from each mouse were plated in human-specific CFU assay as described.16 Before plating, the bnx/hu bone marrow cells were incubated for 4 to 12 hours to remove (by adherence) murine stromal cells, which secrete murine cytokines and invalidate the specificity for growth of human hematopoietic colonies measured in the assay. Colonies were enumerated on day 21, as previously described.16
Inverse PCR.DNA preparation from individual CFU, containing 100 to 1,000 cells, was performed as described.17 DNA from the tissues of each bnx/hu mouse was performed using standard methods as described.18 Half of the DNA sample obtained from each colony or 50 ng DNA from the tissues was subjected to nested inverse PCR reactions as described.17 The resulting PCR products were electrophoresed on a 2% gel (1% Seakem LE agarose and 1% Nu-Sieve; FMC Bioproducts, Rockland, ME) and transferred to nylon membrane (Hybond-N+; Amersham, Arlington Heights, IL). Incubation in SSPE hybridization buffer (10× Denhardt's,17 5× SSPE,17 and 0.5% sodium dodecyl sulfate [SDS]) was performed for at least 4 hours at 55°C with an oligonucleotide probe (5′ GGCAAGCTAGCTTAAGT) specific for LTR sequences end-labeled with γ-32P-ATP by T4 kinase (GIBCO/BRL, Grand Island, NY). After hybridization, blots were washed twice for 5 minutes each at room temperature in SSPE wash buffer no. 1 (2× SSPE, 0.1% SDS). Next, blots were washed for 10 minutes at 55°C in SSPE wash buffer no. 2 (5× SSPE, 0.1% SDS). Exposure to high performance autoradiography film (Hyperfilm-MP; Amersham) was performed for varying lengths of time (10 minutes to 1 week) depending on the strength of the signal. Verification of the identity of clonal patterns by sequencing was performed as described.19
Statistical analyses.The significance of each set of values was assessed using the two-tailed t-test assuming equal variance with the Excel 5.0 software (Microsoft Excel Analysis Toolpack, developed by GreyMatter International, Inc, Cambridge, MA). Average values are listed with standard deviations.
RESULTS
Transduction of human CD34+ progenitors.Human bone marrow was enriched for CD34+ progenitors by immunomagnetic separation. Enriched populations were 90% to 95% CD34+, as shown by FACS analysis.20 The selected cells were transduced by the addition of supernatant from the retroviral vector LN packaged in a GALV pseudotype by the cell line PG13.15 The LN vector carries the bacterial neomycin resistance gene21 and imparts resistance to the selective agent G418. Three supernatant additions were performed at 24-hour intervals over the course of 72 hours. The cytokines IL-3, IL-6, and SCF were present in all cultures. Transductions were performed in suspension culture with or without the inclusion of 100 ng/mL FLT3 ligand, in direct comparison to transduction on stromal support. A portion of each transduced sample was plated in 14-day colony-forming assay with or without the selective agent G418, to assess the extent of gene transfer into committed progenitors. The remainder of each sample was transplanted into a set of bnx mice to allow the subsequent study of more primitive human hematopoietic cells (Fig 1).
Experimental schema. CD34+ progenitors were isolated from human bone marrow by immunomagnetic selection. Cells were transduced by the LN retroviral vector packaged with the GALV pseudotype envelope protein by the PG13 cell line. All transductions were performed in the presence of IL-3 (10 ng/mL), IL-6 (50 U/mL), and SCF (50 ng/mL). Transductions were performed in suspension culture (SNS; supernatant, no stroma), in suspension with addition of 100 ng/mL FLT3 ligand (SNS + FL), or with the support of an irradiated allogeneic bone marrow stromal cell monolayer (supernatant on stroma [SOS]). After transduction, methylcellulose-based CFU assays with or without G418 were performed to assess the extent of gene transfer into colony-forming progenitors. The remainder of each sample was cotransplanted with IL-3–producing primary human marrow stromal cells into a cohort of sublethally irradiated bnx mice. Mice were harvested 7 to 8 months after transplantation, and the extent of human hematopoietic cell engraftment and vector marking in all tissues was assessed.
Experimental schema. CD34+ progenitors were isolated from human bone marrow by immunomagnetic selection. Cells were transduced by the LN retroviral vector packaged with the GALV pseudotype envelope protein by the PG13 cell line. All transductions were performed in the presence of IL-3 (10 ng/mL), IL-6 (50 U/mL), and SCF (50 ng/mL). Transductions were performed in suspension culture (SNS; supernatant, no stroma), in suspension with addition of 100 ng/mL FLT3 ligand (SNS + FL), or with the support of an irradiated allogeneic bone marrow stromal cell monolayer (supernatant on stroma [SOS]). After transduction, methylcellulose-based CFU assays with or without G418 were performed to assess the extent of gene transfer into colony-forming progenitors. The remainder of each sample was cotransplanted with IL-3–producing primary human marrow stromal cells into a cohort of sublethally irradiated bnx mice. Mice were harvested 7 to 8 months after transplantation, and the extent of human hematopoietic cell engraftment and vector marking in all tissues was assessed.
The extent of transduction in each condition before transplantation is shown in Table 1. Stromal support was required to obtain efficient transduction of colony-forming progenitors with retroviral supernatant (average extent of transduction, 4.2% ± 3.6% G418R CFU without stromal support [SNS] and 17.7% ± 5.6% with stroma [SOS], P < .01). No significant increase in the extent of gene transduction into committed colony-forming progenitors was caused by inclusion of FL in the transduction media (SNS + FL) in the absence of stromal support (average, 5.57% ± 2.8% G418R CFU with FL and 4.2% ± 3.6% without FL, P = .6). The average extent of transduction in suspension culture, with or without FL, was not statistically higher than the SHAM background level. There was no significant difference in the composition of the CFU (burst-forming unit-erythroid [BFU-E], CFU-MIX, and CFU–granulocyte-macrophage [CFU-GM]) obtained after transduction in the different conditions. Transduction on stromal support provided the only gene transfer level that was significantly higher than background (average, 17.7% ± 5.6 % G418R CFU with stromal support v 1.8% ± 1.6% G418R CFU in the SHAM condition, P = .002). Therefore, the addition of FL to suspension culture transductions, in the presence of IL-3, IL-6, and SCF, did not result in significant extents of gene transfer into colony-forming progenitors.
Transduction Extent of Colony-Forming Progenitors
| Transduction Condition . | G418R/Total CFU . | % G418R . | Bnx/hu Set No. . |
|---|---|---|---|
| Experiment no. 1 | |||
| SHAM | 3/249 | 1.2 | |
| SNS + 3/6/S | 22/235 | 9.4 | |
| SNS + 3/6/S + FL | 21/229 | 9.2 | |
| SOS + 3/6/S | 61/270 | 22.6 | 43 |
| Experiment no. 2 | |||
| SHAM | 26/630 | 4.1 | |
| SNS + 3/6/S | 18/534 | 3.3 | |
| SNS + 3/6/S + FL | 18/525 | 3.4 | |
| SOS + 3/6/S | 167/738 | 22.6 | 44 |
| Experiment no. 3 | |||
| SHAM | 3/522 | 0.6 | |
| SNS + 3/6/S | 11/918 | 1.2 | |
| SNS + 3/6/S + FL | 23/363 | 6.3 | |
| SOS + 3/6/S | 86/696 | 12.6 | 46 |
| Experiment no. 4 | |||
| SHAM | 8/785 | 1.1 | |
| SNS + 3/6/S | 15/514 | 2.9 | |
| SNS + 3/6/S + FL | 21/623 | 3.4 | |
| SOS + 3/6/S | 113/861 | 13.1 | 48 |
| Transduction Condition . | G418R/Total CFU . | % G418R . | Bnx/hu Set No. . |
|---|---|---|---|
| Experiment no. 1 | |||
| SHAM | 3/249 | 1.2 | |
| SNS + 3/6/S | 22/235 | 9.4 | |
| SNS + 3/6/S + FL | 21/229 | 9.2 | |
| SOS + 3/6/S | 61/270 | 22.6 | 43 |
| Experiment no. 2 | |||
| SHAM | 26/630 | 4.1 | |
| SNS + 3/6/S | 18/534 | 3.3 | |
| SNS + 3/6/S + FL | 18/525 | 3.4 | |
| SOS + 3/6/S | 167/738 | 22.6 | 44 |
| Experiment no. 3 | |||
| SHAM | 3/522 | 0.6 | |
| SNS + 3/6/S | 11/918 | 1.2 | |
| SNS + 3/6/S + FL | 23/363 | 6.3 | |
| SOS + 3/6/S | 86/696 | 12.6 | 46 |
| Experiment no. 4 | |||
| SHAM | 8/785 | 1.1 | |
| SNS + 3/6/S | 15/514 | 2.9 | |
| SNS + 3/6/S + FL | 21/623 | 3.4 | |
| SOS + 3/6/S | 113/861 | 13.1 | 48 |
Methylcellulose-based colony-forming assays were plated 96 hours after the initiation of transduction. Supernatant from the PG13 cell line, packaging the LN retroviral vector, was used in all cases. Transduction conditions used were supernatant addition with stromal support (SOS) or supernatant addition in suspension culture with addition of IL-3, IL-6, and SCF (3/6/S) with or without the addition of FLT3 ligand (SNS and SNS + FL, respectively). Colonies were enumerated on day 14. The percentage of G418-resistant colony-forming progenitors was calculated as (no. of G418-resistant CFU/total no. of CFU) × 100. The values shown were obtained from 3,000 CD34+ cells plated with and without G418 (0.9 mg/mL).
Engraftment of human hematopoietic cells in bnx mice.After in vitro transduction, each sample was cotransplanted with primary human stromal cells engineered to produce human IL-3 into 6-week-old beige/nude/xid mice as described.16 To transplant a series of 12 mice in 3 groups, 4 × 106 purified CD34+ cells were initially inoculated into three separate transduction flasks to transplant 4 mice from each flask. The cell numbers resulting after the in vitro period were not recounted or normalized, but reflect the input cell number. Therefore, differences in the extent of human hematopoietic cell recovery from each mouse reflect changes in the CD34+ progenitors that had occurred during the 72-hour in vitro transduction period, which was followed by the relatively constant environment of highly inbred bnx mice.
Mice were killed 7 to 8 months after xenotransplantation. Marrow was recovered and analyzed for the percentage of human CD45+ cell engraftment by FACS analysis. The human hematopoietic lineages engrafted in each mouse were compared to determine whether the initial in vitro transduction conditions had influenced subsequent differentiation of the transplanted CD34+ cells. Immune-deficient mice that had received human cells transduced in suspension culture in the presence of IL-3, IL-6, and SCF (SNS, N = 10) lacked significant numbers of human cells in all organs 7 to 8 months after transplantation (average, 0.12% human CD45+ cells in the marrow; Table 2). In contrast, human cells transduced under the same conditions with the addition of 100 U/mL FLT3 ligand (SNS + FL) were recovered from the marrow of 9 of 11 mice. Human CD45+ cells, detected by FACS, averaged 3.7% in this group. Nine of ten mice transplanted with human cells transduced on stromal support (SOS) also had significant levels of engrafted human hematopoietic cells in their marrow, ranging from 2.82% to 11.08% and averaging 5.5% (Table 2).
Human Hematopoietic Lineages Recovered From the Bone Marrow of bnx Mice 7 to 8 Months After Transplantation
| Mouse No. . | Transduction Conditions . | Months Engrafted . | % hu CD45+ . | % hu CD4 . | % hu CD8 . | % hu CD33 . | % hu CD19 . | ||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | BM . | spl . | Blood . | . | . | . | . |
| 43A1 | SNS | 8 | 0.4 | — | — | 0.1 | 0.2 | 0.2 | 0.0 |
| 43A2 | SNS | 8 | 0.1 | — | — | 0.0 | 0.0 | 0.0 | 0.0 |
| 44A1 | SNS | 7 | 0.0 | — | — | 0.0 | 0.0 | 0.0 | 0.0 |
| 44A2 | SNS | 7 | 0.1 | — | — | 0.0 | 0.1 | 0.1 | 0.0 |
| 44A3 | SNS | 7 | 0.0 | — | — | 0.0 | 0.0 | 0.0 | 0.0 |
| 46B1 | SNS | 7.5 | 0.1 | — | — | 0.1 | 0.0 | 0.0 | 0.0 |
| 46B2 | SNS | 7.5 | 0.1 | — | — | 0.0 | 0.0 | 0.1 | 0.0 |
| 48A1 | SNS | 8.5 | 0.3 | — | — | 0.1 | 0.2 | 0.1 | 0.0 |
| 48A2 | SNS | 8.5 | 0.0 | — | — | 0.0 | 0.0 | 0.0 | 0.0 |
| 48A3 | SNS | 8.5 | 0.0 | — | — | 0.0 | 0.0 | 0.0 | 0.0 |
| 43B1 | SNS + FL | 8 | 4.5 | 1.7 | — | 1.3 | 2.4 | 0.9 | 0.0 |
| 43B2 | SNS + FL | 8 | 1.8 | — | — | 0.5 | 1.0 | 0.8 | 0.0 |
| 44B1 | SNS + FL | 7 | 0.0 | — | — | 0.0 | 0.1 | 0.0 | 0.0 |
| 44B2 | SNS + FL | 7 | 4.4 | — | — | 1.0 | 2.1 | 1.5 | 0.0 |
| 44B3 | SNS + FL | 7 | 4.0 | — | — | 1.2 | 2.0 | 1.7 | 0.0 |
| 46C1 | SNS + FL | 7.5 | 5.3 | — | 0.3 | 1.2 | 2.2 | 1.5 | 0.0 |
| 46C2 | SNS + FL | 7.5 | 3.2 | — | — | 1.3 | 1.0 | 0.9 | 0.0 |
| 46C3 | SNS + FL | 7.5 | 7.3 | 1.2 | — | 2.6 | 2.5 | 3.1 | 0.1 |
| 48B1 | SNS + FL | 8.5 | 2.9 | — | — | 1.0 | 1.0 | 0.9 | 0.0 |
| 48B2 | SNS + FL | 8.5 | 7.6 | 5.6 | 1.6 | 1.8 | 3.6 | 1.5 | 0.0 |
| 48B3 | SNS + FL | 8.5 | 0.0 | — | — | 0.0 | 0.0 | 0.0 | 0.0 |
| 43C1 | SOS | 8 | 7.3 | 3.2 | 1.0 | 2.9 | 3.6 | 1.4 | 0.0 |
| 43C2 | SOS | 8 | 11.1 | 2.4 | 0.1 | 3.1 | 6.4 | 3.0 | 0.0 |
| 43C3 | SOS | 8 | 3.4 | 0.9 | — | 1.7 | 2.4 | 0.9 | 0.0 |
| 44C1 | SOS | 7 | 7.9 | 1.7 | — | 2.4 | 3.0 | 2.2 | 0.0 |
| 44C2 | SOS | 7 | 2.8 | — | 0.5 | 0.8 | 1.2 | 1.3 | 0.0 |
| 46A1 | SOS | 7.5 | 9.9 | — | 1.1 | 3.5 | 2.5 | 4.4 | 0.0 |
| 46A3 | SOS | 7.5 | 5.3 | 2.1 | 0.4 | 2.2 | 2.1 | 1.3 | 0.1 |
| 48C1 | SOS | 8.5 | 4.1 | — | — | 1.5 | 2.3 | 1.3 | 0.0 |
| 48C2 | SOS | 8.5 | 0.2 | — | — | 0.0 | 0.0 | 0.1 | 0.0 |
| 48C3 | SOS | 8.5 | 3.2 | 0.7 | — | 1.4 | 1.7 | 0.9 | 0.0 |
| Mouse No. . | Transduction Conditions . | Months Engrafted . | % hu CD45+ . | % hu CD4 . | % hu CD8 . | % hu CD33 . | % hu CD19 . | ||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | BM . | spl . | Blood . | . | . | . | . |
| 43A1 | SNS | 8 | 0.4 | — | — | 0.1 | 0.2 | 0.2 | 0.0 |
| 43A2 | SNS | 8 | 0.1 | — | — | 0.0 | 0.0 | 0.0 | 0.0 |
| 44A1 | SNS | 7 | 0.0 | — | — | 0.0 | 0.0 | 0.0 | 0.0 |
| 44A2 | SNS | 7 | 0.1 | — | — | 0.0 | 0.1 | 0.1 | 0.0 |
| 44A3 | SNS | 7 | 0.0 | — | — | 0.0 | 0.0 | 0.0 | 0.0 |
| 46B1 | SNS | 7.5 | 0.1 | — | — | 0.1 | 0.0 | 0.0 | 0.0 |
| 46B2 | SNS | 7.5 | 0.1 | — | — | 0.0 | 0.0 | 0.1 | 0.0 |
| 48A1 | SNS | 8.5 | 0.3 | — | — | 0.1 | 0.2 | 0.1 | 0.0 |
| 48A2 | SNS | 8.5 | 0.0 | — | — | 0.0 | 0.0 | 0.0 | 0.0 |
| 48A3 | SNS | 8.5 | 0.0 | — | — | 0.0 | 0.0 | 0.0 | 0.0 |
| 43B1 | SNS + FL | 8 | 4.5 | 1.7 | — | 1.3 | 2.4 | 0.9 | 0.0 |
| 43B2 | SNS + FL | 8 | 1.8 | — | — | 0.5 | 1.0 | 0.8 | 0.0 |
| 44B1 | SNS + FL | 7 | 0.0 | — | — | 0.0 | 0.1 | 0.0 | 0.0 |
| 44B2 | SNS + FL | 7 | 4.4 | — | — | 1.0 | 2.1 | 1.5 | 0.0 |
| 44B3 | SNS + FL | 7 | 4.0 | — | — | 1.2 | 2.0 | 1.7 | 0.0 |
| 46C1 | SNS + FL | 7.5 | 5.3 | — | 0.3 | 1.2 | 2.2 | 1.5 | 0.0 |
| 46C2 | SNS + FL | 7.5 | 3.2 | — | — | 1.3 | 1.0 | 0.9 | 0.0 |
| 46C3 | SNS + FL | 7.5 | 7.3 | 1.2 | — | 2.6 | 2.5 | 3.1 | 0.1 |
| 48B1 | SNS + FL | 8.5 | 2.9 | — | — | 1.0 | 1.0 | 0.9 | 0.0 |
| 48B2 | SNS + FL | 8.5 | 7.6 | 5.6 | 1.6 | 1.8 | 3.6 | 1.5 | 0.0 |
| 48B3 | SNS + FL | 8.5 | 0.0 | — | — | 0.0 | 0.0 | 0.0 | 0.0 |
| 43C1 | SOS | 8 | 7.3 | 3.2 | 1.0 | 2.9 | 3.6 | 1.4 | 0.0 |
| 43C2 | SOS | 8 | 11.1 | 2.4 | 0.1 | 3.1 | 6.4 | 3.0 | 0.0 |
| 43C3 | SOS | 8 | 3.4 | 0.9 | — | 1.7 | 2.4 | 0.9 | 0.0 |
| 44C1 | SOS | 7 | 7.9 | 1.7 | — | 2.4 | 3.0 | 2.2 | 0.0 |
| 44C2 | SOS | 7 | 2.8 | — | 0.5 | 0.8 | 1.2 | 1.3 | 0.0 |
| 46A1 | SOS | 7.5 | 9.9 | — | 1.1 | 3.5 | 2.5 | 4.4 | 0.0 |
| 46A3 | SOS | 7.5 | 5.3 | 2.1 | 0.4 | 2.2 | 2.1 | 1.3 | 0.1 |
| 48C1 | SOS | 8.5 | 4.1 | — | — | 1.5 | 2.3 | 1.3 | 0.0 |
| 48C2 | SOS | 8.5 | 0.2 | — | — | 0.0 | 0.0 | 0.1 | 0.0 |
| 48C3 | SOS | 8.5 | 3.2 | 0.7 | — | 1.4 | 1.7 | 0.9 | 0.0 |
Bone marrow, spleen, and blood were recovered from bnx mice 7 to 8 months after cotransplantation of transduced human hematopoietic cells and IL-3–producing stromal cells. The percentage of human CD45+ cells in the marrow (BM), spleen (spl), and blood was determined by FACS. A dash indicates that no human hematopoietic cells were detected in that sample. The percentages of human cells that were positive for each lineage marker by FACS analysis of the ungated bone marrow cells are shown. In each experiment, normal human peripheral blood and age-matched, nontransplanted bnx mice were analyzed concurrently to verify the species specificity of the monoclonal antibodies.
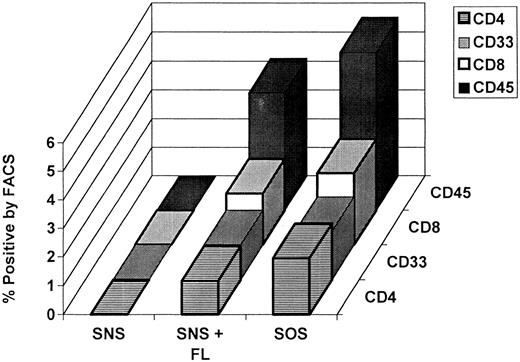
Human hematopoietic cell lineages recovered from the mice were assessed by FACS analysis using monoclonal antibodies specific for human cell surface antigens. In each case, concurrent normal human and nontransplanted age-matched bnx mice were used as controls to verify the species specificity of the antibodies at the concentrations used. The lineages examined were CD19+ B lymphoid, CD4+ and CD8+ T lymphoid, and CD33+ myeloid. The percentages of total bnx/hu bone marrow populations representing human cells of each lineage are shown in Table 2. As we had previously observed in the bnx/hu cotransplantation system,16 there was an absolute lack of development of human CD19+ B lymphocytes in all mice tested. There was no significant difference in the extent of development of the T-cell or myeloid lineages, depending on whether the human cells had been cultured with stromal support and IL-3, IL-6, and SCF or in suspension culture with IL-3, IL-6, SCF, and FL (Fig 2).
Ratio of human hematopoietic lineage to total human cells recovered from long-term engrafted mice. Bone marrow recovered from each bnx/hu mouse was subjected to antibody labeling followed by FACS analysis to determine the percentage of engrafted human hematopoietic cells of each lineage. The averages of each cell type recovered from all mice that received human cells cultured with the same transduction conditions before transplantation are shown.
Ratio of human hematopoietic lineage to total human cells recovered from long-term engrafted mice. Bone marrow recovered from each bnx/hu mouse was subjected to antibody labeling followed by FACS analysis to determine the percentage of engrafted human hematopoietic cells of each lineage. The averages of each cell type recovered from all mice that received human cells cultured with the same transduction conditions before transplantation are shown.
To quantitate the clonogenic human hematopoietic progenitors in the bnx/hu bone marrow, human-specific colony-forming assays were plated from all samples. No human colonies were grown from the marrow of 9 bnx/hu mice from the SNS group (Table 3). Variable numbers of human CFU of all lineages, BFU-E, CFU-GM, and CFU-GEMM, were recovered from mice in the SNS + FL (N = 11) and SOS (N = 10) groups (Table 3). Colonies obtained from the well-engrafted mice showed no significant difference in composition (BFU-E, CFU-MIX, and CFU-GM) that might have been induced by the transduction condition influencing differentiation before transplantation.
Comparison of the Human CD45+ Cell and Clonogenic Progenitor Content in 1 Million Bone Marrow Cells Recovered From Mice That Received Human CD34+ Progenitor Cells Transduced in Suspension With or Without FL Versus on Stromal Support
| bnx No. . | Human CD45+ Cells per 1 × 106bnx BM Cells . | Human CFU per 1 × 106bnx BM Cells . |
|---|---|---|
| Transplanted with human CD34+ cells transduced in suspension culture with IL-3, IL-6, and SCF | ||
| 43A1 | 3,700 | 03-150 |
| 43A2 | 900 | 0 |
| 44A1 | 03-151 | 0 |
| 44A2 | 1,400 | 0 |
| 44A3 | 0 | 0 |
| 46B1 | 1,100 | 0 |
| 46B2 | 900 | 0 |
| 48A1 | 3,100 | 0 |
| 48A2 | 0 | 0 |
| 48A3 | 0 | 0 |
| Average | 1,110 ± 1,324 | 0 ± 0 |
| Transplanted with human CD34+ cells transduced in suspension culture with IL-3, IL-6, SCF, and FL | ||
| 43B1 | 44,900 | 63 |
| 43B2 | 18,100 | 0 |
| 44B1 | 0 | 10 |
| 44B2 | 44,000 | 103 |
| 44B3 | 40,100 | 153 |
| 46C1 | 53,200 | 177 |
| 46C2 | 31,800 | 20 |
| 46C3 | 73,400 | 107 |
| 48B1 | 29,400 | 103 |
| 48B2 | 75,900 | 57 |
| 48B3 | 0 | 0 |
| Average | 37,345 ± 25,299 | 72 ± 62 |
| Transplanted with human CD34+ cells transduced on stromal support with IL-3, IL-6, and SCF | ||
| 43C1 | 73,200 | 90 |
| 43C2 | 110,800 | 223 |
| 43C3 | 34,200 | 7 |
| 44C1 | 78,900 | 240 |
| 44C2 | 28,200 | 213 |
| 46A1 | 99,100 | 326 |
| 46A3 | 52,500 | 170 |
| 48C1 | 40,900 | 203 |
| 48C2 | 1,500 | 0 |
| 48C3 | 32,000 | 233 |
| Average | 55,130 ± 34,493 | 171 ± 106 |
| bnx No. . | Human CD45+ Cells per 1 × 106bnx BM Cells . | Human CFU per 1 × 106bnx BM Cells . |
|---|---|---|
| Transplanted with human CD34+ cells transduced in suspension culture with IL-3, IL-6, and SCF | ||
| 43A1 | 3,700 | 03-150 |
| 43A2 | 900 | 0 |
| 44A1 | 03-151 | 0 |
| 44A2 | 1,400 | 0 |
| 44A3 | 0 | 0 |
| 46B1 | 1,100 | 0 |
| 46B2 | 900 | 0 |
| 48A1 | 3,100 | 0 |
| 48A2 | 0 | 0 |
| 48A3 | 0 | 0 |
| Average | 1,110 ± 1,324 | 0 ± 0 |
| Transplanted with human CD34+ cells transduced in suspension culture with IL-3, IL-6, SCF, and FL | ||
| 43B1 | 44,900 | 63 |
| 43B2 | 18,100 | 0 |
| 44B1 | 0 | 10 |
| 44B2 | 44,000 | 103 |
| 44B3 | 40,100 | 153 |
| 46C1 | 53,200 | 177 |
| 46C2 | 31,800 | 20 |
| 46C3 | 73,400 | 107 |
| 48B1 | 29,400 | 103 |
| 48B2 | 75,900 | 57 |
| 48B3 | 0 | 0 |
| Average | 37,345 ± 25,299 | 72 ± 62 |
| Transplanted with human CD34+ cells transduced on stromal support with IL-3, IL-6, and SCF | ||
| 43C1 | 73,200 | 90 |
| 43C2 | 110,800 | 223 |
| 43C3 | 34,200 | 7 |
| 44C1 | 78,900 | 240 |
| 44C2 | 28,200 | 213 |
| 46A1 | 99,100 | 326 |
| 46A3 | 52,500 | 170 |
| 48C1 | 40,900 | 203 |
| 48C2 | 1,500 | 0 |
| 48C3 | 32,000 | 233 |
| Average | 55,130 ± 34,493 | 171 ± 106 |
Bone marrow was recovered from bnx mice after engraftment periods of 7 to 8 months. The percentage of human CD45+ cells in the marrow (BM) was determined by FACS, and the number of human cells per 1 × 106 bnx bone marrow cells (total, ungated cells from the FACS profile) was calculated. Human-specific colony-forming assays were plated from the marrow of each mouse to determine the number of clonogenic human hematopoietic progenitors recovered from 1 × 106bnx bone marrow cells.
Between 3 × 105 and 9 × 105 bone marrow cells were plated from each mouse, and numbers were normalized to 1 × 106 for equivalent comparison. Therefore, numbers reported as 0 may be as high as 2 colonies.
Ten thousand events were acquired from each sample, and the numbers were extrapolated to 1 × 106, with a level of sensitivity of 100 human cells per 1 million bnx bone marrow cells.
A comparison of the extents of human cell engraftment and clonogenic progenitor content in the three groups of mice is shown in Table 3. Mice transplanted with human CD34+ cells transduced for 72 hours in suspension culture with IL-3, IL-6, and SCF had extremely low human cell engraftment levels (average, 1,110 ± 1,324 human CD45+ cells/1 × 106 bnx marrow cells). Mice transplanted with cells transduced in suspension with the addition of FL had greatly increased extents of human CD45+ cell engraftment (average, 37,345 ± 25,299/1 × 106). Mice transplanted with human CD34+ cells transduced on stromal support had an average of 55,130 ± 34,493 human CD45+ cells per 1 million bnx bone marrow cells (Table 3). Engraftment levels for the SNS + FL and SOS groups were not significantly different, but both were significantly higher than the values obtained from the mice in the SNS group.
The average number of human colonies that developed from 1 × 106 marrow cells recovered from mice in the SOS group was 171 ± 106 (Table 3). The average number of human colonies obtained from the same number of marrow cells from mice in the SNS + FL group was 72 ± 62, which was a significantly lower value (P < .01). In contrast, no clonogenic human hematopoietic progenitors were recovered from mice that had received human CD34+ cells transduced in suspension culture in the absence of FL (SNS, Table 3). We conclude that FL partially replaces stromal support during transduction in maintaining the ability of human hematopoietic cells to retain their clonogenic capacity for extended periods of time (7 to 8 months). However, the effects from inclusion of FL do not completely replace the presence of stromal support on maintenance of human hematopoietic cells able to sustain long-term engraftment of bnx mice, so additional factors may be involved, such as additional cytokines or engagement of integrins.
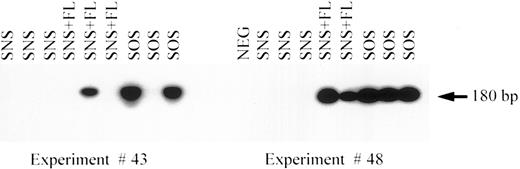
Tissue distribution of vector-marked human cells in bnx mice.It was possible that the differences in culture conditions during transduction, before transplantation, might cause variation in homing and/or sites of subsequent human hematopoiesis. Samples of the bone marrow, blood, and spleen from each bnx mouse were therefore analyzed by FACS for the presence of human cells, and organs (liver, lung, kidney, spleen, blood, and bone marrow) were screened for the presence of LN provirus by PCR for the neo gene as described.16 Marked cells were most commonly detected in the marrow (Fig 3). No cells bearing neo provirus were recovered from the marrrow of mice transplanted with human cells transduced in suspension culture (SNS, Table 4). The addition of FL to the same transduction condition resulted in the presence of vector-marked cells in the marrow of 5 of 11 mice (SNS + FL), whereas the inclusion of stromal support resulted in marked cells in the marrow of 9 of 10 mice (SOS, Table 4). Blood samples with detectable levels of LN provirus were obtained from 2 of 10 mice from the SOS group, but none from the SNS or SNS + FL groups. Marked spleen cells were found in 3 of 10 mice from the SOS group, in 1 of 11 mice from the SNS + FL group, and in none of the mice from the SNS group (Table 4). No human cells bearing LN provirus were recovered from the liver, lung, or kidney of any animal (Table 4). Therefore, the major site for survival of marked human cells was the murine bone marrow, with few cells recovered from the blood and spleen after long-term engraftment.
PCR for the neo gene in bnx/hu bone marrow. DNA was extracted from the bone marrow samples of each mouse and subjected to PCR to detect the presence of the neo gene in the LN provirus. The results from experiments no. 43 and no. 48 are shown. SNS indicates that in vitro transduction of the human hematopoietic cells was performed in suspension culture (supernatant, no stromal support). SNS + FL, same conditions but with the inclusion of 100 ng/mL FLT3 ligand. SOS indicates that the human hematopoietic progenitors were transduced on stromal support (supernatant on stroma).
PCR for the neo gene in bnx/hu bone marrow. DNA was extracted from the bone marrow samples of each mouse and subjected to PCR to detect the presence of the neo gene in the LN provirus. The results from experiments no. 43 and no. 48 are shown. SNS indicates that in vitro transduction of the human hematopoietic cells was performed in suspension culture (supernatant, no stromal support). SNS + FL, same conditions but with the inclusion of 100 ng/mL FLT3 ligand. SOS indicates that the human hematopoietic progenitors were transduced on stromal support (supernatant on stroma).
Posttransplantation bnx/hu Human Cell Engraftment and Marking Levels
| bnx No. . | Transduction . | Months Engrafted . | %G418R CFU4-150 . | Results of PCR for neo Gene . | |||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | BM . | spl . | Blood . | Liver . | Lung . | Kidney . |
| 43A1 | SNS | 8 | 0/0 (0) | − | − | − | − | − | − |
| 43A2 | SNS | 8 | 0/0 (0) | − | − | − | − | − | − |
| 44A1 | SNS | 7 | 0/0 (0) | − | − | − | − | − | − |
| 44A2 | SNS | 7 | 0/0 (0) | − | − | − | − | − | − |
| 44A3 | SNS | 7 | 0/0 (0) | − | − | − | − | − | − |
| 46B1 | SNS | 7.5 | 0/0 (0) | − | − | − | − | − | − |
| 46B2 | SNS | 7.5 | 0/0 (0) | − | − | − | − | − | − |
| 48A1 | SNS | 8.5 | 0/0 (0) | − | − | − | − | − | − |
| 48A2 | SNS | 8.5 | 0/0 (0) | − | − | − | − | − | − |
| 48A3 | SNS | 8.5 | 0/0 (0) | − | − | − | − | − | − |
| 43B1 | SNS + FL | 8 | 0/19 (0) | + | + | − | − | − | − |
| 43B2 | SNS + FL | 8 | 0/0 (0) | − | − | − | − | − | − |
| 44B1 | SNS + FL | 7 | 0/3 (0) | − | − | − | − | − | − |
| 44B2 | SNS + FL | 7 | 0/31 (0) | − | − | − | − | − | − |
| 44B3 | SNS + FL | 7 | 2/46 (4.3) | − | − | − | − | − | − |
| 46C1 | SNS + FL | 7.5 | 1/53 (1.9) | + | − | − | − | − | − |
| 46C2 | SNS + FL | 7.5 | 0/6 (0) | − | − | − | − | − | − |
| 46C3 | SNS + FL | 7.5 | 2/32 (6.3) | + | − | − | − | − | − |
| 48B1 | SNS + FL | 8.5 | 2/31 (6.5) | + | − | − | − | − | − |
| 48B2 | SNS + FL | 8.5 | 0/17 (0) | + | − | − | − | − | − |
| 48B3 | SNS + FL | 8.5 | 0/0 (0) | − | − | − | − | − | − |
| 43C1 | SOS | 8 | 3/27 (11.1) | + | + | + | − | − | − |
| 43C2 | SOS | 8 | 12/67 (17.9) | + | + | + | − | − | − |
| 43C3 | SOS | 8 | 0/2 (0) | + | − | − | − | − | − |
| 44C1 | SOS | 7 | 10/72 (13.9) | + | − | − | − | − | − |
| 44C2 | SOS | 7 | 8/64 (12.5) | + | − | − | − | − | − |
| 46A1 | SOS | 7.5 | 15/98 (15.3) | + | − | − | − | − | − |
| 46A3 | SOS | 7.5 | 8/51 (15.7) | + | + | − | − | − | − |
| 48C1 | SOS | 8.5 | 3/61 (4.9) | + | − | − | − | − | − |
| 48C2 | SOS | 8.5 | 0/0 (0) | − | − | − | − | − | − |
| 48C3 | SOS | 8.5 | 5/70 (7.1) | + | − | − | − | − | − |
| bnx No. . | Transduction . | Months Engrafted . | %G418R CFU4-150 . | Results of PCR for neo Gene . | |||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | BM . | spl . | Blood . | Liver . | Lung . | Kidney . |
| 43A1 | SNS | 8 | 0/0 (0) | − | − | − | − | − | − |
| 43A2 | SNS | 8 | 0/0 (0) | − | − | − | − | − | − |
| 44A1 | SNS | 7 | 0/0 (0) | − | − | − | − | − | − |
| 44A2 | SNS | 7 | 0/0 (0) | − | − | − | − | − | − |
| 44A3 | SNS | 7 | 0/0 (0) | − | − | − | − | − | − |
| 46B1 | SNS | 7.5 | 0/0 (0) | − | − | − | − | − | − |
| 46B2 | SNS | 7.5 | 0/0 (0) | − | − | − | − | − | − |
| 48A1 | SNS | 8.5 | 0/0 (0) | − | − | − | − | − | − |
| 48A2 | SNS | 8.5 | 0/0 (0) | − | − | − | − | − | − |
| 48A3 | SNS | 8.5 | 0/0 (0) | − | − | − | − | − | − |
| 43B1 | SNS + FL | 8 | 0/19 (0) | + | + | − | − | − | − |
| 43B2 | SNS + FL | 8 | 0/0 (0) | − | − | − | − | − | − |
| 44B1 | SNS + FL | 7 | 0/3 (0) | − | − | − | − | − | − |
| 44B2 | SNS + FL | 7 | 0/31 (0) | − | − | − | − | − | − |
| 44B3 | SNS + FL | 7 | 2/46 (4.3) | − | − | − | − | − | − |
| 46C1 | SNS + FL | 7.5 | 1/53 (1.9) | + | − | − | − | − | − |
| 46C2 | SNS + FL | 7.5 | 0/6 (0) | − | − | − | − | − | − |
| 46C3 | SNS + FL | 7.5 | 2/32 (6.3) | + | − | − | − | − | − |
| 48B1 | SNS + FL | 8.5 | 2/31 (6.5) | + | − | − | − | − | − |
| 48B2 | SNS + FL | 8.5 | 0/17 (0) | + | − | − | − | − | − |
| 48B3 | SNS + FL | 8.5 | 0/0 (0) | − | − | − | − | − | − |
| 43C1 | SOS | 8 | 3/27 (11.1) | + | + | + | − | − | − |
| 43C2 | SOS | 8 | 12/67 (17.9) | + | + | + | − | − | − |
| 43C3 | SOS | 8 | 0/2 (0) | + | − | − | − | − | − |
| 44C1 | SOS | 7 | 10/72 (13.9) | + | − | − | − | − | − |
| 44C2 | SOS | 7 | 8/64 (12.5) | + | − | − | − | − | − |
| 46A1 | SOS | 7.5 | 15/98 (15.3) | + | − | − | − | − | − |
| 46A3 | SOS | 7.5 | 8/51 (15.7) | + | + | − | − | − | − |
| 48C1 | SOS | 8.5 | 3/61 (4.9) | + | − | − | − | − | − |
| 48C2 | SOS | 8.5 | 0/0 (0) | − | − | − | − | − | − |
| 48C3 | SOS | 8.5 | 5/70 (7.1) | + | − | − | − | − | − |
Bone marrow, blood, and organs were recovered from bnx mice after engraftment periods of 7 to 8 months. Human-specific colony-forming assays with and without the addition of the selective agent G418 were plated from the bone marrow of each mouse, and the results are shown as the no. of G418-resistant/total human CFU (% G418 − resistant CFU). PCR for the presence of the neo gene, in the LN proviral vector, was performed on each organ. Results of the PCR analyses are indicated as positive (+) or no signal detected (−).
A total of 3 × 105 bnx/hu bone marrow cells plated with and without G418 (0.9 mg/mL active) per sample.
Transduced human colony-forming progenitor recovery from the bone marrow of bnx mice.To further assess the maintenance and survival of vector-marked human hematopoietic progenitors in the bone marrow of each mouse in the three groups, human-specific colony-forming assays were plated with and without the selective agent G418. Mice that had been transplanted with human cells transduced in the absence of stromal support or FL (SNS) had no long-term engrafted human cells able to form colonies in the presence or absence of G418. In contrast, human cells transduced under the same conditions but, with the addition of 100 ng/mL FL (SNS + FL), gave rise to an average of 1.7% G418-resistant CFU after long-term engraftment in the mice (Table 4). This level of transduced long-lived progenitors was significantly lower than that obtained after transduction on stromal support and long-term engraftment (1.7% SNS + FL v 9.8% SOS, P = .001; Table 4). In summary, inclusion of FL during transductions performed in suspension culture did not fully replace stromal support, but allowed some level of gene transfer into primitive cells to be observed.
Clonal integration analysis.The identification of 5 of 11 mice harboring vector-marked human cells in their bone marrow 7 to 8 months after transduction in suspension culture with FL was surprising, due to the low extents of transduction of colony-forming progenitors (Table 1). To assess the number of different marked human hematopoietic cell populations, we performed inverse PCR on samples of the marrow recovered from each mouse, using a protocol modified from that originally published by Rill et al.22 Retroviral vectors integrate into host cell chromosomes at random sites, generating unique restriction fragment length polymorphisms. Integrated vectors can therefore be used as clonal tags to identify all cells derived from a common progenitor.23-26 We used the inverse PCR technique to identify the site of proviral integration in the human cellular DNA by amplifying outward from the retroviral long terminal repeat (LTR).27 The inverse PCR technique allows the determination of the number of individual, marked human progenitors contributing to the neo-positive PCR signals obtained from the bone marrow samples. Using this procedure, individual proviral integrants can be detected from small samples, containing DNA from 100 cells (∼500 pg DNA). We had previously shown that the inverse PCR technique can identify unique proviral integration products through a background of nontransduced murine and human cells.17
Bone marrow samples from each mouse were subjected to inverse PCR (Table 5). Mice that had received human cells transduced in suspension with IL-3, IL-6, and SCF alone had no integrated LN provirus in their marrrow. Mice that had received human cells transduced on stromal support had oligoclonal marking, with 0 to 6 marked human progenitors contributing to hematopoiesis (average, 2.6 proviral integrants per recipient). Mice that had received human cells transduced in suspension culture with the addition of FL had 0 to 2 marked human precursors giving rise to enough progeny to be detected by the inverse PCR technique (average, 1.0 integrant/recipient; Table 5). The average values were not significantly different (P = .04). We conclude that transfer of the neo gene into a limited number of individual human progenitors capable of engrafting bnx mice for extended periods of time occurred using the SOS and SNS + FL, but not the SNS, transduction conditions.
Clonal Integration Analysis of bnx/hu Bone Marrow by Inverse PCR
| Mouse No. . | Transduction Conditions . | Total No. of Proviral Integrants . | Average No. of Integrants/Recipient . |
|---|---|---|---|
| 43A1 | SNS | 0 | |
| 43A2 | SNS | 0 | |
| 44A1 | SNS | 0 | |
| 44A2 | SNS | 0 | |
| 44A3 | SNS | 0 | |
| 48A1 | SNS | 0 | |
| 48A2 | SNS | 0 | |
| 48A3 | SNS | 0 | 0 |
| 43B1 | SNS + FL | 1 | |
| 43B2 | SNS + FL | 2 | |
| 44B1 | SNS + FL | 0 | |
| 44B2 | SNS + FL | 0 | |
| 44B3 | SNS + FL | 1 | |
| 48B1 | SNS + FL | 2 | |
| 48B2 | SNS + FL | 2 | |
| 48B3 | SNS + FL | 0 | 0.88 |
| 43C1 | SOS | 3 | |
| 43C2 | SOS | 2 | |
| 43C3 | SOS | 1 | |
| 44C1 | SOS | 1 | |
| 44C2 | SOS | 4 | |
| 48C1 | SOS | 4 | |
| 48C2 | SOS | 0 | |
| 48C3 | SOS | 6 | 2.63 |
| Mouse No. . | Transduction Conditions . | Total No. of Proviral Integrants . | Average No. of Integrants/Recipient . |
|---|---|---|---|
| 43A1 | SNS | 0 | |
| 43A2 | SNS | 0 | |
| 44A1 | SNS | 0 | |
| 44A2 | SNS | 0 | |
| 44A3 | SNS | 0 | |
| 48A1 | SNS | 0 | |
| 48A2 | SNS | 0 | |
| 48A3 | SNS | 0 | 0 |
| 43B1 | SNS + FL | 1 | |
| 43B2 | SNS + FL | 2 | |
| 44B1 | SNS + FL | 0 | |
| 44B2 | SNS + FL | 0 | |
| 44B3 | SNS + FL | 1 | |
| 48B1 | SNS + FL | 2 | |
| 48B2 | SNS + FL | 2 | |
| 48B3 | SNS + FL | 0 | 0.88 |
| 43C1 | SOS | 3 | |
| 43C2 | SOS | 2 | |
| 43C3 | SOS | 1 | |
| 44C1 | SOS | 1 | |
| 44C2 | SOS | 4 | |
| 48C1 | SOS | 4 | |
| 48C2 | SOS | 0 | |
| 48C3 | SOS | 6 | 2.63 |
Genomic DNA samples extracted from the marrow of each mouse were subjected to clonal analysis by inverse PCR. The number of proviral integrants detected in each sample (no. of PCR product bands/2 LTR bands per proviral integrant = no. of integrants) is listed.
Human CD33+ myeloid and CD3+ lymphoid cells recovered from the bone marrow of the engrafted mice in experiment no. 48 (transplanted with cells from in vitro experiment no. 4) were analyzed individually. Single cells were plated by ACDU into 96-well plates and expanded to a size of 100 to 1,000 cells under lymphoid-specific or human myeloid-specific conditions as described.17 To ensure that growth of the myeloid and lymphoid clones was lineage exclusive, we did cross-plating experiments from bnx/hu marrow and human peripheral blood or bone marrow samples. The human CD45+/CD33+ myeloid cells were deposited by ACDU into the T-cell–specific medium (with IL-2 and phytohemagglutinin) as well as the appropriate myeloid medium. The human CD45+/CD3+ T cells were deposited into wells containing the myeloid methylcellulose medium (containing hydrocortisone as one of the components) and the T-cell–specific medium. Whereas the cells grew well in the media of the appropriate type in each experiment, no expansion of cells from either population was seen in the plating media for the opposite lineage in four crossing experiments. These experiments show the specificity for growth of cells of the appropriate lineage in each medium.
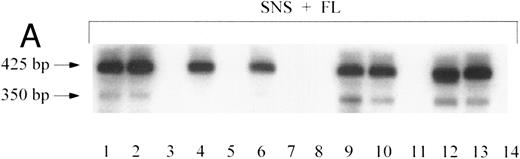
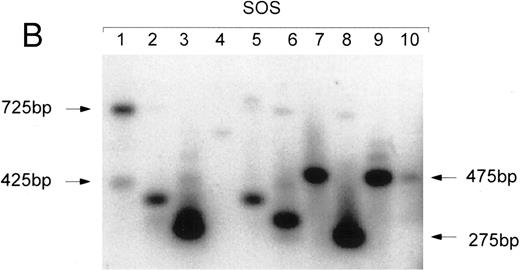
Individual human myeloid and lymphoid colonies grown from the marrow of the mice in experiment no. 48 were subjected to inverse PCR (Table 6). Mice no. 48B1 and 48B2, transplanted with human CD34+ cells transduced in suspension culture with FL, had 2 and 1 distinct integration pattern among the myeloid clones, respectively (Fig 4A), and 0 and 1 pattern in the T-cell clones, with 3 to 8 clones bearing the same integrant (Table 6). Mice no. 48C1 and 48C3, who were transplanted with human cells transduced on stromal support, had 3 and 5 separate proviral integration patterns among the myeloid clones, respectively (Fig 4B), and each had a single integrant in the neo-positive human T-cell clones. Few marked T lymphocyes were recovered in this series of experiments, and none matched the patterns obtained from the corresponding human myeloid clones recovered from the same mouse, which would have indicated that they were derived from the same stem cell (Table 6).
Clonal Integration Patterns Detected in Human Hematopoietic Cells Recovered From Long-Term Engrafted bnx Mice 8.5 Months After Bone Marrow Transplantation
| Mouse No. . | BMT . | No. of Integrants in Total BM . | No. of Integrants in GM Clones . | No. of Integrants in T Clones . | Common Integrant? . |
|---|---|---|---|---|---|
| 48A1 | SNS | 0 | — | — | — |
| 48A2 | SNS | 0 | — | — | — |
| 48A3 | SNS | 0 | — | — | — |
| 48B1 | SNS + FL | 2 | 2 (3 + 7) | 0 | No |
| 48B2 | SNS + FL | 2 | 1 (8) | 1 (3) | No |
| 48B3 | SNS + FL | 0 | — | — | — |
| 48C1 | SOS | 4 | 3 (17 + 1 + 3) | 1 (1) | No |
| 48C2 | SOS | 0 | — | — | — |
| 48C3 | SOS | 6 | 5 (8 + 5 + 2 + 2 + 1) | 1 (3) | No |
| Mouse No. . | BMT . | No. of Integrants in Total BM . | No. of Integrants in GM Clones . | No. of Integrants in T Clones . | Common Integrant? . |
|---|---|---|---|---|---|
| 48A1 | SNS | 0 | — | — | — |
| 48A2 | SNS | 0 | — | — | — |
| 48A3 | SNS | 0 | — | — | — |
| 48B1 | SNS + FL | 2 | 2 (3 + 7) | 0 | No |
| 48B2 | SNS + FL | 2 | 1 (8) | 1 (3) | No |
| 48B3 | SNS + FL | 0 | — | — | — |
| 48C1 | SOS | 4 | 3 (17 + 1 + 3) | 1 (1) | No |
| 48C2 | SOS | 0 | — | — | — |
| 48C3 | SOS | 6 | 5 (8 + 5 + 2 + 2 + 1) | 1 (3) | No |
Individual human T-lymphoid (CD45+/CD3+) and myeloid (CD45+/CD33+) cells recovered from long-term engrafted mice from experiment no. 48 were deposited by ACDU into 96-well plates. A dash indicates that no human cells were recovered from the mice, so subsequent analyses were not possible. Human granulocyte-macrophage clones were grown individually in methylcellulose medium with 0.9 mg/mL G418. Human T lymphocytes were expanded individually in medium containing rHIL-2 + PHA and then shown to contain the neo gene by PCR. Clonal integration analysis by inverse PCR was performed on the total marrow sample, and each human clone recovered from each mouse. The number of proviral integrants in each category are shown as: number of integrants (no. of clones sharing the same pattern). The products of the inverse PCR reactions were analyzed to determine whether common integrants were present in the human GM and T-cell clones from each bnx recipient.
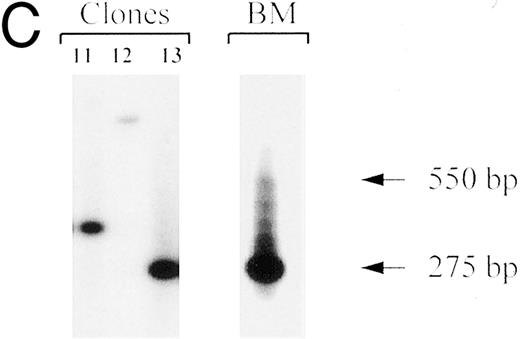
Clonal analysis by inverse PCR of individual human CFU-GM recovered after 8.5 months engraftment in bnx mice. (A) Human CFU-GM from a mouse (no. 48B2) transplanted with human CD34+ progenitors transduced in suspension culture with FL. (B) Human CFU-GM from a mouse (no. 48C3) transplanted with human CD34+ progenitors transduced with stromal support. (C) Individual CFU-GM clones and total bone marrow from mouse no. 48C3.
Clonal analysis by inverse PCR of individual human CFU-GM recovered after 8.5 months engraftment in bnx mice. (A) Human CFU-GM from a mouse (no. 48B2) transplanted with human CD34+ progenitors transduced in suspension culture with FL. (B) Human CFU-GM from a mouse (no. 48C3) transplanted with human CD34+ progenitors transduced with stromal support. (C) Individual CFU-GM clones and total bone marrow from mouse no. 48C3.
An example of the clonal integration patterns obtained from the marrow of a mouse that was well engrafted with vector-marked human cells (48C3) is shown in Fig 4C. The inverse PCR patterns from total bone marrow and 3 individual myeloid clones are shown to demonstrate that bands of a specific molecular weight can be observed through a background of nontransduced human and murine cells. Some clones, such as no. 12 in Fig 4C (which did not have the same integration pattern as any other myeloid or T-cell clone recovered from bnx/hu no. 48C3, Table 6), were only detected by the single-cell cloning, and bands of the corresponding molecular weight were not detected in the bulk marrow sample. From the two clones that were best represented in the marrow (clone no. 11 shared the same clonal integration pattern with 4 other clones, and clone no. 13 had the same pattern as 7 other clones, Table 6), bands of the appropriate molecular weight could be easily observed in the total marrow sample (Fig 4C).
DISCUSSION
The addition of functional genes to human HSCs to augment defective copies (gene therapy) holds much promise. However, major obstacles exist in this field and have frustrated many efforts at correction of single-copy gene defects in HSCs. Retroviral vectors can stably insert a foreign gene into the chromosomes of a target cell and have been used effectively for many gene replacement studies. However, the target cell must undergo the process of DNA replication and nuclear envelope breakdown, so that the large viral nucleoprotein complex can gain access to the host cell chromosomes.1 The majority of HSC are quiescent,28-30 and many are cytokine nonresponsive.31 Several strategies have been used to attempt to prompt quiescent cells into cycle to facilitate retroviral-mediated gene transfer, including stimulation by cytokines and/or stromal support.32-35 Transduction in the presence of the cytokines IL-3, IL-6, and SCF in suspension culture in the strict absence of stromal cells can result in modest levels of gene transfer into CD34+ colony-forming progenitors from granulocyte colony-stimulating factor–mobilized peripheral blood and cord blood, but results in very low levels of gene transfer into progenitors derived from human bone marrow.5-7
Our previous studies showed that the presence of a stromal underlayer had dual benefits during ex vivo transduction of long-lived human CD34+ cells with cell-free retroviral supernatant. In addition to the enhancement of gene transfer into clonogenic progenitors, as had been previously shown by Moore et al,6 the presence of stroma during ex vivo transduction also maintained the ability of the progenitors to achieve long-term engraftment in the bnx/hu system.9 It is not clear whether these two roles are derived from a common activity or from two distinct features of stroma. FL is a recently cloned cytokine, normally produced by the bone marrow stroma, that is a good candidate for promoting division and survival of primitive HSCs.10,36 FL has been shown to stimulate the growth and expansion of primitive hematopoietic progenitors from murine and human bone marrow.10-14 37-39 The present studies show that FL can partially replace the ability of stroma to allow CD34+ cells to produce long-term engraftment in the bnx/hu model. The levels of human cells present in the bnx/hu mice 7 to 8 months after transplantation were twofold lower when they were transduced with FL in suspension culture rather than with stromal support. The resultant lineages did not vary significantly when the transplanted human cells had been cultured in suspension with 3/6/S/FL or with 3/6/S + stromal support.
FL did not replace the ability of stroma to increase gene transfer into short-lived committed progenitors, as measured by direct plating of CFU after transduction. The extent of gene transfer into long-lived human progenitors that engraft in the mice was also lower from transduction using FL, rather than stroma, by three lines of evidence: plating of human-specific CFU with or without G418, the number of mice in each group with neo-positive marrow and tissues by PCR, and the number of integrated vector proviruses in the marrow of mice from each set, as assessed by clonal integration analysis by inverse PCR. The control group that did not contain either FL or stroma during transduction had essentially no engraftment of human cells; thus, the transduction frequency could not be determined. These observations suggest that FL does not fully replace the ability of stroma to enhance gene transfer but, by increasing graft survival, allows some degree of transduced cells to be apparent. A similar conclusion was reached by Fletcher et al,40 who studied the effects of leukemia-inhibitory factor (LIF ) on transduction of murine cells. They found that LIF did not significantly increase the extent of gene transfer, but acted to support stem cell survival. Therefore, they recovered a higher percentage of marked primitive cells when LIF was included in the transduction medium. The effects of FL in our system appear to be comparable.
As we have previously observed, there was a discrepancy between the number of marked progenitors in the marrow and the number of mature marked cells produced. This phenomenon has been observed in bnx/hu mice and in our human gene therapy recipients.41 In bulk bnx/hu bone marrow, as with patient marrow, the total percentage of marked cells is low. Upon cloning, higher numbers of transduced, G418-resistant progenitors are detected. We have determined that there is a disproportionate number of marked progenitors that have not produced mature progeny.41 If a block to differentiation exists in vivo, it appears to be relieved by growth in vitro. In preliminary data, we have observed (from both gene therapy recipients and bnx/hu mice) that the marked progenitors have expanded laterally within the marrow, generating multiple clonogenic progeny with the same integrant, which are not reflected in the blood.41 42 Detailed studies of the clonal diversity of the marked cells in each gene therapy recipient and bnx/hu mouse are in progress.
Because of the ability of stroma to enhance gene transfer into human progenitor cells, a number of current clinical gene therapy trials are using autologous stroma derived from the patient for ex vivo transduction of CD34+ cells.43-45 However, the growth of stroma from patients with disease or after chemotherapy can be problematic.46 Also, the expansion of stroma on a scale adequate for transduction of an entire inoculum of bone marrow or peripheral blood progenitors may not be possible within the period of time between the initial marrow aspirate and the harvest for the trial. The combination of fibronectin, to enhance gene transfer,8 35 and FL, to support progenitor survival, may ultimately replace the use of patient-derived stromal layers. Ongoing studies examining the combination of fibronectin and FL in the bnx/hu and other xenograft models may determine whether this will be possible.
ACKNOWLEDGMENT
We are very appreciative of G. Annett's advice on immunology and antibody labeling of cells. Thank you to K. Weinberg, G. Crooks, S. Walker, and R. Parkman, as always, for useful discussion. This work was made possible by S. Worttman, head of our animal facility, and R. Traub-Workman, who maintains the bnx mouse colony.
Supported by National Institutes of Health (NIH) Grant No. RO1 DK 48700-01, National Institute of Diabetes and Digestive and Kidney Diseases Grant No. RO1 #DK42694, and NIH National Cancer Institute Grant No. SCOR #1-P50-HL54850-01.
Address reprint requests to Jan A. Nolta, PhD, Division of Research Immunology/Bone Marrow Transplantation, Childrens Hospital Los Angeles, 4650 Sunset Blvd, Mailstop #62, Los Angeles, CA 90027.

![Fig. 1. Experimental schema. CD34+ progenitors were isolated from human bone marrow by immunomagnetic selection. Cells were transduced by the LN retroviral vector packaged with the GALV pseudotype envelope protein by the PG13 cell line. All transductions were performed in the presence of IL-3 (10 ng/mL), IL-6 (50 U/mL), and SCF (50 ng/mL). Transductions were performed in suspension culture (SNS; supernatant, no stroma), in suspension with addition of 100 ng/mL FLT3 ligand (SNS + FL), or with the support of an irradiated allogeneic bone marrow stromal cell monolayer (supernatant on stroma [SOS]). After transduction, methylcellulose-based CFU assays with or without G418 were performed to assess the extent of gene transfer into colony-forming progenitors. The remainder of each sample was cotransplanted with IL-3–producing primary human marrow stromal cells into a cohort of sublethally irradiated bnx mice. Mice were harvested 7 to 8 months after transplantation, and the extent of human hematopoietic cell engraftment and vector marking in all tissues was assessed.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/2/10.1182_blood.v89.2.446/4/m_bl_0031f1.jpeg?Expires=1769190694&Signature=DAmN9NKTk7klGdmJJkXfpe7P2M3qQCzFE1iHVPlPfXDB6BqTkVgTI~7s9LYqXEg7MsMPYtBhhb3JD6KQYH6yQBVrqu1zBwPk0~wKZ5H~L9kTsWmf3WtDD83ncJEG5I~SXVk5uBev10Q1BUieqSZcz6uDi-J~IVJZhqvima9r5~nBqmf~OBYUap29-VFFxWfxr4PDaPKx8BuahCBTdwA2DQcENaU0qJjIwGTvCZn~nD2zChGkjM498dJhtUHOMC-Slw9k~YbUe1Wr5ArMK1PoSCHrk0YsgNqGf1s8y7Ujje-fcAa7J5Rr8seTLm9lnQuZy2UU2qoAX7uzOb7n4OE2kQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal