Abstract
Megakaryocyte (MK) progenitors, CD34+CD41+ cells, were isolated from human bone marrow with a purity greater than 98% and a viability of 95%, using affinity techniques with magnetic beads followed by fluorescence-activated cell sorting. These cells were incubated in synthetic media containing the cytokines thrombopoietin (TPO), interleukin-3 (IL-3), stem cell factor (SCF ), and IL-6, obviating the confounding effects of serum growth factors or cytokine secretions of non-MK cells on MK maturation. MK number, MK colony-forming units (CFU-MK), and MK ploidy and phenotype were examined during 7 days in culture. TPO in serum-free cultures without any other exogenously added cytokine supported MK growth and maturation. SCF synergized with TPO to augment MK production and maturation and could partially replace it under some conditions. Both TPO and IL-3 alone increased MK number (12- and 5-fold, respectively) and CFU-MK (∼15-fold each). SCF alone had no effect on MK proliferation in the absence of TPO, but increased both MK number and CFU-MK by 1.5- to 2.0-fold in the presence of TPO. When combined with IL-3, SCF increased both MK number and CFU-MK by 15- to 20-fold in the absence of TPO. In the presence of TPO, the combination of IL-3 and SCF produced only modest increases (1.5- to 2.0-fold) in both MK number and CFU-MK. The proportion of polyploid MK increased greater than fivefold in the presence of TPO. SCF had little effect on MK ploidy in the presence of TPO, but enhanced ploidy twofold to threefold in the absence of TPO. IL-3 alone never increased the level of polyploidization. Rather, it consistently inhibited TPO- and SCF-induced polyploidization of MK. This inhibition was observed in cultures with or without SCF or IL-6. Although IL-3 also supported the proliferation of CD41+ cells and CFU-MK production, the cells that developed under the influence of IL-3 were phenotypically unusual (CD41dim, CD42dim) and of relatively low ploidy. Mature MK were not produced. When added with TPO, IL-3 suppressed polyploidization. Therefore, TPO stimulates MK growth and maturation, whereas IL-3 stimulates growth without maturation and may serve to conserve the immature MK compartment.
RECOMBINANT thrombopoietin (TPO) stimulates the growth and maturation of murine megakaryocytes (MK) in vitro and induces an increase in the number of megakaryocytes and platelets in vivo.1,2 TPO is present in the plasma and serum of thrombocytopenic patients and animals.3,4 MK development is regulated by the interaction of TPO,5,6 a lineage-specific factor known to regulate both megakaryocytopoiesis and thrombopoiesis, and a number of pleotrophic cytokines, including interleukin-3 (IL-3), IL-6, IL-11, and stem cell factor (SCF ).7,8 IL-3 has been shown to play a particularly important role.9 10 Synergistic interactions between TPO and these growth factors appear to play an important role in MK development and platelet production.
Synergistic interactions with other cytokines have made it difficult to analyze the precise role of TPO in MK development. Culturing megakaryocyte precursors in the presence of other bone marrow (BM) cells can lead to complex interactions between exogenous cytokines added as components of the media and endogenous growth factors produced by various bone marrow and stromal cells. There is evidence that the release of hematopoietic growth factors can be induced by T cells, granulocytes, and monocytes11 and that the number of cytokine-secreting cells can be increased by the addition of exogenous growth factors.12 The present manuscript documents these synergistic effects with highly purified CD34+ MK precursors in synthetic media obviating the concern of growth factors introduced by fetal calf serum or complications of other BM cells secreting cytokines induced by the stimulation of the added cytokines.
This study focuses on the effects of TPO, IL-3, SCF, and IL-6 on committed MK progenitors and diploid cells lacking late markers of MK development, but expressing CD41 (glycoprotein [GP] IIb-IIIa). In this study, we show that both TPO and IL-3 stimulate MK production and colony-forming units-MK (CFU-MK). However, only TPO stimulates polyploidization; IL-3 does not. On the contrary, IL-3 inhibits both the maturation and polyploidization induced by TPO, thus contributing to the maintenance of the immature MK compartment.
MATERIALS AND METHODS
BM cells.BM samples were obtained from discarded orthopedic specimens from otherwise normal patients. Sample collection was performed under an Institutional Review Board-approved protocol. BM cells were resuspended in phosphate-buffered saline (PBS; 5 mmol/L EDTA, 0.5% bovine serum albumin [BSA]) and washed twice (1,500 rpm for 10 minutes). A low-density mononuclear cell fraction was obtained by collecting the cells that accumulated at the interface of a one-step Ficoll-Histopaque (1.077 g/mL; Sigma Chemical Inc, St Louis, MO) gradient after centrifugation at 2,000 rpm for 20 minutes at 25°C.
Isolation of CD34+CD41+ MK-lineage cells.CD34+ cells were separated by incubating them with saturating amounts of mouse anti-CD34 monoclonal antibodies (MoAbs; QBEND10) and then allowing the antibody-coated cells to bind to superparamagnetic beads coated with rat antimouse IgG. Cells were separated using the column provided in the MACS system (Myltenyi Biotec, Sunnyvale, CA). In some experiments, the positive fraction recovered from the first column was further purified by passage through a second MACS column. The purity of the CD34-enriched population was estimated by staining with an anti-CD34 MoAb conjugated to phycoerythrin (PE) that reacts with a different epitope than the antibody used for the separation. After MACS column separation, the cells were ∼80% CD34+ (recovery >80%). After a second column, the purity of the CD34+ cells increased to 95%. Fluorescence-activated cell sorting was used to isolate CD41+ cells from the MACS-enriched CD34+ population. CD34-enriched cells recovered from the MACS column were stained with anti-CD41 fluorescein isothiocyanate (FITC) and anti-CD34 PE MoAbs and sorted on a Epics Elite Cell Sorter (Coulter, Hialeah, FL). Debris and dead cells were excluded using scatter gates. Only cells with low orthogonal light-scattering patterns were included in the sorting gates. Double-positive cells (CD41+CD34+) were sorted and reanalyzed on FACScan flow cytometer (Becton Dickinson, San Jose, CA). If the sorted population was less than 95% CD41+, a second sort was performed. The cell populations used in this study were always more than 99% CD34+ and 97% to 99% CD41+.
Culture conditions for MK production.Sorted CD34+CD41+ cells were cultured in serum-free Iscove's modified Dulbecco's medium with L-glutamine supplemented with deionized 10 mg/mL BSA (Boehringer Mannheim, Indianapolis, IN), 25 μg/mL soybean lipids (Boehringer Mannheim), 7.8 μg/mL cholesterol (Sigma), 5.6 μg/mL linoleic acid (Sigma), 1 mmol/L sodium pyruvate (Sigma), 100 μmol/L α-thioglycerol (Sigma), and 300 μg/mL of 30% iron-saturated transferrin (Boehringer Mannheim). On the basis of preliminary experiments, the following growth factors were used alone or in combination: 300 U/mL TPO (a gift from Dr Si Lok, Zymogenetics, Seattle, WA), 10 ng/mL recombinant human IL-6 (Peprotech, Rocky Hill, NJ), 50 ng/mL recombinant human SCF (Peprotech), and recombinant human IL-3 (Immunex, Seattle, WA). In most experiments, IL-3 was used at 1 ng/mL, but a variety of concentrations were tested to establish its optimum effect. The cells were cultured in 96-well plates (Corning, Corning, NY) at 30,000/mL in 250 μL of media per well. The plates were incubated at 37°C for 7 days in an atmosphere of 5% CO2 in air in a humidified incubator. After culture, live cells were counted with a hemocytometer using trypan blue uptake to identify dead cells. To characterize the phenotype and ploidy of the cultured cells, the entire contents of a well were removed and stained as described below.
Culture conditions for CFU-MK.Sorted CD41+CD34+ cells were also analyzed for their capacity to produce MK colonies (CFU-MK) when cultured in a plasma clot.13 The cultures were performed in 96-well plates in 100 μL volume with an input of 100 cells per well. After 7 and 12 days of incubation, the cultures were examined microscopically, and clusters of 3 or more cells were scored as MK colonies. In some experiments, the plasma clot cultures were fixed with methanol and stained with mouse anti-CD61 and goat antimouse Ig coupled to alkaline phosphatase to confirm that all of the colonies found in the cultures were in the MK lineage.
Phenotype analysis.Sorted cells were resuspended in MK medium (Ca++ and Mg++-free PBS containing 13.6 mmol/L sodium citrate, 2.2 μmol/L prostaglandin E1, 1.0 mmol/L theophylline, 3% BSA, and 11 mmol/L glucose) and adjusted to pH 7.3. All reagents were purchased from Sigma.
For flow cytometry, cells were stained with the following antibodies: anti-CD41a (GPIIb) FITC (IOP41a, clone P2, IgG1; Immunotech, Westbrook, MA); anti-CD34 PE (HPCA-2, clone 8Gl2, IgG1; Becton Dickinson); anti-CD42 (GPlb) biotin (IOP42b, clone SZ2), IgG1 (Immunotech); anti-CD62 PE (P-selectin), clone CRC81, IgG1 (Caltag Laboratories, South San Francisco, CA); and anti-CD61 (GPIIIa) prepared in our laboratories (LK-7r)14 and conjugated to FITC. Control samples were stained with mouse IgG1 FITC, IgG1 PE, and IgG1 biotin. Cells were incubated for 30 minutes on ice, washed, resuspended in MK medium, and stained with streptavidin coupled to Tri-Color (Caltag) for 30 minutes. After staining, cells were washed and resuspended in 2% paraformaldehyde in PBS.
DNA analysis.Cell suspensions were stained with anti-CD41 FITC and then fixed and permeabilized with 1% paraformaldehyde by adding, dropwise, an equal volume of 2% paraformaldehyde in PBS containing 160 μg/mL of lysophosphatidylcholine (Lysolecithin; Sigma). After 5 minutes on ice, the cell suspension was diluted with 5 vol of MK medium, washed, and resuspended in PBS (0.1% sodium citrate, 1% BSA) containing 50 μg/mL of propidium iodide (PI; Sigma). The suspension was incubated overnight at 4°C in the dark, after which 100 μg/mL of RNAse (Calbiochem, San Diego, CA) was added. The cells were incubated for 1 hour at room temperature and the suspension was filtered through a 70-μm nylon filter to remove aggregates.
Two-color (FITC and PI) and three-color (FITC, PE, and Tri-Color) flow analysis was performed in a FACScan cytometer equipped with a 15 mW argon-ion laser. Forward and side light scatter and two or three fluorescence signals were recorded from each cell. PI fluorescence was collected in the logarithmic mode. Electronic compensation was used to eliminate spectral overlap between the various fluorochromes.
Morphology of megakaryocytes.CD41+CD34+ sorted cells and cells recovered after 7 days of culture were centrifuged onto glass slides and stained with Wright-Giemsa.
Statistics.Statistical significance was determined using the Student's two-tailed t-test for paired samples.
RESULTS
Isolation of CD34+CD41+ cells.Only 0.1% to 0.5% of low-density BM cells are CD41. Of these, ∼15% express the CD34 antigen characteristic of hematopoietic progenitors. The amount of CD34 expressed correlates inversely with maturity. Immunomagnetic separation increased the proportion of CD34+ cells from 3.0% to 81%. Only bright CD34+ cells are retained by the beads. Fluorescence-activated sorting of the enriched material increased the percentage of CD41+ cells (MK) from 5% to 98% and a second sort increased the enrichment for CD34+ cells to greater than 99% and CD41+ cells to greater than 98%. These cells were used as the starting material in the experiments reported here. A correlated two-parameter histogram of the staining of the isolated cells is shown in Fig 1A. The sorted megakaryocytes were 95% viable as measured by dye exclusion with PI. They are predominantly diploid, with only 0.5% having DNA contents greater than 4N (Fig 1B). Morphologically, they are a relatively homogeneous population of large cells with little evidence of cytoplasmic maturation (Fig 1C). Only 18% ± 7% of these cells express CD42 and 4% ± 2% of these cells express CD62, late MK maturation markers (see below).
Separation of CD34+CD41+ human BM cells. Correlated two-parameter histogram of staining of (A) low-density BM cells after enrichment of the CD34+ population using magnetic beads (MACS) and two cycles of sorting. The separated population is 99% CD34+ and 98% CD41+. (B) DNA content of isolated CD34+CD41+ BM cells. In this sample, 15% of the cells are in S phase and 17% are in G2-M or have reached the 4N stage via endomitosis. Less than 0.5% of the cells in this sample have a DNA content ≥8N. The data were collected in log mode. (C) Morphology of purified CD34+CD41+ BM cells. The CD34+CD41+ cells shown in (A) were deposited on slides by cytocentrifugation and stained with Wright-Giemsa. (Original magnification × 630.)
Separation of CD34+CD41+ human BM cells. Correlated two-parameter histogram of staining of (A) low-density BM cells after enrichment of the CD34+ population using magnetic beads (MACS) and two cycles of sorting. The separated population is 99% CD34+ and 98% CD41+. (B) DNA content of isolated CD34+CD41+ BM cells. In this sample, 15% of the cells are in S phase and 17% are in G2-M or have reached the 4N stage via endomitosis. Less than 0.5% of the cells in this sample have a DNA content ≥8N. The data were collected in log mode. (C) Morphology of purified CD34+CD41+ BM cells. The CD34+CD41+ cells shown in (A) were deposited on slides by cytocentrifugation and stained with Wright-Giemsa. (Original magnification × 630.)
Effect of cytokines on production of MK in liquid culture.Figure 2 shows the production of CD41+ MK after 7 days in liquid culture. Maximum MK production was obtained in cultures containing both TPO and SCF (lines 1 through 4). The addition of either IL-3 (line 2) or IL-6 (line 3) did not further increase the yield of CD41+ cells, but the combination of all four cytokines produced the maximal yield of MK (line 4 compared with line 1; P < .05). TPO alone produced about half as many MK as the combination of TPO and SCF (lines 1 through 4 v lines 5 through 8). The differences between the SCF-supplemented cultures and those receiving TPO alone were highly significant (lines 1 v 5, lines 2 v 6, and lines 4 v 8, P < .001). The difference between line 3 (TPO + IL-6 with SCF ) and line 7 (TPO + IL-6 without SCF ) was in the same direction, but had a P value of .09. SCF alone (line 9) did not support MK development.
Effect of cytokines on the production of MK by CD34+CD41+ progenitors. Highly purified CD34+CD41+ BM cells (30,000/mL) were incubated for 7 days in a serum-free synthetic culture media containing various combinations of cytokines. After culture, cells were counted and analyzed for CD41 and PI by flow cytometry. The resulting two-parameter histogram was gated on CD41+ cells with DNA content ≥2N to exclude cell fragments and other debris. The total number of CD41+ cells recovered from each well has been plotted. Each bar represents the mean of two to four experiments. The error bars indicate the standard deviation of the mean and are shown for all results in which three or more values were available.
Effect of cytokines on the production of MK by CD34+CD41+ progenitors. Highly purified CD34+CD41+ BM cells (30,000/mL) were incubated for 7 days in a serum-free synthetic culture media containing various combinations of cytokines. After culture, cells were counted and analyzed for CD41 and PI by flow cytometry. The resulting two-parameter histogram was gated on CD41+ cells with DNA content ≥2N to exclude cell fragments and other debris. The total number of CD41+ cells recovered from each well has been plotted. Each bar represents the mean of two to four experiments. The error bars indicate the standard deviation of the mean and are shown for all results in which three or more values were available.
However, IL-3 was able to support the production of CD41+ cells in the absence of TPO. Only minimal MK production was obtained with IL-3 alone (line 14); however, when SCF was included, the production of CD41+ cells increased significantly (line 10; P = .01).
Effect of cytokines on CFU-MK production.Discrete colonies were visible after 7 to 10 days in culture in serum-free medium containing all four cytokines. CFU-MK produced by the isolated progenitor cells were small, rarely exceeding 8 cells per colony. Many isolated large MK were present. These cells were also abundant in the liquid phase cultures. They contained large lobulated nuclei and had numerous cytoplasmic granules.
MK burst-forming units (BFU-MK) were not seen in any of these cultures. Figure 3 shows the effects of the cytokines on CFU-MK formation. Again, TPO played a dominant role. Synergy with SCF was again observed despite the inability of SCF to support colony formation by itself (line 9). Although the extent of the SCF-induced augmentation of colony number was smaller than the effect on cell number, the differences were all significant at P values ranging between .02 and .0001. IL-3 or IL-6 produced small, but statistically significant (P < .05) increases in CFU-MK when added in combination with TPO and SCF.
Effect of cytokines on colony formation by CD34+CD41+ MK progenitors. CD34+CD41+ BM cells were incubated for 7 days in serum-free plasma clot culture in 96-well plates (100 cells in 100 μL of media) with different combinations of cytokines. After 7 days, the wells were fixed, stained as described, and examined microscopically. Clusters of 3 or more cells were scored as CFU-MK colonies. The results shown are the mean ± SD of four experiments.
Effect of cytokines on colony formation by CD34+CD41+ MK progenitors. CD34+CD41+ BM cells were incubated for 7 days in serum-free plasma clot culture in 96-well plates (100 cells in 100 μL of media) with different combinations of cytokines. After 7 days, the wells were fixed, stained as described, and examined microscopically. Clusters of 3 or more cells were scored as CFU-MK colonies. The results shown are the mean ± SD of four experiments.
Although the number of CFU-MK found in the plasma clot cultures generally paralleled the total number of CD41+ cells recovered from the suspension cultures, an exception was noted. In the absence of TPO, SCF did not synergize with IL-3 to increase the number of CFU-MK, although together these cytokines increased the number of MK recovered by threefold. Only in the presence of IL-6 (lines 11 and 15) did SCF augment CFU-MK formation (P < .01).
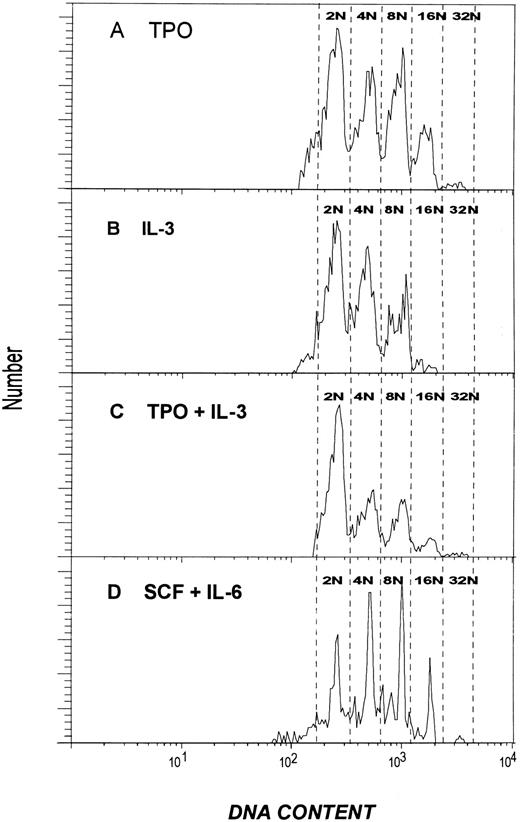
Effect of cytokines on MK ploidy.The effects of the four cytokines on the development of polyploid MK in culture is shown in Figs 4 and 5. Figure 4 shows the percentage of cells that acquired a DNA content greater than 8N. TPO alone produced a near-maximum proportion of polyploid cells (lines 5 and 13). The addition of SCF increased this slightly (P < .01; lines 1 and 5). Because the combination of TPO and SCF increased the total number of CD41+ cells by 67-fold compared with unsupplemented medium (percentage × total, data in Fig 4 multiplied by data in Fig 2), it is clear that this combination was most efficient at inducing polyploidy. The distribution of the DNA content of these cells fell into five patterns (Fig 5). These patterns differed both in the proportion of diploid cells remaining after 1 week in culture and in the degree of polyploidization that was induced. The pattern obtained when cells were cultured with TPO alone is shown in Fig 5A. Similar patterns were obtained with TPO and either IL-6 or SCF (data not shown). Under these conditions, the 8N population generally exceeded the 4N population. In each case, more than 25% of the cells achieved a DNA content of 16N or higher, with a discrete (1.5% to 3%) 32N population. The 32N population was found only after growth in the presence of TPO.
Effect of cytokines on the production of polyploid MK by CD34+CD41+ MK progenitors. CD34+CD41+ BM cells were cultured for 7 days with various combinations of growth factors, enumerated, washed, and stained with anti-CD41 and PI. The proportion of the cells with DNA content greater than 8N was determined. The results shown are the mean ± SD of three to five independent experiments. NA, not available.
Effect of cytokines on the production of polyploid MK by CD34+CD41+ MK progenitors. CD34+CD41+ BM cells were cultured for 7 days with various combinations of growth factors, enumerated, washed, and stained with anti-CD41 and PI. The proportion of the cells with DNA content greater than 8N was determined. The results shown are the mean ± SD of three to five independent experiments. NA, not available.
Histograms illustrating the DNA content of MK produced from diploid CD34+CD41+ cells after culture with various cytokines. (A) Cells cultured with TPO. (B) Cells cultured with IL-3. (C) Cells cultured with TPO and IL-3. (D) Cells cultured with SCF and IL-6. All cultures were performed in serum-free media and harvested after 7 days. The histograms shown are all from the same experiment to facilitate comparison but are representative of the results obtained in two to five experiments. The data were obtained by electronically gating on PI-stained, CD41+ cells after eliminating dead cells and debris by gating on forward and right angle scatter.
Histograms illustrating the DNA content of MK produced from diploid CD34+CD41+ cells after culture with various cytokines. (A) Cells cultured with TPO. (B) Cells cultured with IL-3. (C) Cells cultured with TPO and IL-3. (D) Cells cultured with SCF and IL-6. All cultures were performed in serum-free media and harvested after 7 days. The histograms shown are all from the same experiment to facilitate comparison but are representative of the results obtained in two to five experiments. The data were obtained by electronically gating on PI-stained, CD41+ cells after eliminating dead cells and debris by gating on forward and right angle scatter.
Although SCF alone had no effect on MK growth as measured by number of cells recovered or colony formation (Fig 2), it was able to support the development of polyploid MK and was able to replace TPO when added with IL-6 (Fig 4, line 11 v line 5). The distinctive pattern produced by this combination is shown in Fig 5D. Approximately 20% of the cells are 16N and higher, and a small (1.5%) 32N population was present. Culturing the cells in SCF alone produced a pattern qualitatively similar to that seen with SCF and IL-6, but with a smaller fraction of highly polyploid cells. In the absence of any cytokines, the recovery of viable cells after 7 days was so low as to preclude accurate analysis of the DNA content.
Inhibition of MK polyploidization by IL-3.In the presence of TPO ± SCF, IL-3 reduced the proportion of polyploid cells (Fig 4). In every pairwise comparison (lines 1 v 2, 3 v 4, 5 v 6, 7 v 8, 9 v 10, and 11 v 12), the combination containing IL-3 had fewer polyploid cells than the equivalent combination lacking this cytokine. In all but one pair (lines 9 v 10), the differences were significant, with P values between .01 and .001. The inhibition of TPO-induced polyploidization was even more striking when individual samples were examined for their entire range of DNA ploidy (Fig 5). As shown in Fig 5B, when cells were cultured in the presence of IL-3, the frequency distribution was markedly shifted toward lower ploidy values. Only 4% of the cells are 16N and higher. The 8N population is smaller than the 4N population and no 32N cells are present. This pattern was not altered by the addition of IL-6 (data not shown). Figure 5C shows the results obtained after culturing the cells in the presence of both IL-3 and TPO. Only 7% of the cells were ≥16N. The loss of highly polyploid cells was accompanied by an increase in proportion of diploid cells (42% with both IL-3 and TPO compared with ∼30% in the presence of TPO alone). Although the proportion of higher ploidy cells was reduced whenever IL-3 was present, 32N cells were still detectable in all combinations that contained TPO. All combinations that contained these two cytokines gave a similar pattern (Table 1). Inhibition of polyploidization by IL-3 was seen over a wide range of TPO concentrations ranging from 0.75 U/mL (a value giving suboptimal MK development but approximating the level found in normal human plasma15 ) to 300 U/mL (a concentration producing maximum MK development when used alone). Increasing the concentration of IL-3 to 10 or 50 ng/mL did not further inhibit the polyploidization, and cells cultured in these high concentrations of IL-3 yielded DNA profiles that were indistinguishable from that shown in Fig 5.
Effect of IL-3 on TPO-Induced Polyploidy in MK Cultures
| Concentration IL-3 (ng/mL) . | Percent MK With Ploidy ≥16N . | ||||||
|---|---|---|---|---|---|---|---|
| . | Concentration TPO (U/mL) . | . | . | . | |||
| . | 0 . | 0.75 . | 15.0 . | 300 . | . | . | . |
| 0 | 0 | 5.0 | 17.3 | 18.0 | |||
| 0.3 | 2.5 | 2.3 | 5.5 | 7.5 | |||
| 1.0 | 3.3 | 3.5 | 3.9 | 5.0 | |||
| Concentration IL-3 (ng/mL) . | Percent MK With Ploidy ≥16N . | ||||||
|---|---|---|---|---|---|---|---|
| . | Concentration TPO (U/mL) . | . | . | . | |||
| . | 0 . | 0.75 . | 15.0 . | 300 . | . | . | . |
| 0 | 0 | 5.0 | 17.3 | 18.0 | |||
| 0.3 | 2.5 | 2.3 | 5.5 | 7.5 | |||
| 1.0 | 3.3 | 3.5 | 3.9 | 5.0 | |||
All cultures were performed with isolated CD34+CD41+ cells analyzed after 7 days in culture as described in the text. In replicate analyses of cultured BM cells, the error in the determination of the proportion of cells whose DNA content equals or exceeds 16N is ±10%.
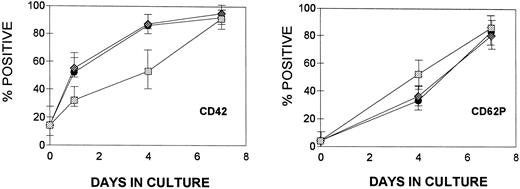
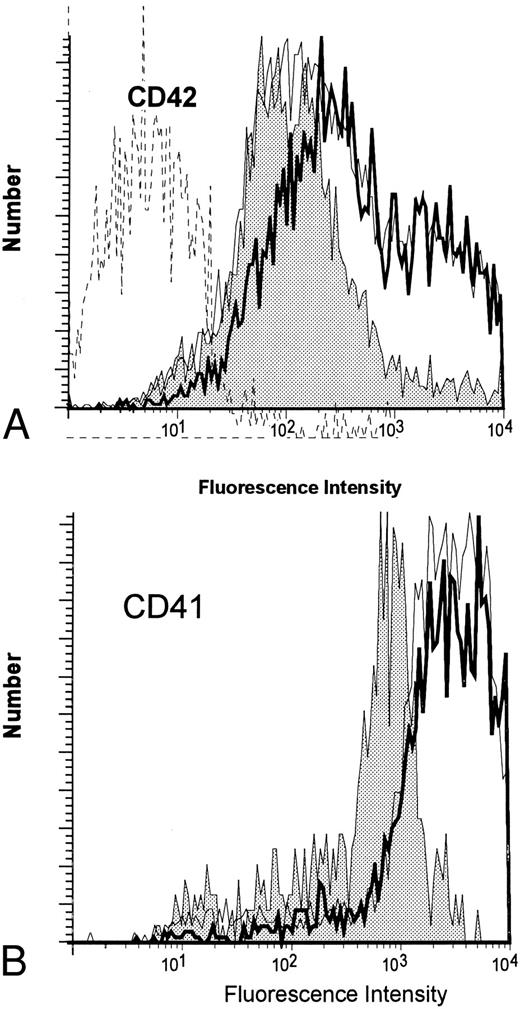
Effect of cytokines on MK maturation.The appearance of CD42 and CD62P (P-selectin) surface antigens was employed to judge MK maturation in suspension cultures (Fig 6). Approximately ∼18% of the isolated CD34+CD41+ progenitor cells express the CD42 antigen. After brief culture (24 hours) in the presence of TPO, this increased to 50% and reached ∼90% by day 4. Cells cultured in IL-3 (without TPO) acquired CD42 slowly compared with those cultured with TPO and never expressed as much antigen as cells exposed to TPO. The brightness of staining by anti-CD42 varied widely in the cultures; by day 7, cells cultured with TPO showed a distinctly bimodal pattern of expression with both dim and bright positive cells (Fig 7A, top) that was not seen in cells cultured in the presence of only IL-3. When IL-3 was added with TPO, both the kinetics of appearance of the marker (Fig 6) and the amount detected on the cell surface (Fig 7A) were similar to that found in the presence of TPO alone.
Kinetics of expression of CD42 and CD62P (P-selectin) by CD34+CD41+ BM cells. At the times indicated, the cultures were harvested and stained simultaneously with anti-CD41, anti-CD42, and anti-CD62P. The stained cells were analyzed in a flow cytometer gating on CD41+ cells. The appearance of CD42 is shown on the left and the appearance of CD62P is shown on the right. The results are reported as the mean ± SD (n = 3 to 5) of the percentage of CD41+ cells expressing either CD42 or CD62P. (▪) TPO; (♦) TPO + IL-3; (▧) IL-3.
Kinetics of expression of CD42 and CD62P (P-selectin) by CD34+CD41+ BM cells. At the times indicated, the cultures were harvested and stained simultaneously with anti-CD41, anti-CD42, and anti-CD62P. The stained cells were analyzed in a flow cytometer gating on CD41+ cells. The appearance of CD42 is shown on the left and the appearance of CD62P is shown on the right. The results are reported as the mean ± SD (n = 3 to 5) of the percentage of CD41+ cells expressing either CD42 or CD62P. (▪) TPO; (♦) TPO + IL-3; (▧) IL-3.
Expression of CD42 and CD41 by MK developing in culture in the presence of TPO and/or IL-3. (A) Histogram of the expression of CD42 after 7 days in culture. (B) Histogram of the expression of CD41 after 7 days in culture. The staining was performed as described in Materials and Methods. (▪) TPO; () IL-3; ( — ) TPO + IL-3; (- - -) isotype control.
Expression of CD42 and CD41 by MK developing in culture in the presence of TPO and/or IL-3. (A) Histogram of the expression of CD42 after 7 days in culture. (B) Histogram of the expression of CD41 after 7 days in culture. The staining was performed as described in Materials and Methods. (▪) TPO; () IL-3; ( — ) TPO + IL-3; (- - -) isotype control.
The presence of P-selectin (CD62P) was also used as a marker of maturity.16 Cells cultured in the presence of TPO express this marker late in development. By day 4, only 30% of the CD41+ MK express this marker on their surface. Cells cultured with IL-3 alone appear to express the antigen somewhat more rapidly than do TPO-treated cells. The intensity of staining was similar to that found on cells cultured with TPO. No inhibitory effect of IL-3 was observed when this cytokine was added with TPO (Fig 6). The relatively rapid appearance of CD62P on cells cultured with IL-3 combined with the reduced quantity of CD42 expressed by these cells results in the predominance of CD41+, CD62P+, CD42dim MK. These cells also differ from MK developing in the presence of TPO in that they consistently express less CD41. This is shown in Fig 7B.
DISCUSSION
We have developed a serum-free culture system that supports the differentiation of highly purified MK progenitors (CD34+CD41+). These precursors (purity >98%) were used to examine the role of TPO and several cytokines (SCF, IL-3, and IL-6) known to synergize with TPO on the proliferation and maturation of MK. The use of highly enriched MK progenitors eliminates the potentially confounding interactions of the cytokines being studied with growth factors produced by different BM cells. The release of these growth factors can be induced by T cells, granulocytes, and monocytes and by the adherent stromal layer of long-term bone-marrow cultures.11,17 The addition of exogenous cytokines to cultures increases the number of myeloid cells secreting growth factors important in megakaryocyte development (IL-la, IL-3, IL-6, and granulocyte-macrophage colony-stimulating factor [GM-CSF]).12 Indeed, cytokines have also been shown to stimulate human megakaryocytes to secrete IL-3 and GM-CSF.18 To circumvent this latter problem, we have cultured the enriched MK at low concentrations (30,000 cells/mL in liquid culture and 1,000 cells/mL in plasma clot). This dilution should serve to minimize the effects of endogenously produced cytokines.
Although this culture system has many advantages, it does not replicate the conditions of normal megakaryocytopoiesis. Only four of the many factors known to play a role in platelet production were studied and several factors known to influence MK progenitors, such as IL-11 and basic fibroblast growth factor, were not included in the medium.19,20 These studies were all performed in the absence of a stromal layer that could greatly influence MK development. They were also performed in the presence of relatively high levels of the cytokines that were present continuously during the culture period. Nevertheless, the reductionist approach used here appears to have provided significant insight into the developmental pathway of normal megakaryocytopoiesis. The high degree of enrichment of the MK progenitor population used reduces the difficulty in interpreting results obtained with heterogeneous populations containing MK at various levels of maturation, including both CD41−CD34+ and CD41+CD34+ progenitors.18 21 An ancillary benefit of the use of this purified population of committed progenitors is the elimination of the requirement of immunohistochemical identification of MK in the semisolid culture assay system. All of the colonies that grew in these cultures expressed serologic markers associated with the MK lineage and therefore did not have to be distinguished from the myeloid colonies that develop in cultures of unseparated CD34+ cells.
Although virtually none of the cells is polyploid at the time of isolation, some (18% ± 7%) of the freshly isolated cells already express CD42. After 3 to 5 days of culture, 4N and 8N cells appear even in the absence of either TPO or SCF. It is reasonable to conclude that some of the CD41+CD34+ cells in the original isolate are somewhat more mature and that these CD42+ cells are already committed to the process of endomitotic reduplication of their DNA and would do so in the absence of any additional cytokine stimulation.
The effect of different cytokines on proliferation and maturation of megakaryocytes has been extensively investigated on unfractionated BM, low-density mononuclear cells, and CD34+-enriched fractions of BM cells.20-23 Recently, Debili et al24 have examined some of the effects of cytokines on separated CD34+ MK-lineage cells. Megakaryocytopoiesis, both in vivo and in vitro, is influenced by a variety of growth factors.8,25,26 Our results, like those of Debili et al,24 indicate that TPO alone, under conditions that minimize autocrine and paracrine effects, can support both the proliferation and maturation of MK. In addition, we have shown that CD34+,CD41+, CD42−, P-selectin− MK progenitor cells can be expanded in culture, acquire CD42 and P-selectin, and become highly polyploid when cultured in medium containing TPO. Both mitotic and endomitotic DNA replication was stimulated and plasma clot cultures produced platelet-like particles (data not shown).
Although TPO appears to be the principal factor controlling MK development, several other factors play critical roles. SCF synergized with TPO to increase both the number of MK and the number of CFU-MK produced in these cultures. Although both the colony number and the MK number were increased, the effect on MK number was greater than on the colony count, indicating that SCF was acting to increase the number of mitotic divisions that took place before terminal differentiation and polyploidization occurred in these colonies. A similar conclusion has been reached in a murine model using unfractionated BM.1
SCF by itself was able to induce the development of polyploid MK. This effect is striking, because this agent alone cannot support MK proliferation or CFU-MK formation. The effect on ploidy could only be seen in the absence of TPO, but SCF in the presence of IL-6 produced as high a proportion of polyploid cells as did TPO alone. SCF also appeared to mimic other aspects of TPO activity in that combinations of SCF and IL-3 supported the development of as many MK as did combinations of TPO and IL-3 (without SCF ).
The effects of IL-3 on the development of these committed MK progenitors were complex. IL-3 alone supported the development of substantial numbers of MK colonies and enhanced activity of SCF in terms of both the total number of MK and the number of CFU-MK recovered from the cultures. It also stimulated a small increase in the number of CFU-MK produced by TPO. MK precursors cultured with IL-3 alone do not mature normally. Although most of the cells express CD42, both the rate of acquisition and the amount of this GP expressed at the cell surface is lower than in TPO-treated cells. CD41 expression is also quantitatively reduced in these cells. Because P-selectin expression proceeds essentially normally, the predominant population of MK that develop in these cultures express normal quantities of P-selectin but low levels of CD42 and CD41. This phenotype is uncommon among TPO-treated cells and normal marrow. These results indicate that the program of MK development does not require tight coordination of CD42 and CD62P expression and suggest that these processes may be regulated independently.
The most striking effect of IL-3 was on MK DNA content. In each combination tested and at all concentrations, the presence of IL-3 in the culture media led to a reduction in the number of polyploid cells with a dramatic loss of the higher ploidy classes. This inhibition occurred in the absence of obvious alterations in TPO-induced maturation, because P-selectin expression was unimpeded and CD42 expression only modestly reduced. The failure of IL-3 to support extensive endomitotic replication is also apparent in recent reports by Debili et al24 and Broudy et al.1 The cells that developed in the presence of TPO and IL-3 resemble fetal MK27,28 in that they are small and predominantly diploid, but are otherwise mature. Thus, IL-3 functions to conserve the immature MK compartment.
Like many of the hematopoietic cytokines, both TPO and IL-3 signal through the STAT3 and STAT5 pathways.29-31 Both quantitative and qualitative differences in STAT use have been observed.31 Multiple isoforms of STAT5 exist, and it appears that immature hematopoietic cells use different sets of STAT5 proteins than the more mature elements.32 The similarities of CFU-MK response to TPO and IL-3 and the difference in capacity to induce polyploidy may be explained by either quantitative differences in the use of the different pathways or by the association of different STAT5 isoforms in the developing MK. In this context, it is interesting to note that SCF, which mimics some but not all of the effects of TPO, does not act through the STAT5 pathway.29 Understanding of the differential effects of these cytokines will require a detailed analysis of how the signaling pathways in the cells change as the cells mature.
ACKNOWLEDGMENT
The gracious cooperation of Drs J. Fetto, J. Lamont, P. Ort, and N. Testa (Department of Orthopedics, New York University Medical Center, New York, NY) made this work possible. The Kaplan Cancer Center operates the core flow cytometry facility at the New York University Medical Center.
Supported by National Institutes of Health Grants No. DK43376, DK49895, and HL13336; by National Institute of Drug Abuse Grant No. DA04315; and by grants from the Boulanger-Glazier Immunology Research Fund and the Dorothy and Seymour Weinstein Fund. A.D. was supported by a training grant (5 T32 CA09161) from the National Cancer Institute.
Address reprint requests to Simon Karpatkin, MD, Department of Medicine, New York University Medical Center, 550 First Ave, New York, NY 10016.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal