Abstract
To compare the signal transduction pathways used by erythropoietin (Epo) and interleukin-6 (IL-6), the cDNA for the murine Epo receptor (Epo-R) was introduced into an IL-6–responsive plasmacytoma cell line (TEPC-2027) by retrovirally mediated gene transfer. G418-resistant clones were amplified in IL-6 and studied for their ability to grow and differentiate in response to Epo. Epo-R synthesized from the viral gene showed the same affinity for Epo as did the receptor on erythroid cells; however, the numbers of Epo receptors expressed on the cell membrane varied among clones. After a delay of 3 to 5 days in the presence of Epo, all the clones studied proliferated as well in response to Epo as in response to IL-6. In response to IL-6, Stat3 was activated and JunB mRNA was accumulated, whereas in response to Epo, Jak2 and Stat5 were activated and JunB mRNA was not accumulated in Epo-R–expressing TEPC (Epo-R/TEPC) cells. These results suggest that Epo and IL-6 transduced their proliferative signals through different pathways. Further studies showed that, in Epo-R/TEPC cells, Epo neither induces the synthesis of erythroid-specific mRNA nor modifies the synthesis of γ1 Ig heavy chain, suggesting that ectopic expression of the Epo-R in plasmacytoma cells does not modify their differentiative potential. The data show that Epo induces a proliferative response without differentiation providing a new cellular model for evaluating molecular events specific for proliferation.
HEMATOPOIETIC cell proliferation and differentiation are in large part controlled by growth factors that transduce proliferation and/or differentiation signals via specific cell surface receptors.1 Most cytokine receptors involved in the regulation of hematopoietic growth and differentiation belong to the cytokine receptor superfamily. This family is characterized by the absence of intrinsic tyrosine kinase activity, by conserved structures including the WSXWS motif, and by four positionally conserved cysteine residues.2 Furthermore, certain similarities are observed in the cytoplasmic domain of these receptors. In a 60 amino acid segment located in the membrane proximal domain, there are two conserved subdomains of amino acids. One subdomain, designated box 1, consists of a ProXXPro sequence after a series of hydrophobic amino acids. The other subdomain, termed box 2, consists of a group of hydrophobic amino acids followed by one or two charged amino acids.3,4 Included in this receptor family are the receptors for interleukin-2 (IL-2), IL-3, IL-4, IL-5, IL-6, IL-7, granulocyte-macrophage colony-stimulating factor (GM-CSF ), leukemia-inhibitory factor (LIF ), ciliary neutrophic factor (CNTF), oncostatin M (OSM), erythropoietin (Epo), thrombopoietin (TPO), prolactin, and growth hormone.5-7
At least two general mechanisms are involved in the transmission of signals from these receptors to the nucleus: the ras-MAP kinase8 and the Jak-Stat signaling pathways.9 The ras-MAP kinase pathway depends on the activation of protein kinases that phosphorylate target transcription factors. The Jak-Stat pathway results in phosphorylation of signal transducers and activators of transcription (Stat) by tyrosine kinases of the Jak family. These Stat transcriptional factors bind the GAS family of DNA response elements, first characterized in the interferon α/β and γ signal transduction pathway.10
Epo is a glycoprotein acting mainly on late erythroid progenitor cells and regulating their growth and differentiation.11 Epo binding to its receptor (Epo-R) induces the activation of the receptor-associated Jak2 kinase,12,13 possibly through ligand-induced receptor dimerization.14,15 Binding of Epo also induces the phosphorylation of the Epo-R itself,16-19 phospholipase Cγ,20 rasGap,21 Vav,22 Shc,23 Stat5,24-26 and c-Cbl27 and the activation of the phosphatidylinositol (PI) 3-kinase28-31 and the Ras/Map kinase pathway.32 33
IL-6 is required for B- and T-cell growth and differentiation, neuronal and macrophage differentiation, Ig production, and the acute phase response.34,35 IL-6 acts synergistically with other colony-stimulating factors (CSFs) to induce proliferation of hematopoietic progenitor cells.36 IL-6 also promotes the growth of murine hybridoma and plasmacytoma as well as human myeloma cell lines.37 The IL-6 receptor is composed of two chains: a 80-kD ligand binding chain and a 130-kD signal transducing chain (gp130).38 Epo binding and IL-6 binding induce the activation of Jak2, but IL-6 binding also activates Jak1 and Tyk2, depending on the cell context.39 However, although Epo activates Stat5, IL-6 has been shown to activate Stat1 (ISGF3, ap91) and Stat3 (APRF ).
Epo-R cDNA introduced into IL-3–dependent cell lines allows them to grow in the presence of Epo,40 suggesting a common growth signaling mechanism for Epo-R and IL-3–R. In contrast, introduction of the Epo-R cDNA into an IL-2–dependent murine cytotoxic T-cell line CTLL-2 gave controversial results. Showers et al41 reported that CTLL-2 cells expressing the Epo-R (CTLL-2/Epo-R) proliferate in response to Epo, whereas Yamamura et al42 and Sakamaki et al43 reported that CTLL-2/Epo-R do not proliferate in response to Epo, suggesting that CTLL-2 cells lack a critical component required for Epo signaling.
In the present study, we introduced a normal murine Epo-R cDNA using a retroviral vector into an IL-6–dependent plasmacytoma cell line (TEPC/2027) to determine if Epo is able to transduce proliferative and/or differentiative signals in these IL-6–dependent plasmacytoma cells modified by gene transfer. We show that, although ectopic expression of the Epo-R in TEPC cells confers proliferative responsiveness to Epo, these cells do not synthesize erythroid-specific mRNA and do not modify their stage of differentiation in response to Epo.
MATERIALS AND METHODS
Cytokines and antibodies.Recombinant human Epo (rhEpo) was a generous gift from Cilag (Paris, France) and Dr Schoepe (Boerhinger Mannheim, Mannheim, Germany) and was used at a concentration of 1 U/mL. Murine IL-6, provided as a conditioned medium of the P388D1 cell line,44 was used to maintain TEPC cells in culture. Recombinant IL-6 was used (500 U/mL; Genzyme, Paris, France) to stimulate the growth of TEPC cells in proliferation or phosphorylation analysis.
Human GM-CSF was a generous gift from Amgen (Thousand Oaks, CA) and was used at a concentration of 10 ng/mL.
Rabbit antibodies against Epo-R were previously described.45 Antibodies against murine Jak2 and phosphotyrosine (4G10) were from UBI (Lake Placid, NY). Anti-Stat1 antibody was purchased from Transduction Laboratories (Lexington, KY). Anti-Stat3 antibody was obtained from UBI. Anti-Stat5 antibody was a generous gift from Dr B. Groner.
Cells and cell culture.The IL-6–dependent mouse plasmacytoma cell line TEPC-2027,46 which constitutively produces IgG1, was maintained in minimum essential medium (MEM) supplemented with 10% (vol/vol) fetal calf serum (FCS) and 10% conditioned medium from the P388 D1 cell line.44 This line and virus-producing cell lines were maintained in RPMI-1640 medium supplemented with 10% (vol/vol) FCS. UT-7 cells47 were cultured in MEM supplemented with 10% (vol/vol) FCS and 10 ng/mL of GM-CSF. MEM cells were maintained in MEM supplemented with 10% (vol/vol) FCS.
Retroviral constructs.Retroviral constructs were previously described.48 Briefly, the retroviral vector encoding the gene for the Epo-R was constructed by inserting a 1,500-bp fragment of the murine Epo-R cDNA (Sal I fragment) into the Xho I site of the pBT-Zen-SVNEO vector. This construct or a control construct containing the neomycin-resistance gene only were transfected into the Ψ2 packaging cell line.49 Individual neomycin-resistant clones were tested for their viral production on NIH3T3 cells and the clones that produced the greatest quantity of infectious particles in the supernatant were selected. A NEO clone (Ψ2NEO) and an Epo-R clone (Ψ2Epo-R) that produced the same quantity of infectious virus (2 × 105 particles/mL) were used.
Retroviral infection of TEPC cells.Retroviral infections were performed by coculturing TEPC cells (105 cells/mL) with irradiated (10 Gy) virus-producing cells (Ψ2NEO or Ψ2Epo-R) for 48 hours. At the end of the coculture period, TEPC cells were cloned in methylcellulose in the presence of IL-6 and G418 (1 mg/mL; GIBCO, Grand Island, NY). After 7 days, G418-resistant colonies were picked and expanded in liquid culture in the presence of IL-6 + G418. Seven independent Epo-R-infected (Epo-R/TEPC) clones and one NEO-infected (NEO/TEPC) clone were analyzed further.
Cell proliferation assay.To determine the proliferative effects of Epo on Epo-R/TEPC cells, the cells were washed twice and resuspended in MEM supplemented with 10% FCS in the presence of IL-6 or rhEpo or without growth factor. Cells (5 × 104/mL) were plated in 25-cm2 flasks and counted every day for 10 days.
DNA and RNA blot analysis.DNA from Epo-R and NEO/TEPC clones was prepared as previously described.50 Ten micrograms of DNA was digested by Bgl II, separated on a 1% agarose gel, and transferred onto a nylon membrane (Hybond N; Amersham, Paris, France). DNA probes were labeled with (α32P)-dCTP using the Readiprime labeling kit (Amersham).
RNA from each clone was prepared by denaturation in guanidium thiocyanate followed by pelleting through a cesium chloride cushion.51 TEPC clones were starved for growth factors for 18 hours before stimulation in MEM containing 0.1% bovine serum albumin, 22.5 mmol/L NaHCO3 and 100 μg/mL transferrin. Total RNA (6 μg) was separated on a 1% agarose-formaldehyde gel and transferred onto a nylon membrane. RNA probes for c-myc, c-fos, and junB were transcribed from linearized plasmid containing the appropriate coding sequences using SP6 RNA polymerase in the presence of 32P UTP. A 526-bp GATA-1 probe was obtained by polymerase chain reaction. The murine β globin probe was obtained from the pSP64M β 133 plasmid linearized by EcoRI.52 γ1 chain mRNA was detected with a specific 2.5-kb cDNA probe.53 All DNA probes were labeled with (α32P)-dCTP using the Readiprime labeling kit (Amersham).
Epo binding assay and Scatchard analysis.Cells grown in the presence of 500 μg/mL of G418, either with 1 U/mL Epo or 10% conditioned medium containing IL-6, were washed twice in phosphate-buffered saline and cultured for 18 hours in MEM containing 0.1% bovine serum albumin, 22.5 mmol/L NaHCO3 , and 100 μg/mL transferrin in the absence of growth factors and FCS. The cells were then washed with binding medium (RPMI 25 mmol/L HEPES, 5% FCS, and 0.1% NaN3 ) and incubated in duplicate with 125I-rhEpo (30 μCi/μg; 0.2 pmol/L to 2 nmol/L) in 100 μL of binding medium at 4°C for 18 hours or at 37°C for 3 hours. To determine the nonspecific binding of 125I-rhEpo, a 100-fold excess of unlabeled Epo (20 pmol/L to 200 nmol/L) was included in the assay mixture. After centrifugation through a dibutyl-dinonylphtalate cushion (d = 1.015), both cell-bound and free radioactivity were measured in a gamma counter.
Cross-linking studies.Human Epo was labeled with Iodogen to a specific activity of 30 to 40 μCi/μg.54 Cross-linking was performed with disuccinimidyl suberate (DSS), as previously described.45 A 100-fold excess of unlabeled Epo was added in control assay mixtures to ensure the specificity of the binding.
Analysis of Epo-R phosphorylation.Cells were starved for growth factors and cultured 18 hours in MEM containing 5% FCS and 0.1% P388D1 conditioned medium. The cells (5 × 107) were then stimulated with 500 U/mL IL-6, 10 U/mL rhEpo, or medium without FCS for 5 minutes at 37°C in a final volume of 5 mL. Incubation was stopped by diluting the cells to 40 mL with cold phosphate-buffered saline and centrifugation. The pellet was lysed for 30 minutes in cold NP-40 buffer (20 mmol/L Tris-HCl, pH 8, 137 mmol/L NaCl, 2.7 mmol/L KCl, 10% glycerol, and 1% NP-40) containing protease and phosphatase inhibitors (1 mmol/L Na2VO4 , 1 mmol/L phenylmethyl sulfonyl fluoride [PMSF ], 1 mmol/L O-phenanthroline, 10 μg/mL aprotinin, 100 μg/mL leupeptin, and 2 mmol/L EDTA). The supernatants were collected after centrifugation for 30 minutes at 4°C and then immunoprecipated with anti–Epo-R antibodies. The immunoprecipitated proteins were first directly analyzed by Western blot using an antiphosphotyrosine antibody (4G10; UBI) and a horseradish peroxidase (HRP)-coupled antirabbit antibody with the chemoluminescent ECL kit (Amersham).18 To verify the nature of the band shown by this first immunoprecipitation (IP), proteins immunoprecipitated with anti–Epo-R antibodies were denatured in Laemmli buffer without β-mercaptoethanol (β-ME) at 100°C for 5 minutes to cleave potential disulfide bonds, submitted to a second immunoprecipitation with the same anti–Epo-R antibody, and analyzed by Western blot with an antiphosphotyrosine antibody.
Analysis of Jak2 phosphorylation.Cells were starved of growth factors for 18 hours, stimulated, and lysed as described above. Immunoprecipitation was performed with anti-Jak2 antibodies and the immunoprecipitates were analyzed by Western blot using an antiphosphotyrosine antibody (4G10; UBI) and an HRP-coupled antirabbit antibody with the chemoluminescent ECL kit (Amersham).
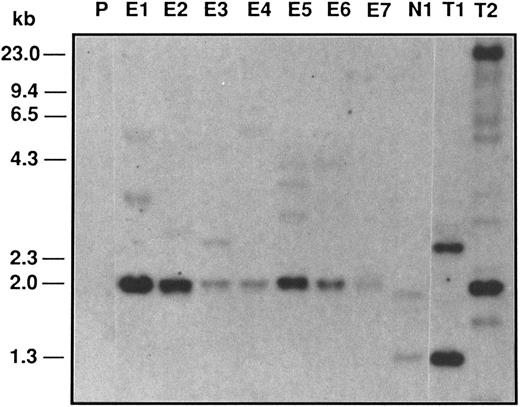
Southern blot analysis of Epo-R–infected TEPC clones. Ten micrograms of Bgl II-digested genomic DNAs from different clones was analyzed on 1% agarose gel, blotted, and hybridized with a 32P NEO DNA probe. P, parental TEPC cells; E1 to E7, Epo-R–infected clones; N1, NEO-infected clone; T1, NEO-infected FDCP-1 clone; T2, Epo-R virus-producing cells.
Southern blot analysis of Epo-R–infected TEPC clones. Ten micrograms of Bgl II-digested genomic DNAs from different clones was analyzed on 1% agarose gel, blotted, and hybridized with a 32P NEO DNA probe. P, parental TEPC cells; E1 to E7, Epo-R–infected clones; N1, NEO-infected clone; T1, NEO-infected FDCP-1 clone; T2, Epo-R virus-producing cells.
Electrophoretic mobility shift assays.Cells were starved of growth factors for 18 hours and stimulated as described above. Nuclear extracts were prepared by lysing the cells in buffer A (50 mmol/L Tris-HCl, pH 7.9, 10 mmol/L KCl, 0.2% NP-40, 10% glycerol, 1 mmol/L EDTA, 1 mmol/L dithiothreitol, 1 mmol/L PMSF, and 1 mmol/L Na2VO4 ) and extracting the pelleted nuclei with a hypertonic buffer B (350 mmol/L NaCl, 20% glycerol, 20 mmol/L HEPES, pH 7.9, 10 mmol/L KCl, 1 mmol/L EDTA, 1 mmol/L PMSF, and 1 mmol/L Na2VO4 ). The extracts were centrifuged at 20,000g for 5 minutes and the supernatants were immediately frozen in liquid nitrogen and stored at −80°C. The oligonucleotide sequence used was derived from the human interferon regulatory factor-1 gamma activation sequence (IRF-1 GAS; 5′-GATCCATTTCCCCGAAATGA-3′).25 Probe was end-labeled with (γ-32P) ATP and 40,000 cpm was added to each sample. Extracts (105 cells/point) were incubated for 30 minutes at 4°C in 20 μL of binding buffer containing 2 μg poly(dI-dC). Complexes were separated on 6% nondenaturing polyacrylamide gels in 0.5× TBE and detected by autoradiography. For supershift assays, nuclear extracts were incubated with the probe and an excess of the indicated antibodies.
RESULTS
Murine Epo-R cDNA was introduced into TEPC cells by retroviral infection. Different clones were selected in the presence of IL-6 and G418 and 7 clones were amplified and further studied.
Southern blot analysis shows different integration sites in the infected clones.Southern blotting was used to examine the retroviral insertion sites in 7 Epo-R virus-infected TEPC clones. Genomic DNA of NEO and Epo-R/TEPC clones were digested by Bgl II, which cuts twice in the construct; electrophoresed; blotted; and hybridized with a NEO probe (Fig 1). A common band of 2.0 kb was detected in all 7 clones, corresponding to an internal Bgl II fragment of the Epo-R-NEO construct. The upper bands corresponded to the 3′ proviral junction fragment. The size and the number of these bands were different in each of the 7 clones, showing that these clones resulted from different infection events. In NEO-infected cells, the NEO probe showed a band of 1.3 kb corresponding to the internal Bgl II fragment and another band corresponding to the 3′ integration site.
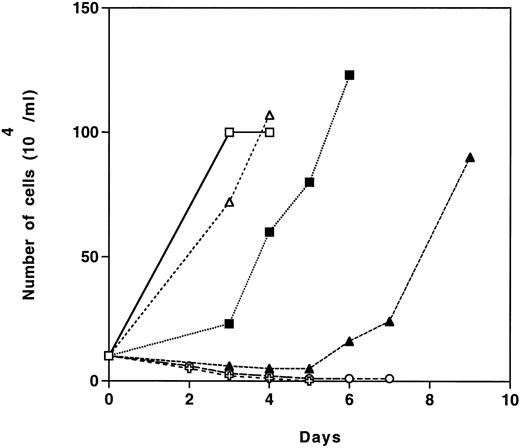
Epo-R/TEPC clones show a delay in proliferation when first exposed to Epo.The proliferative response of NEO- and Epo-R–infected TEPC clones was studied in the presence of Epo or IL-6 + G418. Cells from two G418-resistant clones (E1 and E2), which were grown in IL-6 and selected in the presence of G418, were washed and seeded in liquid culture in the presence of G418 and either IL-6 or Epo. The results presented in Fig 2 show that the two Epo-R clones tested showed a delay in proliferation of 3 to 5 days when stimulated with Epo, but then grew as well in the presence of Epo as in the presence of IL-6. Epo-R/TEPC cells did not proliferate after 48 hours without growth factor. In contrast, parental TEPC cells and NEO/TEPC cells from clone N1 responded only to IL-6 (data not shown).
Proliferative response of infected clones to Epo or IL-6. Cells from different G418-resistant clones grown in IL-6 were washed and 5 × 104 cells/mL were grown in MEM containing 500 μg/mL of G418, 10% FCS, and 1 U/mL rhEpo (solid symbols) or 10% P388D1 conditioned medium (open symbols) or without growth factor (✙ and ○) as described in the Materials and Methods. E1 (squares) and E2 (triangles) are Epo-R–expressing TEPC clones.
Proliferative response of infected clones to Epo or IL-6. Cells from different G418-resistant clones grown in IL-6 were washed and 5 × 104 cells/mL were grown in MEM containing 500 μg/mL of G418, 10% FCS, and 1 U/mL rhEpo (solid symbols) or 10% P388D1 conditioned medium (open symbols) or without growth factor (✙ and ○) as described in the Materials and Methods. E1 (squares) and E2 (triangles) are Epo-R–expressing TEPC clones.
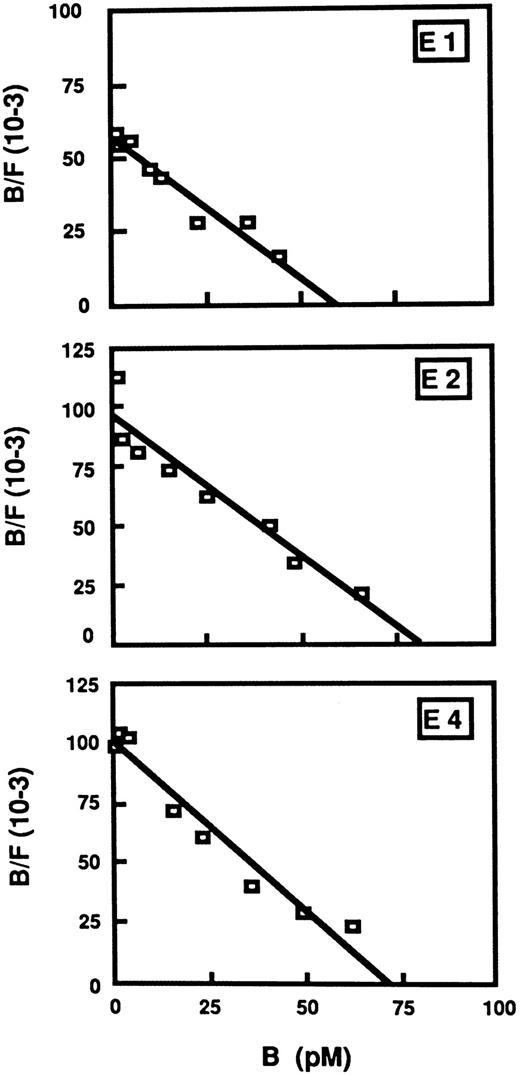
Epo-R/TEPC clones express a single class of high-affinity receptors.The number of receptors expressed on the surface of Epo-R–infected cells and the affinity of these receptors for Epo were determined by Scatchard analysis (Fig 3). Binding of 125I-rhEpo to Epo-R/TEPC clones (E1, E2, and E4) was concentration-dependent and was competed for by a 100-fold excess of unlabeled rhEpo. No specific binding was observed with N1 (NEO/TEPC). This experiment showed the presence of 3,750 ± 400 receptors/cell for the E1 clone and 5,100 ± 520 for the E2 clone. All of these receptors have similar affinity dissociation constant (kd = 0.7 nmol/L for E4 and 1.1 nmol/L for E1).
Scatchard plots of 125I-rhEpo to Epo-R–expressing TEPC clones E1, E2, and E4. Cells (106) were incubated with 125I-rhEpo with or without a 100-fold excess of unlabeled rhEpo (20 pmol/L to 200 nmol/L) for 3 hours at 37°C in RPMI containing 25 mmol/L HEPES, 5% FCS, 0.1% NaN3 . B/F means bound radioactivity against free radioactivity.
Scatchard plots of 125I-rhEpo to Epo-R–expressing TEPC clones E1, E2, and E4. Cells (106) were incubated with 125I-rhEpo with or without a 100-fold excess of unlabeled rhEpo (20 pmol/L to 200 nmol/L) for 3 hours at 37°C in RPMI containing 25 mmol/L HEPES, 5% FCS, 0.1% NaN3 . B/F means bound radioactivity against free radioactivity.
Iodinated Epo cross-linking studies.Cross-linking experiments with DSS were performed to determine if Epo-R is associated with other proteins in E1 Epo-R/TEPC clones. Cross-linked products were immunoprecipitated using anti–Epo-R antibodies and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). As shown in Fig 4, in addition to the Epo-R, two proteins exhibiting apparent molecular masses of 85 and 100 kD (after subtraction of the molecular mass of Epo) were immunoprecipitated (Fig 4A, lane 1 and Fig 4B, lane 3). Proteins of the same molecular weight were precipitated in the same conditions in parental UT-7 cells stimulated by Epo (Fig 4B, lane 1). These proteins were not detected either in the NEO clone (Fig 4B, lane 2) or after the addition of 100-fold excess of unlabeled Epo (Fig 4A, lane 2).
Cross-linking experiments. (A) E1 TEPC clones were labeled with iodinated Epo and then cross-linked with DSS in the absence (lane 1) or presence (lane 2) of a 100-fold excess of unlabeled Epo. (B) UT-7 cells (lane 1), NEO-infected clone N1 (lane 2), and Epo-R–infected clone (lane 3) were labeled with iodinated Epo and then cross-linked with DSS in the absence of 100-fold excess of unlabeled Epo. Epo-binding proteins were immunoprecipitated with anti–Epo-R antibodies bound to Sepharose beads. After denaturation, immunoprecipitated products were separated by SDS-PAGE.
Cross-linking experiments. (A) E1 TEPC clones were labeled with iodinated Epo and then cross-linked with DSS in the absence (lane 1) or presence (lane 2) of a 100-fold excess of unlabeled Epo. (B) UT-7 cells (lane 1), NEO-infected clone N1 (lane 2), and Epo-R–infected clone (lane 3) were labeled with iodinated Epo and then cross-linked with DSS in the absence of 100-fold excess of unlabeled Epo. Epo-binding proteins were immunoprecipitated with anti–Epo-R antibodies bound to Sepharose beads. After denaturation, immunoprecipitated products were separated by SDS-PAGE.
(A) Tyrosine phosphorylation of Epo-R. Factor-deprived E4 cells were incubated with medium (control), 500 U/mL IL-6 (lane IL-6), or 10 U/mL Epo [lanes Epo and Epo(D)] for 5 minutes. Cell lysates were prepared as described in the Materials and Methods and proteins immunoprecipitated with anti–Epo-R antibodies once (Epo) or twice [Epo(D)] were separated by SDS-PAGE under reducing conditions and blotted. The filter was probed with an antiphosphotyrosine antibody (4G10). (B) Tyrosine phosphorylation of Jak2. Factor-deprived E4 cells were incubated with medium (control), 500 U/mL IL-6 (lane IL-6), or 10 U/mL Epo (lane Epo). After lysis, proteins were immunoprecipitated with anti-Jak2 antibodies, separated by SDS-PAGE under reducing conditions, and blotted. The filter was probed with an antiphosphotyrosine antibody (4G10).
(A) Tyrosine phosphorylation of Epo-R. Factor-deprived E4 cells were incubated with medium (control), 500 U/mL IL-6 (lane IL-6), or 10 U/mL Epo [lanes Epo and Epo(D)] for 5 minutes. Cell lysates were prepared as described in the Materials and Methods and proteins immunoprecipitated with anti–Epo-R antibodies once (Epo) or twice [Epo(D)] were separated by SDS-PAGE under reducing conditions and blotted. The filter was probed with an antiphosphotyrosine antibody (4G10). (B) Tyrosine phosphorylation of Jak2. Factor-deprived E4 cells were incubated with medium (control), 500 U/mL IL-6 (lane IL-6), or 10 U/mL Epo (lane Epo). After lysis, proteins were immunoprecipitated with anti-Jak2 antibodies, separated by SDS-PAGE under reducing conditions, and blotted. The filter was probed with an antiphosphotyrosine antibody (4G10).
Electrophoretic mobility shift assay. Nuclear extracts prepared from E4 TEPC clone were incubated in the presence of the 5′ end-labeled IRF-1 probe and electrophoresed on a 6% polyacrylamide gel. The bound IRF-1 complexes (C1, C2, and C3) and the supershifted IRF-1 complexes (S1 and S3) are indicated. Extract from noninduced (lane 1), IL-6–induced (lanes 2 through 5), or Epo-induced (lanes 6 through 9) cells were incubated with antibodies specific for Stat1 (lanes 3 and 7), Stat3 (lanes 4 and 8), and Stat5 (lanes 5 and 9) or without antiserum (lanes 2 and 6).
Electrophoretic mobility shift assay. Nuclear extracts prepared from E4 TEPC clone were incubated in the presence of the 5′ end-labeled IRF-1 probe and electrophoresed on a 6% polyacrylamide gel. The bound IRF-1 complexes (C1, C2, and C3) and the supershifted IRF-1 complexes (S1 and S3) are indicated. Extract from noninduced (lane 1), IL-6–induced (lanes 2 through 5), or Epo-induced (lanes 6 through 9) cells were incubated with antibodies specific for Stat1 (lanes 3 and 7), Stat3 (lanes 4 and 8), and Stat5 (lanes 5 and 9) or without antiserum (lanes 2 and 6).
Time course of induction of immediate-early gene expression after Epo or IL-6 stimulation of Epo-R/TEPC clones. Epo-R/TEPC cells were washed, grown in the absence of growth factors for 18 hours, and then stimulated with IL-6 or Epo for 20 minutes to 4 hours. Total RNA was extracted and electrophoresed on a 1% agarose gel. (A) c-myc induction. A c-myc probe detects normal (2.4 kb) and abnormal (4.0 kb) transcripts. (B) JunB and c-fos induction. A junB probe detects a 1.8-kb transcript. A c-fos probe detects a 2.2-kb transcript. Lanes 1, before stimulation; lanes 2, 20 minutes; lanes 3, 1 hour; lanes 4, 3 hours; lanes 5, 4 hours of Epo or IL-6 stimulation. NIH/3T3 fibroblasts were used as control for the expression of c-fos and JunB. These fibroblasts were deprived of FCS (lanes 1) and stimulated by FCS for 20 minutes (lanes 2), 1 hour (lanes 3), and 3 hours (lanes 4).
Time course of induction of immediate-early gene expression after Epo or IL-6 stimulation of Epo-R/TEPC clones. Epo-R/TEPC cells were washed, grown in the absence of growth factors for 18 hours, and then stimulated with IL-6 or Epo for 20 minutes to 4 hours. Total RNA was extracted and electrophoresed on a 1% agarose gel. (A) c-myc induction. A c-myc probe detects normal (2.4 kb) and abnormal (4.0 kb) transcripts. (B) JunB and c-fos induction. A junB probe detects a 1.8-kb transcript. A c-fos probe detects a 2.2-kb transcript. Lanes 1, before stimulation; lanes 2, 20 minutes; lanes 3, 1 hour; lanes 4, 3 hours; lanes 5, 4 hours of Epo or IL-6 stimulation. NIH/3T3 fibroblasts were used as control for the expression of c-fos and JunB. These fibroblasts were deprived of FCS (lanes 1) and stimulated by FCS for 20 minutes (lanes 2), 1 hour (lanes 3), and 3 hours (lanes 4).
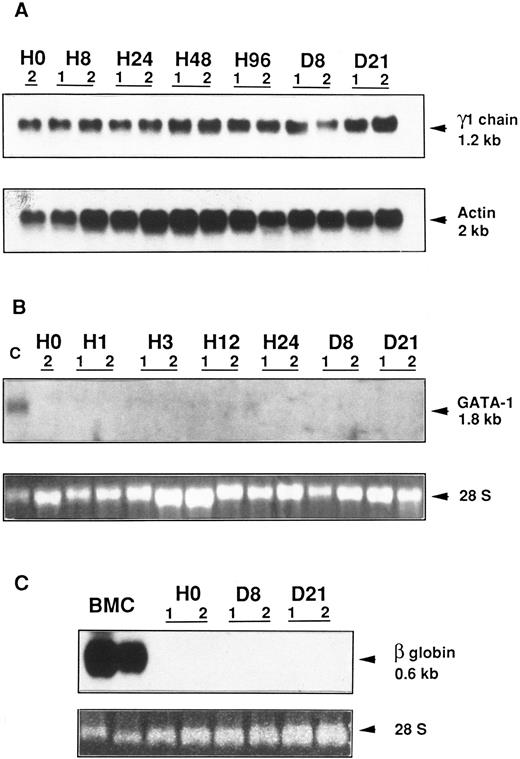
Northern blot analysis of γ1 heavy Ig chain, GATA-1, and β globin gene expression in an Epo-R/TEPC clone. Epo-R/TEPC cells (clone E4), which were growth factor deprived for 18 hours, were stimulated with either IL-6 or Epo for different times ranging from 1 or 8 hours to 21 days. Total RNA was extracted and subjected to Northern blotting. (A) Analysis of γ1 Ig heavy chain expression. A 1.2-kb mRNA was detected after 18 hours (H0) of growth factor starvation and from 8 hours (H8) to 21 days (D21) after Epo (lanes 1) or IL-6 (lanes 2) stimulation. Hybridation of the same filter with an actin probe quantifies the amount of RNA loaded. (B) Analysis of GATA-1 gene expression. A mRNA of 1.8 kb was detected in MEL cells (lane C). No transcript was detected in Epo-R/TEPC cells from 1 hour (H1) to 21 days (D21) after Epo (lanes 1) or IL-6 (lanes 2) stimulation. 28 S RNAs are shown to evaluate the amount of RNA loaded. (C) Analysis of β globin gene expression. A 0.6-kb transcript was detected in bone marrow cells (lanes BMC). No globin mRNA was detected in Epo-R/TEPC cells from day 8 (D8) to day 21 (D21) after Epo (lanes 1) or IL-6 (lanes 2) stimulation. 28 S RNAs are shown to evaluate the amount of RNA loaded.
Northern blot analysis of γ1 heavy Ig chain, GATA-1, and β globin gene expression in an Epo-R/TEPC clone. Epo-R/TEPC cells (clone E4), which were growth factor deprived for 18 hours, were stimulated with either IL-6 or Epo for different times ranging from 1 or 8 hours to 21 days. Total RNA was extracted and subjected to Northern blotting. (A) Analysis of γ1 Ig heavy chain expression. A 1.2-kb mRNA was detected after 18 hours (H0) of growth factor starvation and from 8 hours (H8) to 21 days (D21) after Epo (lanes 1) or IL-6 (lanes 2) stimulation. Hybridation of the same filter with an actin probe quantifies the amount of RNA loaded. (B) Analysis of GATA-1 gene expression. A mRNA of 1.8 kb was detected in MEL cells (lane C). No transcript was detected in Epo-R/TEPC cells from 1 hour (H1) to 21 days (D21) after Epo (lanes 1) or IL-6 (lanes 2) stimulation. 28 S RNAs are shown to evaluate the amount of RNA loaded. (C) Analysis of β globin gene expression. A 0.6-kb transcript was detected in bone marrow cells (lanes BMC). No globin mRNA was detected in Epo-R/TEPC cells from day 8 (D8) to day 21 (D21) after Epo (lanes 1) or IL-6 (lanes 2) stimulation. 28 S RNAs are shown to evaluate the amount of RNA loaded.
Epo induces Epo receptor and Jak2 phosphorylation in Epo-R/TEPC clones.To determine if the Epo-R expressed from the viral gene is phosphorylated in response to Epo, immunoprecipitation and Western blot analyses were performed on Epo-R/TEPC clone E4 cells. NEO/TEPC cells were used as a negative control. As shown in Fig 5A, after stimulation with Epo, a protein of 70 kD, corresponding to the molecular mass of Epo-R, was phosphorylated (lane Epo). No signal was observed after stimulation of the Epo-R/TEPC cells with IL-6 (lane IL-6) or of the N1 NEO clone, irrespective of the growth factor used (data not shown).
To ensure that this p70 was Epo-R protein, immunoprecipitated proteins were denatured in boiling Laemmli buffer and subjected to a second immunoprecipitation with anti–Epo-R antibodies and labeled with an antiphosphotyrosine antibody on Western blot. Under these conditions, the phosphorylated protein [lane Epo (D)] had the same molecular weight, confirming that this protein is Epo-R.
To determine if Jak2 is phosphorylated in response to Epo and IL-6, the same type of experiment was performed, ie, immunoprecipitation with an anti-Jak 2 antibody and Western blot analysis with an antiphosphotyrosine antibody. As shown in Fig 5B, stimulation with Epo, but not with IL-6, increases the phosphorylation level of both a protein of 140 kD corresponding to Jak2 and a phosphorylated protein of 70 kD reminiscent of Epo-R. Residual phosphorylation of Jak2 in unstimulated Epo-R/TEPC cells may be due to incomplete deactivation of Jak2 in cells cultured in medium containing 0.1% P388D1 conditioned medium.
Stat5 is activated after Epo stimulation of Epo-R/TEPC clones.Nuclear extracts prepared from E4 Epo-R/TEPC cells stimulated either by Epo or IL-6 possessed DNA binding activity with the IRF-1 GAS oligonucleotide (Fig 6).
Two major complexes (C1 and C2) appear in response to IL-6 (lane 2); whereas no complexes exist without stimulation (lane 1), one complex (C3) appears in response to Epo (lane 6). To characterize the proteins that are associated with the shifted complexes, supershift experiments were performed with antibodies raised against various Stat proteins (Stat1, 3, or 5). As shown in Fig 6, the intensity of complex C1 decreased when incubated with anti-Stat1 antibody (lane 3). On the same gel exposed for longer time, a third tiny complex migrating faster than C1 was observed that was supershifted in the presence of anti-Stat1 (data not shown). Similarly, the intensity of complexes C1 and C2 decreased when incubated with anti-Stat3 antibody, and a supershifted complex (S1) was detected (lane 4). In contrast, no supershifted complex was observed in the presence of anti-Stat5 antibody. These data indicate that, in TEPC cells, IL-6 induces DNA binding complexes containing a majority of heterodimers Stat1/Stat3 (C1 complex) and homodimers Stat3/Stat3 (C2 complex). No Stat5 seems to be activated in response to IL-6. On the other hand, our results show that Epo stimulation induces DNA binding complex (C3) that is recognized by anti-Stat5 antibody (lane 9, supershifted complex S3), suggesting that DNA binding activity immunologically related to Stat5 is activated in response to Epo in TEPC cells expressing Epo-R.
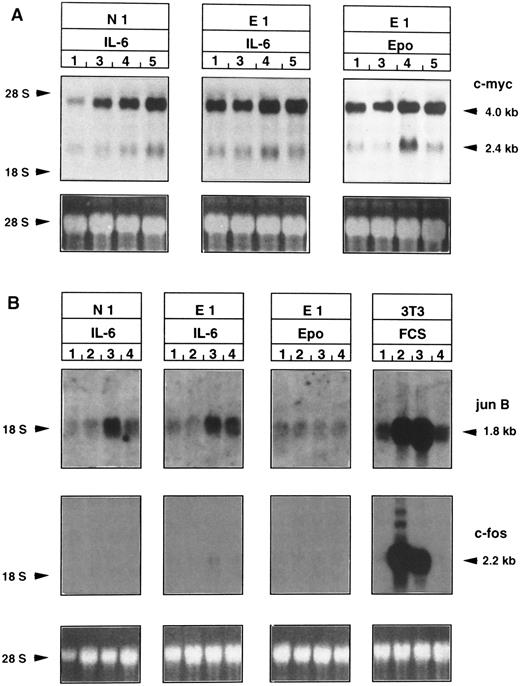
Epo induces c-myc, but neither junB nor c-fos mRNA accumulation in Epo-R/TEPC clones.To compare the signal transduction pathways of Epo and IL-6 in Epo-R/TEPC cells, the expression of immediate early genes (c-myc, junB, and c-fos) was analyzed by Northern blotting after stimulation by Epo or IL-6 (Fig 7). Starved Epo-R-TEPC clones were stimulated with Epo or IL-6 for 1, 2, or 4 hours for the analysis of c-myc or for 20, 60, or 180 minutes for the analysis of junB and c-fos. Two species of mRNA were detected with a c-myc probe (Fig 7A), ie, 2.4- and 4.0-kb species, as previously described by Bauer et al.55 The 4.0-kb species appears to be more abundant than the 2.4-kb species, and the 2.4-kb species appeared to be regulated by both growth factors.
Expression of the junB gene (Fig 7B) was increased in Epo-R and N1 clones stimulated with IL-6, but not after stimulation with rhEpo (not in N1, data not shown, or E1 subclones), suggesting that the signal transduction pathways are different, at least in part, for IL-6 and Epo.
c-fos expression (Fig 7B) could not be detected either in parental TEPC cells or in Epo-R/TEPC cells after Epo or IL-6, whereas NIH3T3 cells, used as a positive control, expressed a 2.2-kb transcript corresponding to the expected size of c-fos mRNA.
Epo does not induce murine erythroid specific gene expression and does not modify γ1 Ig heavy chain gene expression.To test the possibility that Epo could modulate IL-6–specific mRNA or induce the synthesis of some erythroid-specific mRNA in Epo-R/TEPC cells, γ1 chain, GATA-1 (an erythroid transcription factor) and β major globin gene expression were analyzed various times after Epo stimulation (Fig 8). The level of γ1 chain mRNA was not modified by growth factor starvation or by Epo or IL-6 stimulation, suggesting that, in this particular plasmacytoma cell line, γ1 chain expression is not directly regulated by IL-6. GATA-1 transcripts were not observed from 1 hour to 21 days after Epo stimulation in Epo-R–expressing TEPC cells. Noninduced murine erythroleukemic (MEL) cells were used as a positive control. From 8 hours to 21 days after Epo stimulation, no expression of the β major globin gene was induced. Murine bone marrow cells were used as a positive control.
DISCUSSION
The goal of this study was to investigate the functional capacity of the Epo-R when expressed in an IL-6–dependent lymphoid context and to characterize the molecules involved in the signaling pathway. To this end, an IL-6–dependent plasmacytoma cell line, TEPC-2027, was infected with a retrovirus carrying the Epo-R cDNA. Our results show that, after a latency of 3 to 5 days, the Epo-R/TEPC cell clones grew equally well in response to both IL-6 and Epo. Therefore, in this plasmacytoma cell line, the Epo-R is expressed at the mRNA and protein level, is correctly processed, and is translocated to the cell surface. Moreover, our results indicate that, although Epo-R and IL-6–R do not belong to the same subgroup of the hematopoietic cytokine receptor superfamily2 and do not share a common β subunit nor common Jak/Stat pathway, both receptors induce a proliferative signal in Epo-R–expressing TEPC cells. This result was not obvious, because it has been shown that expression of the wild-type Epo-R, contrary to the chimeric receptor Epo-R/IL-2–R, does not always provide dependence on Epo in T cells.56 It has been suggested that this absence of a response of T-lymphoid cells to Epo is due to the absence of interaction between an intracellular region of Epo-R and Jak2.57
Migliaccio et al58 have shown that, in a factor-dependent cell line, the major regulatory step determining the response to Epo is the efficiency of Epo-R protein translocation to the cell surface. They hypothesized that mechanisms could affect lineage-specific translocation and that the regulation of these mechanisms will be different in the cells of different lineages. We showed that high-affinity Epo-R are expressed at the cell surface of retrovirally infected TEPC cells. The number of these receptors varies from one clone to another, suggesting that either the level of retroviral gene expression is dependent on the integration site or the regulation of transport of the receptor to the membrane varies from clone to clone.
Whatever the number of receptors expressed at the cell surface, Epo-R/TEPC cells grew in the presence of Epo, suggesting that forced expression of the cloned Epo-R chain in TEPC cells is sufficient to permit the transduction of an efficient proliferative signal.
The possibility that Epo itself induces formation of noncovalent Epo-R homodimers on the cell surface and that this dimerization is sufficient to generate a high-affinity receptor has been proposed14 15 and is consistent with our results. However, using cross-linking experiments, we have shown that, in Epo-R/TEPC cells, the Epo-R was associated with proteins of the same molecular weight as observed in control erythroid cells, suggesting that accessory Epo-R chains are expressed in the TEPC cell line. Expression of these accessory chains should not be a limiting element for Epo signaling in TEPC cells.
Because we showed that Epo-R is efficient in transducing a proliferative response in plasmacytoma cells, we tried to characterize the molecules involved in the signal transducing pathway of Epo in Epo-R/TEPC cells.
The first event induced by binding of Epo to its receptor in Epo-R/TEPC cells appears to be the same as that observed in cells normally responding to Epo. The Epo-R is phosphorylated within 5 minutes after Epo stimulation.12 16 Jak2 is also phosphorylated in response to Epo. Our results show that phosphorylation of Jak2 is greater after Epo stimulation than after IL-6 or in control unstimulated cells. These results suggest that Epo and IL-6 do not activate the same members of Jak family. Supporting this hypothesis, we have shown (Fig 5B) that anti-Jak2 antibodies could immunoprecipitate two phosphorylated proteins (Jak2 and Epo-R), indicating that phosphorylated Epo-R is associated with phosphorylated Jak2, whereas gp130 is not.
Because different Jak kinases are activated in response to Epo and IL-6,12,39 we examined the STAT substrates that are activated by these kinases. Our results show that, in Epo-R/TEPC cells, Stat5 is activated in response to Epo, whereas Stat3 is activated in response to IL-6, suggesting that Epo and IL-6 transduce their signals via different pathways. It is of interest that TEPC cells, which normally respond to IL-6 and do not activate Stat5, are able to respond to Epo by activating Stat5. Therefore, although Stat5 is expressed in TEPC cells, it is not activated when IL-6 induces cell growth, indicating that different species of Stat are recruited by IL-6 and Epo-R. This is confirmed by the fact that gp130, the IL-6 signal-transducing chain, contains cytoplasmic sequences capable of binding Stat3, whereas the Epo-R contains binding sequences for Stat5 but not for Stat3.59
Expression of immediate-early genes (which usually are induced in response to growth factors) was subsequently studied. The TEPC cell line has been derived from a tumor that has undergone a translocation of the Ig heavy chain locus located 5′ to the c-myc gene promoter, resulting in the formation of two c-myc mRNA species.55 In Epo-R/TEPC cells, the normal mRNA of 2.4 kb is normally regulated60 in response to either Epo or IL-6, whereas the abnormal species of 4 kb seems to be slightly regulated by growth factors. The precise role of abnormal c-myc expression in TEPC cell proliferation is unknown.
Although DA-3 cells expressing retroviral Epo-R show a transient expression of c-fos in response to Epo,61 Epo-R/TEPC cells do not express c-fos after IL-6 or Epo stimulation. It has been shown that expression of v-ras in fibroblasts62 and v-abl in hematopoietic cells63 inhibits the growth factor-induced upregulation of c-fos expression. The constitutive expression of the rearranged myc gene may play the same role in the inhibition of c-fos expression in TEPC cells.
JunB mRNA accumulation was found after IL-6 stimulation in agreement with the observation that cytokines sharing the gp130 receptor chain induce expression of immediate-early genes such as JunB.64 In contrast, Epo stimulation was unable to induce JunB mRNA accumulation in Epo-R/TEPC cells. It has been recently shown that Epo increases the activity of the transcription factor AP-1 in both transformed and normal erythroid cells.65 However, this increase in AP-1 activity may not be transcriptionally related and is not linked to an increase in JunB expression. Furthermore, the transcription of JunB is controlled through an IL-6–specific cis-acting regulatory element (IRE) located at positions −199 and −91.66 Gel retardation analysis has shown that this IRE (TGTCAGGAA) binds complexes containing Stat3. Because JunB mRNA is not accumulated in response to Epo and JunB IRE does not contain the specific Stat5 binding sequence (TTCXXXGAA), it seems reasonable that the promoter region of JunB does not bind Stat5.
To determine if Epo-R expression has a specific role in the signal transduction pathways that lead to differentiation, the induction of γ1 heavy chain, GATA-1, and β globin mRNA were examined at various times after Epo or IL-6 stimulation. No γ1 heavy chain, GATA-1, or β globin mRNA accumulation was observed either after Epo or IL-6.
The absence of γ1 heavy chain mRNA accumulation is surprising given the demonstration of the cycle-dependent regulation in Epstein-Barr virus-infected B cells67 and the induction of γ1 mRNA synthesis by IL-6 stimulation in lymphoblastoid cells.68
It has been already described that the transcription of GATA-1 and β globin gene is induced by Epo in IL-3–dependent cell lines retrovirally infected with the murine Epo-R cDNA.69-72 In TEPC cells, a terminally differentiated cell line, transcription of erythroid-specific genes is not induced in response to Epo, reflecting probably the inability of these cells to change their differentiation state.
Finally, in TEPC cells expressing the murine Epo-R, Epo induces cellular proliferation, but is unable to induce the expression of erythroid-specific mRNA. Therefore, erythroid differentiation depends on the cell type, and an erythroid cellular environment seems to be necessary. The molecular mechanisms responsible for this limitation are presently unknown.
ACKNOWLEDGMENT
We thank N. Doyen (Institut Pasteur, Paris, France) for the gift of γ1 chain probe, N. Reich (U91, Créteil, France) for the β globin probe, and S. Burstein for helpful discussions.
Supported by grants from Institut National de la Santé et de la Recherche Médicale (INSERM) and the Association pour la Recherche sur le Cancer (ARC 6277). F.F. received a fellowship from the Institut National de la Santé et de la Recherche Médicale.
Address reprint requests to Frédéric Féger, INSERM U362, Institut Gustave Roussy, 94800 Villejuif, France.



![Fig. 5. (A) Tyrosine phosphorylation of Epo-R. Factor-deprived E4 cells were incubated with medium (control), 500 U/mL IL-6 (lane IL-6), or 10 U/mL Epo [lanes Epo and Epo(D)] for 5 minutes. Cell lysates were prepared as described in the Materials and Methods and proteins immunoprecipitated with anti–Epo-R antibodies once (Epo) or twice [Epo(D)] were separated by SDS-PAGE under reducing conditions and blotted. The filter was probed with an antiphosphotyrosine antibody (4G10). (B) Tyrosine phosphorylation of Jak2. Factor-deprived E4 cells were incubated with medium (control), 500 U/mL IL-6 (lane IL-6), or 10 U/mL Epo (lane Epo). After lysis, proteins were immunoprecipitated with anti-Jak2 antibodies, separated by SDS-PAGE under reducing conditions, and blotted. The filter was probed with an antiphosphotyrosine antibody (4G10).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/2/10.1182_blood.v89.2.435/4/m_bl_0011f5.jpeg?Expires=1767855361&Signature=5KDZOEAtwRU9sbPm~qKkbeZ5FA8NhmrZjFoC7JXzrel4xQsIObeKVmoRxd09x9Np82mH4KyG2apsb1MDAWXWGLTWXb2LvbimP~F~ImR01sIBrwh~N58o5tYxDhdrKvJOFiaRn~F868zVboM3R7mipjfHHRP-xaWwwv3vNfuQIPXTmhxHMaNAjHltTf0PUAZJP2HXOD2duuF-nTudi~YgsOl01otGnUdZt72VqRpYz5J7Tgst7m-qeNO6vcXBMuT7JZbi8W59MiY9cOsEe64midR3~-E0erUOhxu1cg-3vbmquOwmbZ8Llxj20xnPDc69VPStmzZwMlcpZ12lPmPFYw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal