Abstract
We have previously reported that 3′-azido 3′-deoxythymidine (AZT) can possess a significant antineoplastic activity when combined with drugs that disrupt de novo thymidylate synthesis, such as 5-fluorouracil and methotrexate (MTX). The aim of the present study was to evaluate the efficacy and the tolerance of the combination AZT + MTX in human immunodeficiency virus (HIV)-related non-Hodgkin's lymphoma (NHL). Twenty-nine patients (22 men and 7 women), either newly diagnosed or pretreated, have been enrolled in the trial; the median age was 34 years, 45% had acquired immunodeficiency syndrome before lymphoma and 19 patients had less than 100 CD4 lymphocytes/μL. Histologic diagnoses were mainly Burkitt (27%) and diffuse large B-cell lymphoma (45%); extranodal involvement was present in 20 patients. The treatment plan included three weekly courses of MTX at 1 g/m2 (days 1, 8, and 15) plus oral AZT at 2 g/m2 (days 1, 2, and 3), 4 g/m2 (days 8, 9, and 10), and 6 g/m2 (days 15, 16, and 17), plus leucovorin rescue. From the eleventh patient on, in case of complete or partial remission, the treatment was continued with three additional courses, using AZT at the maximum dose. In 26 evaluable patients, the total (complete + partial) response rate was 77% (95% confidence interval, 58% to 89%), with complete remission (CR) in 46% of the patients (95% confidence interval, 29% to 65%). The median CR duration was 12.8 months. Grade III-IV neutropenia and anemia were observed in 52% and 31% of the courses, respectively. There was one therapy-related death due to bacteremia followed by septic shock; the only other recorded infection was a herpes vaginalis. In conclusion, we suggest that AZT + MTX is an effective and well-tolerated regimen in HIV-related NHL.
3′-AZIDO 3′-DEOXYTHYMIDINE (AZT) is a thymidine analogue that has proven useful in the treatment of acquired immunodeficiency syndrome (AIDS)-related complex (ARC) and AIDS.1 This compound, after intracellular phosphorylation by mammalian thymidine kinase, acts as a false substitute for human immunodeficiency virus (HIV) reverse transcriptase and is incorporated into pro-viral DNA, blocking chain elongation.2 AZT was originally developed as an antineoplastic agent,3 but further investigations showed that its activity was rather unimpressive, because the drug represents a poor substrate for human DNA polymerase2 so that, under normal conditions, only small amounts can be incorporated into the DNA of tumor cells. However, it has been recently shown that the inhibition of de novo thymidylate biosynthesis can markedly increase AZT phosphorylation and metabolism and finally leads to an enhanced incorporation of the drug into DNA. Earlier studies have shown, both in vitro and in murine models, that the cytotoxic activity of AZT can be synergistically increased by 5-fluorouracil (5FU).4 Subsequent phase I-II clinical trials in patients with solid tumors5,6 showed biologic activity and neglegible toxicity of the drug combination. Our group and others have shown the efficacy of the combination of AZT with methotrexate (MTX) both in vitro in solid tumors and in leukemia cell lines7 and in vivo in murine models.8
The purpose of the present study was to further investigate the activity of AZT + MTX in HIV-related high-grade non-Hodgkin's lymphomas (NHL). Compared with those occurring in noninfected individuals, AIDS lymphomas have, in general, a poorer prognosis.9 This could be attributed to several reasons: first, the features of HIV-related NHL are those of a highly aggressive malignancy; second, and most importantly, AIDS patients have a poor tolerance to conventional chemotherapy,10 because pre-existing impairment of whole bone marrow function and immunodeficiency11 can cause life-threatening infections. Furthermore, concomitant administration of antiretroviral therapy, especially with AZT, elicits unacceptable cumulative toxicity,12 so that the drug often has to be withdrawn during chemotherapy. The novelty of our study consists in exploiting the antineoplastic effects of AZT by combining it with MTX. The choice of this drug, rather than 5FU, was based on previously reported studies showing the activity of MTX as a single agent (20% complete remission rate) in poor-risk NHL patients.13 14 At the same time, we also aimed at evaluating the effects, if any, of the drug combination on HIV infection.
PATIENTS AND METHODS
Elegibility criteria.The study included HIV-seropositive patients (15 to 65 years of age) with high-grade NHL either newly diagnosed or pretreated (relapsed/refractory). Further required criteria were a performance status ≤3 (World Health Organization [WHO] grading system), a hemoglobin (Hb) level of ≥8 g/dL, a white blood cell (WBC) count of ≤2.0 × 109/L, a platelet count of ≤50 × 109/L, normal cardiac or renal functions, a bilirubin level of ≥34 μmol/L, alanine aminotransferase (ALT)/aspartate aminotransferase (AST) ≤2× the upper limit of normal, and no active opportunistic infections. Before treatment, patients underwent conventional staging procedures including bone marrow trephine biopsy, computer tomography scan, chest x-ray, and abdominal ultrasonography. If central nervous system (CNS) involvement was suspected or in patients with Burkitt's lymphoma, a diagnostic cerebrospinal fluid (CSF) evaluation was performed.
Treatment regimen.Patients received three weekly courses of MTX at 1 g/m2 (on days 1, 8, and 15), plus oral AZT four times daily at 2 g/m2 (days 1, 2, and 3), 4 g/m2 (days 8, 9, and 10), and 6 g/m2 (days 15, 16, and 17). Leucovorin (50 mg) was administered intravenously twice daily 12 hours after the end of MTX infusion on days 1, 2, 8, 9, 15, and 16, based on previously reported doses and schedules.13,15 From the eleventh patient on, if a complete or partial response was obtained, within 1 month from the end of the first three cycles, the treatment was continued with three additional courses, using AZT at the maximum dose. In patients with CNS involvement or Burkitt's lymphoma, intrathecal MTX at 10 mg plus dexamethasone at 4 mg were administered once weekly for 6 weeks. At least 1 month after response assessment to 3 (patients no. 1 through 10) or 6 (patients no. 11 through 29) courses of AZT + MTX, in case of complete or partial remission, radiotherapy with 40 CGy in 2 Gy/d fractions was scheduled in the site of previous bulky disease or in single-site residual disease. In case of no response or partial response after the completion of 3 or 6 courses of AZT + MTX, patients were submitted to etoposide + mitoxantrome + cyclophosphamide + vincristine + prednisone + bleomycin (VNCOP-B) treatment regimen.16
Antiretroviral therapy.Antiretroviral therapy with AZT at 500 mg/d was continued throughout all the treatment period. In patients receiving didanosine (DDI) before chemotherapy, the drug was withheld and substituted with AZT until completion of the entire treatment, after which it was started again. Patients who did not receive any antiretroviral therapy before the beginning of the study subsequently received AZT if the CD4 lymphocyte count was less than 400/μL.
Supportive treatment.Every kind of supportive treatment was allowed, including antibiotic and antifungal (oral amphotericin B or nistatin) prophylaxis and Pneumocystis carinii pneumonia prophylaxis (trimetoprim-sulfamethoxazole or inhaled pentamidine). Granulocyte colony-stimulating factors (G-CSF; 5 g/kg) was administered according to the physician's discretion. Packed red blood cells (RBC) or platelets transfusions were to be administered when the Hb level was less than 7.5 g/dL or the platelet count was less than 20 × 109/L.
Clinical and laboratory evaluation.Physical examination, WBC count and differential, lymphocyte subpopulations, Hb level, platelet count, liver and renal function, lactate dehydrogenase (LDH) level, and serum electrolytes were evaluated before treatment and weekly thereafter. HIV-1 core antigen was monitored every other week by an anti–p-24 enzyme immunoassay (Du Pont, Wilmington, DE). A complete restaging procedure was performed after 3 and 6 courses. All pathologic and cytologic material was reviewed by a single hemopatologist and was classified according to the Revised European American Lymphoma (REAL) classification.17
Evaluation of the outcome.A complete response (CR) was defined as the complete disappearance of all detectable disease for at least 4 weeks after the end of treatment. A partial response (PR) was defined as greater than 50% reduction in the area of measurable disease, calculated by multiplying two perpendicular dimensions lasting more than 4 weeks after completion of therapy. No response or disease progression was defined as no change or increase in the size of the initial lesions or appearance of new lesions. Toxicity was evaluated by using the WHO grading system.
Statistical analysis.Kaplan-Meier analysis18 was used to estimate disease-free survival and overall survival, which was calculated from the time at which patients started the treatment. The Fisher exact test was used to evaluate the association between baseline characteristics and achievement of CR. Differences between baseline and posttreatment CD4 lymphocyte counts and p24 antigen were evaluated by Wilcoxon test; P values were always two-tailed and were considered significant when less than .05.
RESULTS
Patient characteristics.From July 1992 to July 1995, 29 HIV-seropositive patients with high-grade NHL entered the study after giving their informed consent (Table 1). Their median age was 34 years. Twenty-two of them were men and 50% were previous intravenous drug users (IVDU). A history of AIDS before lymphoma was reported in 13 patients (45%), in 11 cases for opportunistic infections (Pneumocistis carinii pneumonia [PCP] or mycobacteriosis), whereas 2 patients had Kaposi sarcoma (KS). Sixteen patients were receiving antiretroviral therapy when the lymphoma was diagnosed, 11 with AZT at 500 mg/d (mean duration, 21.5 months) and 5 with DDI at 200 mg/d (mean duration, 16 months); no patient was suffering from hematologic or other toxicity due to antiretroviral therapy. The most common histologic subtypes of lymphoma were Burkitt's (27%) and diffuse large B-cell lymphoma (DLBCL; 45%). Extranodal disease was very common, as it is frequently reported in these patients.19 Bulky disease was present in 45% of the cases. Twenty-two patients (76%) were diagnosed as having a stage IV lymphoma, with bone marrow involvement in 34%.
Patient Characteristics
| . | No Prior CHT . | Prior CHT . | Total . |
|---|---|---|---|
| Total no. | 21 | 8 | 29 |
| Male/female | 16/5 | 6/2 | 22/7 |
| Median age in years (range) | 32 | 36 | 34 |
| (23-48) | (28-49) | (23-49) | |
| Risk group | |||
| Intravenous drug user | 13 | 2 | 15 |
| Homosexual | 4 | 3 | 7 |
| Heterosexual | 4 | 3 | 7 |
| Prior AIDS | |||
| Opportunistic infections | 9 | 2 | 11 |
| Kaposi sarcoma | 0 | 2 | 2 |
| Prior antiretroviral therapy | |||
| AZT (dose/duration) | 7 | 4 | 11 |
| (500 mg/22 mo) | (500 mg/20 mo) | (500 mg/21.5 mo) | |
| DDI (dose/duration) | 3 | 2 | 5 |
| (200 mg/19 mo) | (200 mg/15 mo) | (200 mg/16 mo) | |
| CD4 mean | 164 ± 182 | 49.9 ± 52 | 132.7 ± 164 |
| CD4 median (range) | 164 | 48.7 | 132.7 |
| (4.7-605) | (4.3-167) | (4.3-605) | |
| CD4 <100/μL | 12 | 7 | 19 |
| LDH | |||
| Normal | 8 | 5 | 13 |
| > Normal | 13 | 3 | 16 |
| Performance status | |||
| 0-1 | 8 | 4 | 12 |
| 2-3 | 13 | 4 | 17 |
| Stage | |||
| I | 2 | 0 | 2 |
| II | 4 | 1 | 5 |
| III | 0 | 0 | 0 |
| IV | 15 | 7 | 22 |
| Histology | |||
| Burkitt | 6 | 2 | 8 |
| DLBCL | 9 | 4 | 13 |
| Lymphoblastic | 0 | 1 | 1 |
| PTCL | 3 | 0 | 3 |
| ALC | 1 | 1 | 2 |
| Unclassified | 2 | 0 | 2 |
| Extranodal disease | 19 | 7 | 26 |
| Bulky disease (>10 cm) | 9 | 4 | 13 |
| Previous treatment | |||
| F-MACHOP | 1 | 1 | |
| αIFN | 1 | 1 | |
| CVP | 3 | 3 | |
| ABVD | 1 | 1 | |
| CHOP | 1 | 1 | |
| VCR + P | 1 | 1 |
| . | No Prior CHT . | Prior CHT . | Total . |
|---|---|---|---|
| Total no. | 21 | 8 | 29 |
| Male/female | 16/5 | 6/2 | 22/7 |
| Median age in years (range) | 32 | 36 | 34 |
| (23-48) | (28-49) | (23-49) | |
| Risk group | |||
| Intravenous drug user | 13 | 2 | 15 |
| Homosexual | 4 | 3 | 7 |
| Heterosexual | 4 | 3 | 7 |
| Prior AIDS | |||
| Opportunistic infections | 9 | 2 | 11 |
| Kaposi sarcoma | 0 | 2 | 2 |
| Prior antiretroviral therapy | |||
| AZT (dose/duration) | 7 | 4 | 11 |
| (500 mg/22 mo) | (500 mg/20 mo) | (500 mg/21.5 mo) | |
| DDI (dose/duration) | 3 | 2 | 5 |
| (200 mg/19 mo) | (200 mg/15 mo) | (200 mg/16 mo) | |
| CD4 mean | 164 ± 182 | 49.9 ± 52 | 132.7 ± 164 |
| CD4 median (range) | 164 | 48.7 | 132.7 |
| (4.7-605) | (4.3-167) | (4.3-605) | |
| CD4 <100/μL | 12 | 7 | 19 |
| LDH | |||
| Normal | 8 | 5 | 13 |
| > Normal | 13 | 3 | 16 |
| Performance status | |||
| 0-1 | 8 | 4 | 12 |
| 2-3 | 13 | 4 | 17 |
| Stage | |||
| I | 2 | 0 | 2 |
| II | 4 | 1 | 5 |
| III | 0 | 0 | 0 |
| IV | 15 | 7 | 22 |
| Histology | |||
| Burkitt | 6 | 2 | 8 |
| DLBCL | 9 | 4 | 13 |
| Lymphoblastic | 0 | 1 | 1 |
| PTCL | 3 | 0 | 3 |
| ALC | 1 | 1 | 2 |
| Unclassified | 2 | 0 | 2 |
| Extranodal disease | 19 | 7 | 26 |
| Bulky disease (>10 cm) | 9 | 4 | 13 |
| Previous treatment | |||
| F-MACHOP | 1 | 1 | |
| αIFN | 1 | 1 | |
| CVP | 3 | 3 | |
| ABVD | 1 | 1 | |
| CHOP | 1 | 1 | |
| VCR + P | 1 | 1 |
Abbreviations: PTCL, peripheral T-cell lymphoma; ALC, anaplastic large-cell lymphoma; CHT, chemotherapy.
Twenty-one patients were newly diagnosed, whereas 7 had been previously treated with various chemotherapy schedules and 1 with α-interferon for KS. Median CD4 lymphocytes counts were 132.7/μL, with 19 patients with less than 100 CD4/μL. Fourteen of 27 patients had a detectable p24 antigenemia.
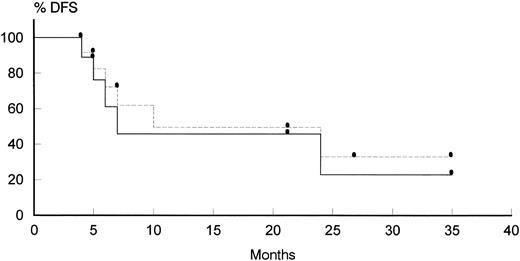
Evaluation of the response.The characteristics and the outcome of each individual who entered the study are reported on Tables 2 and 3. Of 29 patients, 26 were considered evaluable for response, whereas 3 patients were administered only 1 course of therapy, after which they were withdrawn from study for progression of KS (patient no. 3), treatment refusal (patient no. 13), and septic shock occurring just after the end of the first course of treatment (patient no. 28). Radiotherapy was delivered only in 3 of 8 elegible patients, that was because 1 patient (patient no. 14) had more than one site of localized residual disease, 3 patients (patients no. 9, 10, and 19) had an early relapse, and patient no. 29 refused to undergo further therapy. A CR was achieved in 12 patients (46%; 95% confidence interval [CI], 29% to 65%), whereas 8 patients obtained a PR (31%; 95% CI, 16.5% to 50%); the overall response rate was 77%. No difference in the response rate was observed comparing patients who had received previous chemotherapy (71%, with 43% CR; 95% CI, 15% to 75%) and those who had not received previous treatment (79%, with 47% CR; 95% CI, 27% to 68%). All the same, the remission rate in patients treated with 3 courses of therapy (77%, with 44% CR; 95% CI, 18% to 73%) was comparable to that obtained in patients who were scheduled to receive 6 courses (76%, with 47% CR; 95% CI, 26% to 69%). A significantly lower percentage of CRs was observed only in case of bulky disease (P = .04), whereas other initial parameters that have been described to influence patients outcome,20 such as CD4 counts, seemed to have no effect. The median overall CR duration was 12.8 months (12.2 months considering only untreated patients; Fig 1). Patients who have been treated with 3 courses of therapy (patients no. 1 through 10) apparently have a longer CR duration (14.5 months) than those who have received 6 courses (patients no. 11 through 29; 10.5 months); this difference could be probably attributed to the longer median follow-up of the first group of patients (12.4 v 11.7 months).
Detailed Clinical Characteristics of the Patients With No Previous Chemotherapy
| Patient No. . | Sex/Age . | RF . | Prior AIDS . | CD4/μL . | p24/μL . | LDH . | Histology . | Extranodal Site . | Bulky . | BM . | Stage . | PS . | Response . | RT/Response . | Duration (mo) . | Survival (mo) . | Status . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | (other than BM) . | . | . | . | . | . | . | . | . | . |
| 2 | M/48 | HO | OI | 139 | 0 | 470 | PTCL | — | − | + | IV | 2 | CR | — | 35+ | 36+ | A/W |
| 4 | F/38 | IVDU | — | 4.7 | 0 | 1380 | DLBCL | Epiglottis | − | + | IV | 2 | CR | — | 4.5 | 8 | D/L |
| 7 | M/42 | HO | OI | 522 | 33 | 810 | Burkitt | Pleura | − | − | IV | 3 | NR | — | — | 2 | D/L |
| 8 | M/29 | IVDU | OI | 32 | 0 | 959 | Burkitt | — | + | + | IV | 1 | CR | Yes/CR | 4 | 7 | D/L |
| 9 | F/34 | IVDU | — | 125 | NA | 1189 | DLBCL | — | + | − | II | 1 | PR | — | 2 | 12 | D/L |
| 10 | M/33 | IVDU | OI | 5.1 | 164 | 368 | DLBCL | — | + | − | II | 3 | PR | — | 2 | 4 | D/L |
| 11 | M/35 | IVDU | OI | 38.5 | 0 | 218 | DLBCL | Stomach | − | − | II-E | 2 | CR | — | 24 | 26 | D/WS |
| 12 | M/40 | IVDU | — | 16 | 0 | 490 | DLBCL | — | − | + | IV | 2 | CR | — | 7 | 9 | LFU |
| 13 | F/33 | HE | OI | 22 | 0 | 875 | U | Gluteus | − | − | I-E | 2 | NE | — | — | — | — |
| 14 | F/23 | HE | — | 70 | 90 | 140 | U | Liver | + | − | IV | 0 | PR | — | 2 | 18 | D/L |
| 16 | F/29 | HE | — | 55 | 16 | 563 | Burkitt | Uterine cervix | − | − | I-E | 1 | CR | — | 21+ | 23+ | A/W |
| 17 | M/33 | HO | OI | 313 | 0 | 391 | DLCBL | — | + | + | IV | 0 | PR | Yes/PR | 2 | 16+ | A/W |
| 18 | M/28 | IVDU | OI | 5 | 312 | 577 | PTCL | Skin | − | − | IV | 2 | CR | — | 6 | 8 | D/INF |
| 20 | M/35 | IVDU | OI | 80 | 221 | 900 | DLBCL | CNS | − | − | IV | 3 | NR | — | — | 4 | D/L |
| 21 | M/31 | IVDU | — | 300 | 0 | 586 | DLBCL | Stomach | − | − | II-E | 3 | PR | — | 1 | 10 | D/L |
| 22 | M/33 | IVDU | — | 364 | 0 | 1283 | DLBCL | Gut | + | − | IV | 0 | PR | Yes/PR | 2 | 13.5+ | A/W |
| 23 | M/35 | HE | — | 35 | ND | 307 | Burkitt | Stomach | + | − | IV | 2 | NR | — | — | 13+ | A/W |
| 25 | M/38 | HO | — | 98 | 130 | 3724 | ALC | — | − | + | IV | 1 | NR | — | — | 8+ | A/L |
| 27 | M/34 | IVDU | — | 395 | 17 | 402 | PTCL | Liver | − | − | IV | 1 | CR | — | 5+ | 7+ | A/W |
| 28 | M/30 | IVDU | — | 225 | 0 | 450 | Burkitt | Gut | + | − | IV | 3 | NE | — | — | — | — |
| 29 | M/36 | IVDU | — | 605 | 0 | 812 | Burkitt | Stomach | + | − | IV | 3 | CR | — | 4+ | 6+ | A/W |
| Patient No. . | Sex/Age . | RF . | Prior AIDS . | CD4/μL . | p24/μL . | LDH . | Histology . | Extranodal Site . | Bulky . | BM . | Stage . | PS . | Response . | RT/Response . | Duration (mo) . | Survival (mo) . | Status . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | (other than BM) . | . | . | . | . | . | . | . | . | . |
| 2 | M/48 | HO | OI | 139 | 0 | 470 | PTCL | — | − | + | IV | 2 | CR | — | 35+ | 36+ | A/W |
| 4 | F/38 | IVDU | — | 4.7 | 0 | 1380 | DLBCL | Epiglottis | − | + | IV | 2 | CR | — | 4.5 | 8 | D/L |
| 7 | M/42 | HO | OI | 522 | 33 | 810 | Burkitt | Pleura | − | − | IV | 3 | NR | — | — | 2 | D/L |
| 8 | M/29 | IVDU | OI | 32 | 0 | 959 | Burkitt | — | + | + | IV | 1 | CR | Yes/CR | 4 | 7 | D/L |
| 9 | F/34 | IVDU | — | 125 | NA | 1189 | DLBCL | — | + | − | II | 1 | PR | — | 2 | 12 | D/L |
| 10 | M/33 | IVDU | OI | 5.1 | 164 | 368 | DLBCL | — | + | − | II | 3 | PR | — | 2 | 4 | D/L |
| 11 | M/35 | IVDU | OI | 38.5 | 0 | 218 | DLBCL | Stomach | − | − | II-E | 2 | CR | — | 24 | 26 | D/WS |
| 12 | M/40 | IVDU | — | 16 | 0 | 490 | DLBCL | — | − | + | IV | 2 | CR | — | 7 | 9 | LFU |
| 13 | F/33 | HE | OI | 22 | 0 | 875 | U | Gluteus | − | − | I-E | 2 | NE | — | — | — | — |
| 14 | F/23 | HE | — | 70 | 90 | 140 | U | Liver | + | − | IV | 0 | PR | — | 2 | 18 | D/L |
| 16 | F/29 | HE | — | 55 | 16 | 563 | Burkitt | Uterine cervix | − | − | I-E | 1 | CR | — | 21+ | 23+ | A/W |
| 17 | M/33 | HO | OI | 313 | 0 | 391 | DLCBL | — | + | + | IV | 0 | PR | Yes/PR | 2 | 16+ | A/W |
| 18 | M/28 | IVDU | OI | 5 | 312 | 577 | PTCL | Skin | − | − | IV | 2 | CR | — | 6 | 8 | D/INF |
| 20 | M/35 | IVDU | OI | 80 | 221 | 900 | DLBCL | CNS | − | − | IV | 3 | NR | — | — | 4 | D/L |
| 21 | M/31 | IVDU | — | 300 | 0 | 586 | DLBCL | Stomach | − | − | II-E | 3 | PR | — | 1 | 10 | D/L |
| 22 | M/33 | IVDU | — | 364 | 0 | 1283 | DLBCL | Gut | + | − | IV | 0 | PR | Yes/PR | 2 | 13.5+ | A/W |
| 23 | M/35 | HE | — | 35 | ND | 307 | Burkitt | Stomach | + | − | IV | 2 | NR | — | — | 13+ | A/W |
| 25 | M/38 | HO | — | 98 | 130 | 3724 | ALC | — | − | + | IV | 1 | NR | — | — | 8+ | A/L |
| 27 | M/34 | IVDU | — | 395 | 17 | 402 | PTCL | Liver | − | − | IV | 1 | CR | — | 5+ | 7+ | A/W |
| 28 | M/30 | IVDU | — | 225 | 0 | 450 | Burkitt | Gut | + | − | IV | 3 | NE | — | — | — | — |
| 29 | M/36 | IVDU | — | 605 | 0 | 812 | Burkitt | Stomach | + | − | IV | 3 | CR | — | 4+ | 6+ | A/W |
Abbreviations: HO, homosexual; HE, heterosexual; OI, opportunistic infection; BM, bone marrow; PTCL, peripheral T-cell lymphoma; ALC, anaplastic large-cell lymphoma; U, unclassified lymphoma; L, lymphoblastic lymphoma; ND, not done; NR, no response; NE, not evaluable; D/L, dead with lymphoma; A/W, alive and well; D/WS, dead with wasting syndrome; LFU, lost to follow-up; D/INF, dead with infection.
Detailed Clinical Characteristics of the Patients With No Previous Chemotherapy
| Patient No. . | Sex/Age . | FR . | Prior AIDS . | CD4/μL . | p24/μL . | LDH . | Histology . | Extranodal Site (other than BM) . | Bulky . | BM . | Stage . | PS . | Response . | RT/Response . | Duration (mo) . | Survival (mo) . | Status . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/28 | HO | OI | 54 | 169.4 | 1539 | Burkitt | Liver | + | − | IV | 3 | NR | — | — | 6 | D/L |
| 3 | M/49 | HO | KS | 4.3 | 3.5 | 720 | DLBCL | Palatum | + | + | IV | 3 | NE | — | — | — | — |
| 5 | M/32 | IVDU | — | 39 | 0 | 195 | Burkitt | CNS | − | − | IV | 1 | CR | — | 27+ | 28+ | A/W |
| 6 | M/40 | IVDU | — | 14 | 128 | 263 | ALC | Skin | − | + | IV | 1 | PR | — | 6+ | 9+ | D/L |
| 15 | F/29 | HE | — | 69 | 15.5 | 1410 | DLBCL | Stomach | − | + | IV | 2 | CR | — | 10+ | 13+ | D/L |
| 19 | M/39 | HO | KS | 42 | 98 | 258 | L | — | + | − | II | 1 | PR | — | 1+ | 9+ | D/L |
| 24 | F/34 | HE | OI | 10 | 24 | 351 | DLCBL | Stomach | + | − | IV | 2 | NR | — | — | 8+ | D/L |
| 26 | M/37 | HE | — | 167 | 0 | 372 | DLCBL | — | − | + | IV | 0 | CR | — | 7+ | 9+ | A/W |
| Patient No. . | Sex/Age . | FR . | Prior AIDS . | CD4/μL . | p24/μL . | LDH . | Histology . | Extranodal Site (other than BM) . | Bulky . | BM . | Stage . | PS . | Response . | RT/Response . | Duration (mo) . | Survival (mo) . | Status . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/28 | HO | OI | 54 | 169.4 | 1539 | Burkitt | Liver | + | − | IV | 3 | NR | — | — | 6 | D/L |
| 3 | M/49 | HO | KS | 4.3 | 3.5 | 720 | DLBCL | Palatum | + | + | IV | 3 | NE | — | — | — | — |
| 5 | M/32 | IVDU | — | 39 | 0 | 195 | Burkitt | CNS | − | − | IV | 1 | CR | — | 27+ | 28+ | A/W |
| 6 | M/40 | IVDU | — | 14 | 128 | 263 | ALC | Skin | − | + | IV | 1 | PR | — | 6+ | 9+ | D/L |
| 15 | F/29 | HE | — | 69 | 15.5 | 1410 | DLBCL | Stomach | − | + | IV | 2 | CR | — | 10+ | 13+ | D/L |
| 19 | M/39 | HO | KS | 42 | 98 | 258 | L | — | + | − | II | 1 | PR | — | 1+ | 9+ | D/L |
| 24 | F/34 | HE | OI | 10 | 24 | 351 | DLCBL | Stomach | + | − | IV | 2 | NR | — | — | 8+ | D/L |
| 26 | M/37 | HE | — | 167 | 0 | 372 | DLCBL | — | − | + | IV | 0 | CR | — | 7+ | 9+ | A/W |
Previous chemotherapy included F-MACHOP (patient no. 1), α-interferon (patient no. 3), CVP (patients no. 5, 6, and 15), Vincristine + prednisone (patient no. 19), ABVD (patient no. 24), and CHOP (patient no. 26).
Abbreviations: HO, homosexual; HE, heterosexual; OI, opportunistic infection; BM, bone marrow; PTCL, peripheral T-cell lymphoma; ALC, anaplastic large-cell lymphoma; U, unclassified lymphoma; L, lymphoblastic lymphoma; ND, not done; NR, no response; NE, not evaluable;D/L, dead with lymphoma; A/W, alive and well; D/WS, dead with wasting syndrome; LFU, lost to follow-up; D/INF, dead with infection.
Disease-free survival in patients achieving CR. (Solid line) previously untreated patients; (dotted line) pretreated + untreated patients.
Disease-free survival in patients achieving CR. (Solid line) previously untreated patients; (dotted line) pretreated + untreated patients.
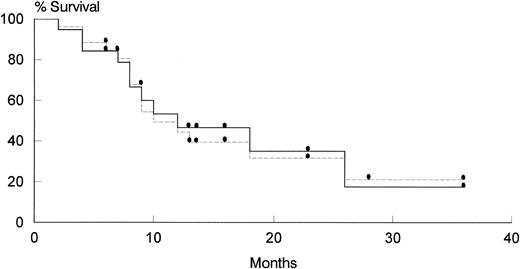
Survival and causes of death.As shown on Fig 2, the median survival of patients treated with AZT + MTX was 12 months. At present, 9 patients are alive and well, with a median follow-up of 16.8 months (range, 6 to 36 months); 6 of them are in continuous CR after AZT + MTX, whereas 2 had a PR and 1 was resistant; these latter patients have been subsequently treated with salvage VNCOP-B protocol. One patient is alive with lymphoma, 13 died of lymphoma, 1 was lost to follow-up, and 2 patients died of causes other than lymphoma (1 of disseminated micobacteriosis and 1 of wasting syndrome after having been in CR for 6 and 21 months, respectively).
Overall survival of patients evaluable for response. (Solid line) previously untreated patients; (dotted line) pretreated + untreated patients.
Overall survival of patients evaluable for response. (Solid line) previously untreated patients; (dotted line) pretreated + untreated patients.
Toxicity.A summary of adverse events occurring during the therapy with MTX + AZT is reported on Table 4. All of the patients who entered the protocol were considered evaluable for toxicity. A total number of 119 courses was administered. Hematologic toxicity included leukopenia with grade III-IV neutropenia (polymorphonuclear lymphocytes <0.5 × 109/L), which was observed in 52% of the patients after 23 courses (19%), and grade III-IV anemia, which occurred after 11 courses in 31% of the patients. Neutropenia caused a delay in the administration of the following course in 8 cases (median delay, 18.6 days). To improve the hematologic tolerance of this protocol, G-CSF was administered after 82 courses; in none of these cases was the treatment postponed. Neutropenia was more common in pretreated patients (12 of 31 v 11 of 88 courses; P = .001) and in patients no. 1 through 10 (12 of 28 v 13/91 courses; P = .001); however, this could be attributed to a more frequent administration of G-CSF both in untreated patients and in patients no. 11 through 29. In fact, the incidence of anemia is not significantly different in the various groups of patients. A fatal bacteremia with septic shock occurred in 1 patient (patient no. 28) just after the end of the first course of treatment. Another patient (patient no. 16) had a herpes vaginalis that promptly resolved with local therapy. No other intercurrent infections were recorded. Because ascites/effusions were not considered exclusion criteria, we treated 1 patient (patient no. 7) showing a pleural effusion; no toxicity was observed and the patient died of lymphoma 2 months after the start of therapy. However, although no toxic effect was recorded, caution is recommended in these patients, because third space retention of administered MTX could potentially cause a prolongation of plasmatic half-life of the drug.15 One patient (patient no. 22) presented with a peripheral neuropathy characterized by symmetrical distal weakness, sensory loss, and reduction of the knee jerk reflex, whereas the results of an electrodiagnostic examination were normal. Even though peripheral neuropathy has not been described to be associated with AZT, the treatment was interrupted and the symptoms resolved within 2 months. Mild nausea was recorded in 2 patients, 1 of whom suffered also from headache. No other toxic effect, such as oral mucositis, was observed in patients treated with AZT + MTX.
Evaluation of Toxicity
| Patient No. . | Courses . | Neutropenia (grade III-IV) . | G-CSF (courses) . | Anemia (grade III-IV) . | RBC (U) . | Other (grade) . |
|---|---|---|---|---|---|---|
| 1 | 3 | 1/3 | 0/3 | 1/3 | 2 | — |
| 2 | 3 | 1/3 | 0/3 | 1/3 | 2 | — |
| 3 | 1 | 1/1 | 0/1 | 1/1 | 1 | — |
| 4 | 3 | 1/3 | 2/3 | 2/3 | 4 | — |
| 5 | 3 | 3/3 | 0/3 | 0/3 | 0 | — |
| 6 | 3 | 3/3 | 0/3 | 1/3 | 2 | — |
| 7 | 3 | 1/3 | 0/3 | 0/3 | 0 | — |
| 8 | 3 | 0/3 | 2/3 | 0/3 | 0 | — |
| 9 | 3 | 1/3 | 2/3 | 0/3 | 0 | — |
| 10 | 3 | 0/3 | 2/3 | 0/3 | 0 | — |
| 11 | 3 + 3 | 0/6 | 5/6 | 0/6 | 0 | — |
| 12 | 3 + 3 | 0/6 | 5/6 | 1/6 | 1 | — |
| 13 | 1 | 1/1 | 1/1 | 0/1 | 0 | — |
| 14 | 3 + 3 | 0/6 | 5/6 | 0/6 | 0 | Nausea (II), headache (I) |
| 15 | 3 + 3 | 0/6 | 5/6 | 0/6 | 0 | — |
| 16 | 3 + 3 | 0/6 | 5/6 | 0/6 | 0 | Herpes vaginalis (II) |
| 17 | 3 + 3 | 0/6 | 5/6 | 0/6 | 0 | — |
| 18 | 3 + 3 | 2/6 | 6/6 | 1/6 | 3 | — |
| 19 | 3 + 3 | 0/6 | 5/6 | 0/6 | 0 | — |
| 20 | 3 | 1/3 | 2/3 | 1/3 | 2 | — |
| 21 | 3 + 3 | 0/6 | 5/6 | 0/6 | 0 | — |
| 22 | 3 + 2 | 0/5 | 4/5 | 0/5 | 0 | Peripheral neuropathy (III) |
| 23 | 3 | 2/3 | 2/3 | 0/3 | 0 | — |
| 24 | 3 | 2/3 | 2/3 | 2/3 | 4 | — |
| 25 | 3 | 0/3 | 2/3 | 0/3 | 0 | — |
| 26 | 3 + 3 | 2/6 | 5/6 | 0/6 | 0 | — |
| 27 | 3 + 3 | 1/6 | 5/6 | 0/6 | 0 | Nausea (II) |
| 28 | 1 | 0/1 | 0/1 | 0/1 | 0 | Septic shock (IV) |
| 29 | 3 + 3 | 0/6 | 5/6 | 0/6 | 0 | — |
| Total courses | 119 | 23 (19%) | 82 (69%) | 11 (9%) | 21 units | |
| Total patients | 29 | 15 (52%) | 22 (76%) | 9 (31%) | 9 (31%) |
| Patient No. . | Courses . | Neutropenia (grade III-IV) . | G-CSF (courses) . | Anemia (grade III-IV) . | RBC (U) . | Other (grade) . |
|---|---|---|---|---|---|---|
| 1 | 3 | 1/3 | 0/3 | 1/3 | 2 | — |
| 2 | 3 | 1/3 | 0/3 | 1/3 | 2 | — |
| 3 | 1 | 1/1 | 0/1 | 1/1 | 1 | — |
| 4 | 3 | 1/3 | 2/3 | 2/3 | 4 | — |
| 5 | 3 | 3/3 | 0/3 | 0/3 | 0 | — |
| 6 | 3 | 3/3 | 0/3 | 1/3 | 2 | — |
| 7 | 3 | 1/3 | 0/3 | 0/3 | 0 | — |
| 8 | 3 | 0/3 | 2/3 | 0/3 | 0 | — |
| 9 | 3 | 1/3 | 2/3 | 0/3 | 0 | — |
| 10 | 3 | 0/3 | 2/3 | 0/3 | 0 | — |
| 11 | 3 + 3 | 0/6 | 5/6 | 0/6 | 0 | — |
| 12 | 3 + 3 | 0/6 | 5/6 | 1/6 | 1 | — |
| 13 | 1 | 1/1 | 1/1 | 0/1 | 0 | — |
| 14 | 3 + 3 | 0/6 | 5/6 | 0/6 | 0 | Nausea (II), headache (I) |
| 15 | 3 + 3 | 0/6 | 5/6 | 0/6 | 0 | — |
| 16 | 3 + 3 | 0/6 | 5/6 | 0/6 | 0 | Herpes vaginalis (II) |
| 17 | 3 + 3 | 0/6 | 5/6 | 0/6 | 0 | — |
| 18 | 3 + 3 | 2/6 | 6/6 | 1/6 | 3 | — |
| 19 | 3 + 3 | 0/6 | 5/6 | 0/6 | 0 | — |
| 20 | 3 | 1/3 | 2/3 | 1/3 | 2 | — |
| 21 | 3 + 3 | 0/6 | 5/6 | 0/6 | 0 | — |
| 22 | 3 + 2 | 0/5 | 4/5 | 0/5 | 0 | Peripheral neuropathy (III) |
| 23 | 3 | 2/3 | 2/3 | 0/3 | 0 | — |
| 24 | 3 | 2/3 | 2/3 | 2/3 | 4 | — |
| 25 | 3 | 0/3 | 2/3 | 0/3 | 0 | — |
| 26 | 3 + 3 | 2/6 | 5/6 | 0/6 | 0 | — |
| 27 | 3 + 3 | 1/6 | 5/6 | 0/6 | 0 | Nausea (II) |
| 28 | 1 | 0/1 | 0/1 | 0/1 | 0 | Septic shock (IV) |
| 29 | 3 + 3 | 0/6 | 5/6 | 0/6 | 0 | — |
| Total courses | 119 | 23 (19%) | 82 (69%) | 11 (9%) | 21 units | |
| Total patients | 29 | 15 (52%) | 22 (76%) | 9 (31%) | 9 (31%) |
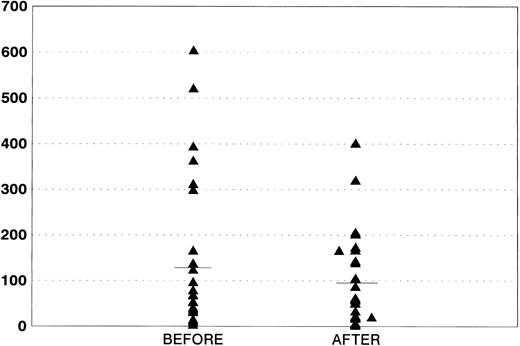
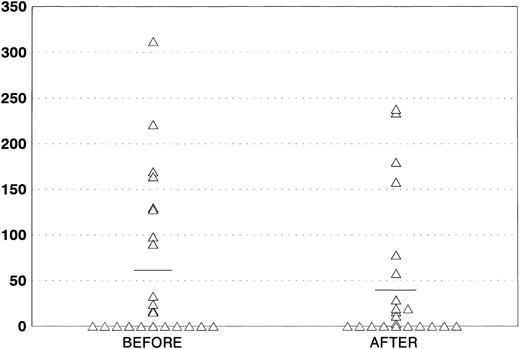
Effects on HIV infection.WBC counts and lymphocyte subpopulations were evaluated in all of the patients at diagnosis and after completion of therapy. As shown on Fig 3, a significant decrease in CD4 counts was observed after treatment (mean CD4, 132.7 v 98.4; P = .04), which is in line with previously reported studies.21 p24 antigenemia was serially evaluated in 25 patients (Fig 4). We showed a decrease, although not significant, in the amount of protein after the end of treatment (59.07 v 44.70 pg/mL). Three patients who were p24-negative before the therapy became subsequently positive, whereas 2 positive patients had their p24 levels decrease to undetectable levels after AZT + MTX treatment.
Quantitative evaluation of p24 antigenemia before and after AZT + MTX treatment.
DISCUSSION
Approximately 3% to 10% of HIV-infected individuals develop an NHL during the course of their disease.22 AIDS-related NHL display several peculiar characteristics, including presentation in advanced stage at a young age, frequent extranodal involvement with bone marrow and CNS as preferential sites, and B symptoms.19,23 The commonest pathologic findings are a B phenotype and Burkitt's or DLBCL histotype.24 Despite the progress achieved in recent years in the treatment of NHL occurring in HIV-seronegative patients, results obtained in AIDS patients are often rather disappointing. In fact, the treatment of these patients with conventional chemotherapy schedules results in unacceptable percentages of therapy-related deaths,10 whereas the efficacy shown by less dose-intensive schedules is rather controversial, as CR rates varied from 30% to 50%.25-27 Encouraging results have been obtained using an infusional polychemotherapy schedule,21 even though hematologic toxicity required a dose reduction in the majority of the patients and 23% infectious morbidity has been reported.28 Alternatively, according to the commonly accepted risk factors for AIDS lymphomas,29 including CD4 counts, performance status, and prior AIDS, patients could be considered as high risk or low risk. Consequently, more or less intensive chemotherapy schedules could be administered.30 The real advantages of this approach are still being debated. However, it is generally accepted that antiretroviral therapy with AZT has to be interrupted during chemotherapy, which could potentially represent a problem in schemes lasting several weeks. Our study has the advantage of exploiting the antineoplastic activity of AZT by combining it with MTX, so that antiretroviral therapy can be continued throughout the whole treatment period.
In the present study, we have obtained a 77% response rate with 46% CR. These results are in line with those reported by several groups20,26,31 and are better than those achieved by others,25,27 even though it is difficult to make a correct comparison because of the differences in the trials, especially in terms of patient selection and dose intensity. However, one must also take into account that all of our patients showed at least one high risk feature. In addition, it should be pointed out that our therapeutic schedule was extremely well tolerated. The patients did not require any dose reduction. We had 1 therapy-related death and 1 herpes vaginalis in 29 patients, but no other infectious episodes were observed. The therapy was administered totally in an outpatient setting. Marrow toxicity was very mild, which is in agreement with what has been reported using AZT + 5FU5,6 and with what has been described in animal models.8 G-CSF was used after 69% of the courses to improve or to prevent therapy-related neutropenia. As previously reported by other groups,32 the administration of this cytokine was safe, because we did not observe any significant change in the overall amount of p24 protein that could have represented a sign of increased viral replication. In conclusion, AZT + MTX is an effective and well-tolerated regimen in HIV-related NHL. The mild toxicity of this protocol prompts us to further improve it by adding chemotherapeutic agents with a possible synergism with this drug combination.
ACKNOWLEDGMENT
The authors gratefully acknowledge Dr Paul Calabresi and Dr James W. Darnowski (Brown University, Providence, RI) for their contribution in protocol development.
Supported in part by Istituto Superiore di Sanità-Progetto Terapia Antivirale-AIDS.
Address reprint requests to Patrizia Tosi, MD, Istituto di Ematologia “L. e A. Seràgnoli”, Policlinico S. Orsola, Via Massarenti, 9, 40138 Bologna, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal