Abstract
Cortactin is a potent filamentous actin-binding protein acting as a prominent substrate of Src tyrosine kinases. We have evaluated cortactin expression in a series of murine tissues and shown an abundant expression of cortactin in megakaryocytes and platelets. Cortactin, but not its related protein HS1, is upregulated during the phorbol 12-myristate 13-acetate (PMA)-mediated maturation of a human megakaryoblastic cell line CMK. Although the expression of Src-related kinases is also upregulated more rapidly than cortactin in PMA-treated CMK cells, tyrosine phosphorylation of cortactin appears to be only transiently elevated 4 days after PMA stimulation. In addition, cortactin expression is induced by thrombopoietin and interleukin-3 in megakaryocytes derived from murine bone marrow cells. Thus, cortactin represents a megakaryocyte-specific gene in bone marrow and the interaction of Src kinases with cortactin may be involved in the maturation of megakaryocytes.
CORTACTIN WAS initially recognized as a major phosphotyrosyl protein in v-src–transformed chicken embryo fibroblasts.1 Recent evidence has further shown that cortactin acts as a promising authentic substrate for pp60c-src in a variety of cells.2-8 Cortactin is also implicated in extracellular ligand-mediated signalings. Tyrosine phosphorylation of murine cortactin is elevated during the late G1 phase in FGF-1–stimulated cells.9 Induction of tyrosine phosphorylation of cortactin was also observed in cells stimulated with epidermal growth factor,6 platelet agonists,10,11 integrin-mediated cell adhesion,12 and bacterial-mediated cell invasion.13
Cortactin has a unique structure containing six and one half tandem 37-amino-acid repeats and a src-homologous 3 (SH3) domain at the carboxyl terminus. It is structurally related to a hematopoietic cell-specific gene, HS1, which is about 60% homologous to cortactin amino acid sequences but contains only three and one half copies of 37-amino-acid tandem repeats.14,15 Like cortactin, HS1 is also an effective substrate for protein tyrosine kinases.16-18 The level of HS1 tyrosine phosphorylation is significantly increased after cross-linking of the surface IgM on B lymphocytes16 or T-cell antigen receptor and CD3 complex on T lymphocytes.17 A recent study with mice lacking the murine homologue HS1 gene has further indicated that HS1 is important for antigen receptor-induced clonal expansion of both splenic B and T lymphocytes as well as deletion of peritoneal B lymphocytes17 and suggested that HS1 may be involved in both proliferation and apoptosis signalings.17 19
However, the role of cortactin in normal physiologic activities is still not clear. Cortactin has been detected in various types of cells in tissue culture, including fibroblasts,1,2 epithelial cells,13 a subset of breast tumor cell lines,20 endothelium (X. Zhan, unpublished data), some plasmacytoma cell lines,21 and platelets.3 Because many of those cells are either established or transformed cell lines, it is not clear whether cortactin expression detected in those cells reflects their tissue origins or an adaptation to tissue culture conditions. A recent report has indicated that cortactin may be involved in cell differentiation. Upregulation of cortactin has been detected in the osteroclast differentiation from monocytes.22 In the present study, we have examined cortactin expression in murine tissues. Our data show that, although cortactin is present in many tissues, in bone marrow it is specifically expressed in megakaryocytes and suggest that cortactin may play an important role in the maturation of megakaryocytes leading to functional platelets.
MATERIALS AND METHODS
Antibodies.The polyclonal antibody (C001) directed against a recombinant murine cortactin and the polyclonal antibody (2719) directed against a cortactin specific peptide were prepared as previously described.23 The monoclonal cortactin antibody 4F11 was purchased from Upstate Biotechnology Inc (Lake Placid, NY). The c-Src antibody (c-Src-2) was purchased from Santa Cruz Biotechnology Inc (Santa Cruz, CA). This reagent also recognizes Src-related proteins such as c-Yes and c-Fyn.
Immunohistochemical staining.Four-micron histologic sections of adult murine or rat tissues were dehydrated with alcohol and treated with methanol containing 0.3% hydrogen peroxide for 30 minutes. After rinsing with phosphate-buffered saline (PBS), the slides were blocked with PBS containing 10% normal goat serum and 1% bovine serum albumin (BSA) for 20 minutes and subsequently incubated with C001 antibody at 1 μg/mL for 30 minutes followed by washing three times with PBS. The slides were treated with biotinylated goat antirabbit IgG antibody for 30 minutes and the conjugated antibodies were detected with an avidin biotin complex reagent (Vector Laboratory Inc, Burlingame, CA) according to the procedure recommended by the manufacturer.
Tissue culture.CMK and CMK11-5 cells (gift of R. Deguchi, Mochida Pharmaceutical Co, Tokyo, Japan) were maintained in RPMI 1640 medium supplemented with 10% (vol/vol) fetal bovine serum at a concentration of 1 to 5 × 105 cells/mL. For the in vitro differentiation assay, cell cultures at a density of 106 cells/mL were treated with 10 ng/mL of phorbol 12-myristate 13-acetate (PMA) for the time as indicated.
Immunologic analysis.PMA-treated CMK cells were harvested and counted. For each time point, 3 × 106 cells were lysed in 50 mmol/L Tris, pH 8.0, containing 134 mmol/L NaCl, 0.5% deoxycholate, 1% NP-40, 0.1% sodium dodecyl sulfate (SDS), 50 mmol/L NaF, 200 μmol/L orthovanadate, 1 mmol/L phenylmethylsulfonyl fluoride, and 4 μg/mL aprotinin. The lysates were clarified by centrifugation for 10 minutes at 13,000g. The clarified cell lysates were incubated with 1 μg/mL 4F11 monoclonal antibody (in some experiments, the lysates were incubated with 5 μg/mL of 2719 polyclonal antibody) and 5 μg/mL goat antimouse IgG antibody (Pierce, Rockford, IL) for 2 hours. Complexes of antigens and antibodies were precipitated with protein A-Sepharose beads (Pharmacia Biotech Inc, Piscataway, NJ) and resolved by SDS-polyacrylmide gel electrophoresis (SDS-PAGE) followed by a transfer to a nitrocellulose membrane. Antigens were first probed with C001 polyclonal antibody (in some experiments, 4F11 antibody was used) and then with a secondary antibody conjugated with horseradish peroxidases and were finally visualized by enhanced chemiluminescence (Amersham Corp, Arlington Heights, IL).
Analysis of cortactin in bone marrow cells.Recombinant human thrombopoietin (Tpo), human interleukin-3 (IL-3), and human IL-11 were purchased from R&D System Inc (Minneapolis, MN). Murine bone marrow was harvested by flushing femurs from Balb/c mice (Jackson Laboratory, Bar Harbor, ME) with Iscove's modified Dulbecco's medium supplemented with 10% fetal bovine serum plus 50 μmol/L β-mercaptoethanol and plated in a 10-cm tissue culture dish in the same medium for 8 hours. The suspended cells were collected and replated to a 24-well plate containing different cytokines or combinations. After 4 days, cells were collected and cytospun to a glass cover slide. The slide was then fixed immediately in a formalin solution for 1 hour at room temperature in 75% ethanol at 4°C for 24 hours and stored in a specimen storage medium (250 mmol/L sucrose, 7 mmol/L MgCl2 , 50% glycerol) at −20°C. Cells were then stained with C001 anticortactin antibody as described above.
Northern blotting analysis.Cytoplasmic RNAs from PMA-treated CMK cells were prepared by the protocol as previously described.24 Ten micrograms of RNA was fractionated on a formaldehyde-containing agarose gel (1%), followed by a transfer to a nitrocellulose membrane. A 32P-labeled cDNA fragment of 2.9 kb encoding the murine cortactin2 was used as probe for cortactin RNA detection. The probe for HS1 was generated by polymerase chain reaction (PCR) in combination with reverse transcription of mRNA derived from human umbilical vein endothelium. The 5′ primer (sense) in PCR has sequence GCAGTCGGCTTTGATTAT. The 3′ primer (antisense) has sequence CCTCTTTGTCACAGCCTT. The amplified DNA was subcloned into PCR-II plasmid (Invitrogen, San Diego, CA) and confirmed with DNA sequencing. For the detection of cortactin mRNA, the prehybridization and hybridization were performed in the buffer containing 50% formamide, 5× SSC, 2× Denhardt's solution, and 100 μg/mL single-stranded carrier DNA at temperature of 37°C for 1 hour and 15 hours, respectively. The membrane was rinsed at the same temperature sequentially with a buffer containing 2× SSC, 0.1% SDS and a buffer containing 0.1× SSC, 0.1% SDS. For the detection of HS1 mRNA, a parallel membrane was subjected to prehybridization and hybridization at 42°C and washed at 68°C in the same buffers. The results of blots were semiquantitated by a densitometry analysis using NIH Image software (National Institutes of Health, Bethesda, MD).
RESULTS
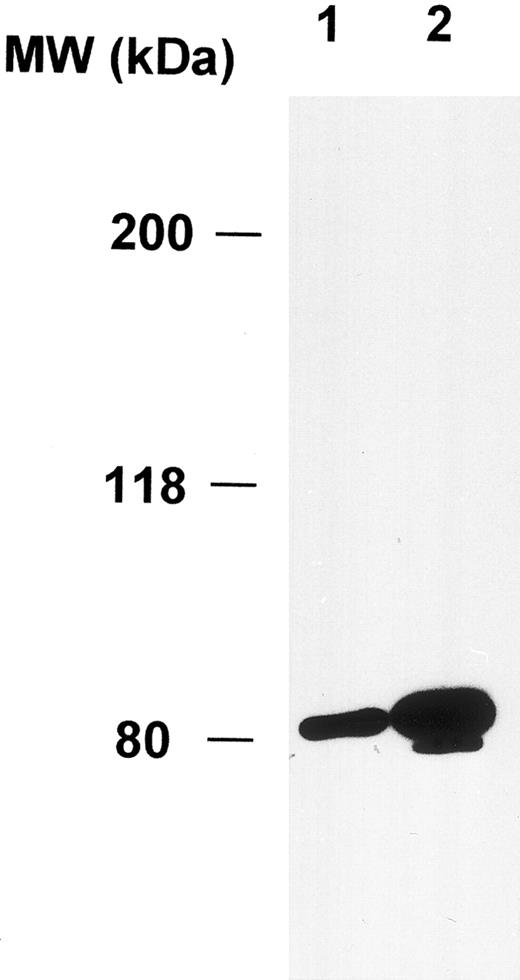
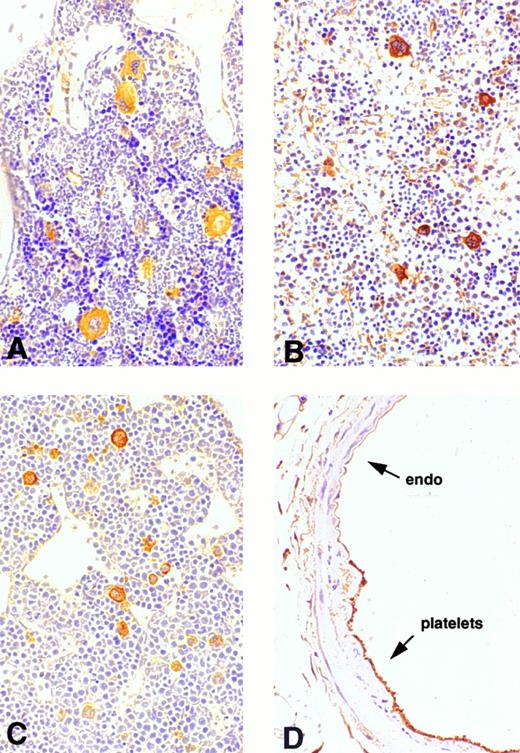
Expression of cortactin in megakaryocytes and platelets.To explore the physiologic function of cortactin, we examined cortactin expression by an immunohistochemistry assay in various adult murine organs, including brain, liver, lung, heart, kidney, and bone marrow. A purified polyclonal antibody directed against recombinant murine cortactin was used in the study.23 The specificity of this antibody was verified by probing a whole lysate from murine fibroblast cells in immunoblots, which detected only cortactin characteristic bands, p80 and p85 (Fig 1). The immunohistochemical study using this antibody showed a weak cortactin staining in various cells, including axons in the cortex and the hippocampus of the brain, endothelium in the heart, bronchial epithelium in the lung, and periosteum near joints in the sternal and vertebral bones. However, the most significant staining was detected within megakaryocytes, which are unequivocally recognizable by their morphologic criteria (giant size, multiple and lobulated nuclei), as shown in the bone marrow (Fig 2A). Because adult spleen and embryonic liver also serve as hematopoietic sites for all blood cell lines in mice, these organs were included in the study. As shown in Fig 2B and C, cells in these organs stained positive for cortactin all display the characteristic morphology of megakaryocytes. A few small cells in bone marrow and spleen were also stained weakly and presumably are megakaryocyte-related cells. The staining in bone marrow was specific for cortactin, because we did not observe staining in other types of hematopoietic cells, including precursors of white and red blood cells, in which HS1, a cortactin-related protein, is highly expressed.14 Consistent with abundant cortactin proteins in megakaryocytes, we also observed a strong cortactin staining on aggregated platelets adhering to the wall of de-endothelialized rat (and mouse; not shown) carotid arteries 24 hours after balloon injury (Fig 2D).
Polyclonal anticortactin antibody detects specifically cortactin proteins in murine fibroblasts. Murine fibroblasts (1 × 106 3T3 cells) were directly lysed in 300 μL of the SDS sample buffer. Ten microliters (lane 1) and 20 μL (lane 2) of the lysate were analyzed by Western blotting using purified polyclonal anticortactin antibody (C001) at the concentration of 1 μg/mL.
Polyclonal anticortactin antibody detects specifically cortactin proteins in murine fibroblasts. Murine fibroblasts (1 × 106 3T3 cells) were directly lysed in 300 μL of the SDS sample buffer. Ten microliters (lane 1) and 20 μL (lane 2) of the lysate were analyzed by Western blotting using purified polyclonal anticortactin antibody (C001) at the concentration of 1 μg/mL.
Immunohistochemical analysis of cortactin expression in megakaryocytes. Mature megakaryocytes stain positively in bone marrow, spleen, and liver. Platelets that cover the de-endothelialized intima (arrow) stain positively as well. (A) Bone marrow (original magnification × 160); (B) spleen (original magnification × 20); (C) liver of a mouse embryo (12.5 days, original magnification × 30); (D) a balloon-injured rat carotid artery (original magnification × 120) 24 hours after injury.
Immunohistochemical analysis of cortactin expression in megakaryocytes. Mature megakaryocytes stain positively in bone marrow, spleen, and liver. Platelets that cover the de-endothelialized intima (arrow) stain positively as well. (A) Bone marrow (original magnification × 160); (B) spleen (original magnification × 20); (C) liver of a mouse embryo (12.5 days, original magnification × 30); (D) a balloon-injured rat carotid artery (original magnification × 120) 24 hours after injury.
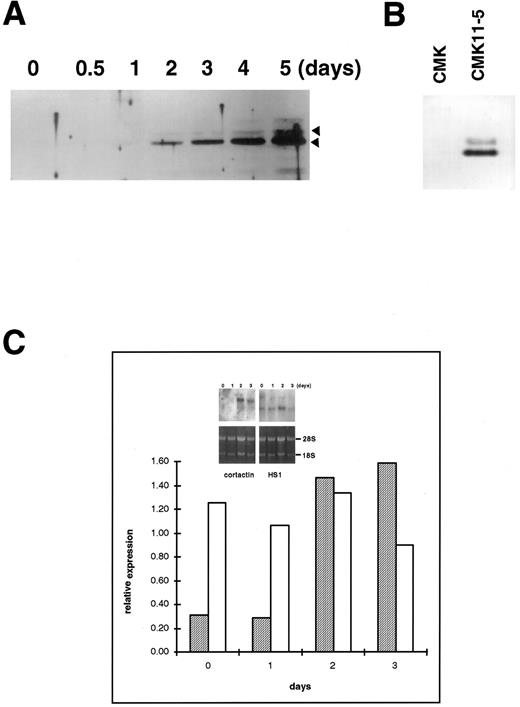
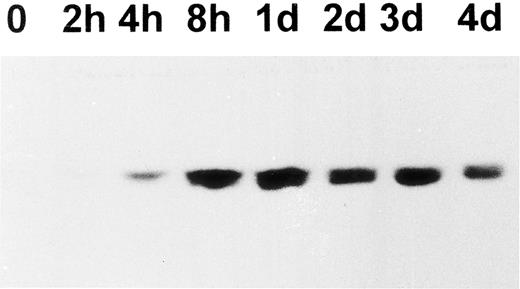
Upregulation of cortactin and c-Src expression during the differentiation of a human megakaryoblastic cell line, CMK.To evaluate the hypothesis that the expression of cortactin may be regulated during the course of megakaryocytes maturation, we examined a human megakaryoblastic cell line, CMK, that can be induced by phorbol esters to differentiate as characterized by expressing megakaryocyte characteristic proteins such as GPIIb-IIIa (αIIbβ3 ) and platelet peroxidase activity.25,26 As shown in Fig 3A (also Fig 5B), cortactin-related proteins were barely detectable in normal CMK cells, but they increased dramatically by at least 10 times at 2 days after PMA treatment and a maximum level occurred during 4 to 5 days after PMA treatment. Furthermore, a substantial amount of cortactin was detected in CMK11-5 cells (Fig 3B), a subclone of CMK cells associated with more characteristics of mature megakaryocytes.27 The increase in the expression of cortactin protein in response to PMA was apparently parallel to the increase in its mRNA expression as shown by a Northern blotting analysis (Fig 3C). In contrast, the PMA treatment had little effect on HS1 mRNA expression, which is readily detectable in nonstimulated cells (Fig 3C). Because previous reports have shown that the quantitative levels of HS1 mRNA are parallel to those of the protein expression,14,21 the detected HS1 mRNA in CMK cells likely reflects its protein expression. It is also in agreement with the notion that HS1 is a ubiquitous protein in hematopoietic lineage.14
Upregulation of cortactin expression in CMK cells in response to differentiation stimulus. (A) CMK cells were treated with PMA (10 nmol/L) for the time period as indicated, lysed, and immunoprecipitated with 4F11. The precipitates were analyzed in an immunoblotting using C001 polyclonal antibody. The detail procedure was described in the Materials and Methods. The data shown here are representative of four experiments. (B) CMK and CMK11-5 cells at an exponential growth stage in serum were analyzed for cortactin expression by immunoblotting analysis. The data are representative of two experiments, showing much more of cortactin proteins in the highly differentiated CMK subclone, CMK11-5. (C) Expression of mRNAs for cortactin and HS1 in PMA-treated CMK cells. The Northern blotting analysis was performed as described in the Materials and Methods. The intensities of mRNA for cortactin and HS1 were normalized with the ribosomal RNA 28S and plotted after a densitometry analysis. The autoradiography of the Northern blot is shown in the inset. The cortactin RNA is shown as a single 3.5-kb transcript, and HS1 RNA is shown as a single 2.0-kb band. The panels on the bottom show ethidium bromide staining of ribosomal RNA in agarose gel as the loading control. The results are representative of three experiments.
Upregulation of cortactin expression in CMK cells in response to differentiation stimulus. (A) CMK cells were treated with PMA (10 nmol/L) for the time period as indicated, lysed, and immunoprecipitated with 4F11. The precipitates were analyzed in an immunoblotting using C001 polyclonal antibody. The detail procedure was described in the Materials and Methods. The data shown here are representative of four experiments. (B) CMK and CMK11-5 cells at an exponential growth stage in serum were analyzed for cortactin expression by immunoblotting analysis. The data are representative of two experiments, showing much more of cortactin proteins in the highly differentiated CMK subclone, CMK11-5. (C) Expression of mRNAs for cortactin and HS1 in PMA-treated CMK cells. The Northern blotting analysis was performed as described in the Materials and Methods. The intensities of mRNA for cortactin and HS1 were normalized with the ribosomal RNA 28S and plotted after a densitometry analysis. The autoradiography of the Northern blot is shown in the inset. The cortactin RNA is shown as a single 3.5-kb transcript, and HS1 RNA is shown as a single 2.0-kb band. The panels on the bottom show ethidium bromide staining of ribosomal RNA in agarose gel as the loading control. The results are representative of three experiments.
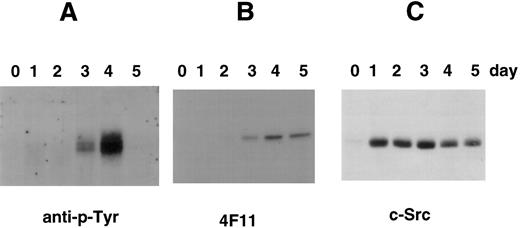
Tyrosine phosphorylation of cortactin in CMK cells stimulated with PMA. (A) CMK cells were treated with PMA for the time periods as indicated, lysed, and subject to immunoprecipitation with the polyclonal antibody 2719 against a cortactin-specific peptide.23 The immunoprecipitates were fractionated with an SDS-PAGE (7.5%) and immunoblotted with an antiphosphotyrosine antibody (anti–p-Tyr). (B) The membrane used above was stripped and reblotted with 4F11 monoclonal antibody. (C) CMK cells stimulated with PMA in parallel were lysed directly with SDS-sample buffer and analyzed for c-Src–related kinases using c-Src-2 antibody.
Tyrosine phosphorylation of cortactin in CMK cells stimulated with PMA. (A) CMK cells were treated with PMA for the time periods as indicated, lysed, and subject to immunoprecipitation with the polyclonal antibody 2719 against a cortactin-specific peptide.23 The immunoprecipitates were fractionated with an SDS-PAGE (7.5%) and immunoblotted with an antiphosphotyrosine antibody (anti–p-Tyr). (B) The membrane used above was stripped and reblotted with 4F11 monoclonal antibody. (C) CMK cells stimulated with PMA in parallel were lysed directly with SDS-sample buffer and analyzed for c-Src–related kinases using c-Src-2 antibody.
Because cortactin is a substrate of pp60c-src, we also examined the expression of Src-related kinases using an antibody against the C-terminus of pp60c-src, a conserved region among other Src-like kinases including c-Yes and c-Fyn. As shown in Fig 4, the expression of Src kinases is dramatically increased after PMA treatment. However, the enhancement can be detected as early as 2 hours and reached maximum levels by 24 hours, which was significantly earlier than the time of reaching maximum levels of the cortactin expression. The induction of Src kinases in differentiated CMK cells is consistent with previous reports regarding abundant pp60c-src present in platelets.28 29 To evaluate whether the expressed Src-related kinases are functionally active for cortactin, we analyzed tyrosine phosphorylation of cortactin. As shown in Fig 5, phosphotyrosyl cortactin became evident in 3 days after PMA treatment. However, it was dramatically enhanced at day 4 and increased approximately 10 times than the value on day 3. In contrast, the protein level of cortactin on day 4 is only about 2 to 3 times that measured on day 3. Strikingly, we failed to detect phosphotyrosyl cortactin on day 5, when there were still significant amounts of cortactin and Src kinases expressed (Fig 5B and C), suggesting that the interaction of Src kinases and cortactin is only transient during PMA-mediated differentiation and may be associated with a specific period for the maturation of megakaryocytes.
Western blotting analysis of pp60c-src expression in PMA-treated CMK cells. CMK cells were treated with PMA. At the time indicated, 5 × 105 cells were harvested and lysed in SDS sample buffer. The presence of c-Src–related proteins in the lysates was analyzed using a purified c-Src polyclonal antibody (c-Src-2; Santa Cruz Biotechnology Inc). The results shown here are representative of three experiments.
Western blotting analysis of pp60c-src expression in PMA-treated CMK cells. CMK cells were treated with PMA. At the time indicated, 5 × 105 cells were harvested and lysed in SDS sample buffer. The presence of c-Src–related proteins in the lysates was analyzed using a purified c-Src polyclonal antibody (c-Src-2; Santa Cruz Biotechnology Inc). The results shown here are representative of three experiments.
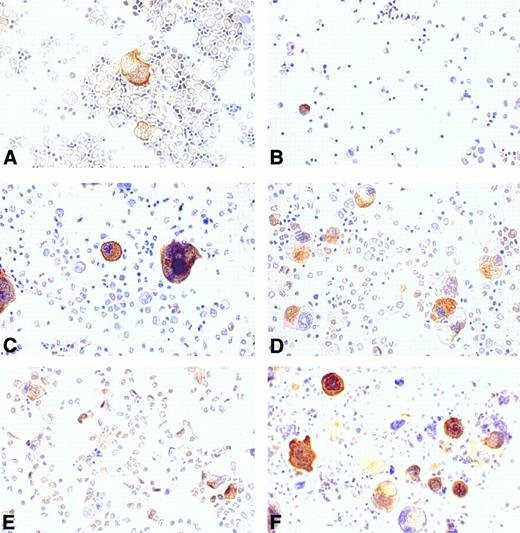
Regulation of cortactin expression in bone marrow cells by hematopoietic factors.Recent compelling evidence has shown that megakaryocytopoiesis is a complicated process regulated by several hematopoietic factors such as IL-3, IL-11, and Tpo. To evaluate the effects of these factors on cortactin expression, we cultured the cells derived from murine bone marrow in a medium supplemented with different types of hematopoietic factors for 4 days and subsequently stained them with the cortactin antibody. The cells freshly isolated from bone marrow exhibited a megakaryocyte-specific staining similar to that in bone marrow tissues (Fig 6A). There were some weak nuclear stainings in cells that resemble stem cells. At day 4, the control culture grown in medium with 10% fetal bovine serum alone had virtually no megakaryocyte-like cells and little cortactin staining (Fig 6B). In contrast, the culture in the presence of Tpo showed a significant increase in the number of cortactin-positive cells. Although those cells stained positive for cortactin are large and exhibit megakaryocyte-like nuclear and cytoplasmic morphology, many of them differ slightly in appearance from mature megakaryocytes in the fresh bone marrow preparation (Fig 6C). It should be noted that about 5% of the megakaryocyte-like cells were not obviously stained positive for cortactin. The culture in the presence of IL-3 alone exhibited a similar increase in the number of stained cells, albeit the average size of positive cells was significantly smaller than that in the culture stimulated with Tpo (Fig 6D). The cultures with IL-11 alone did not show a dramatic difference compared with the cultures grown in medium with serum only (Fig 6E). However, the combination of Tpo and IL-11 produced the highest increase in cells stained positive for cortactin (Fig 6F ). In contrast, the culture stimulated with the combination of Tpo and IL-3 did not show a significant difference in the size and the morphology of cortactin-containing cells compared with those cultured with Tpo or IL-3 alone (data not shown).
Regulation of cortactin expression by hematopoieticfactors in bone marrow. Bone marrow-derived cells were prepared and cultured for 4 days as described in the Materials and Methods. (A) Fresh bone marrow cells; (B) cells in 10% fetal bovine serum; (C) cells in Tpo (200 U/mL); (D) cells in IL-3 (3 ng/mL); (E) cells in IL-11 (50 ng/mL); and (F ) cells in Tpo plus IL-11. The results shown here are representative of two experiments.
Regulation of cortactin expression by hematopoieticfactors in bone marrow. Bone marrow-derived cells were prepared and cultured for 4 days as described in the Materials and Methods. (A) Fresh bone marrow cells; (B) cells in 10% fetal bovine serum; (C) cells in Tpo (200 U/mL); (D) cells in IL-3 (3 ng/mL); (E) cells in IL-11 (50 ng/mL); and (F ) cells in Tpo plus IL-11. The results shown here are representative of two experiments.
DISCUSSION
Our data presented here show that cortactin is specifically expressed in megakaryocytes in bone marrow and that its expression is upregulated during the course of megakaryocyte maturation. First, immunostaining with our polyclonal cortactin antibodies stained only megakaryocyte-like cells in bone marrow, spleen, and embryo livers. Those cells stained positive for cortactin are also stained with a polyclonal antibody to GPIIb-IIIa, confirming that they are megakaryocytes (data not shown). Second, the expression of cortactin is dramatically upregulated during PMA-mediated differentiation of a megakaryoblastic cell line, CMK. However, this finding does not imply that cortactin is simply an PMA-inducible gene. Indeed, the treatment of a T-lymphocyte cell line with PMA for a similar time course did not show any induction of cortactin expression (data not shown). Also, the increased cortactin expression appeared to be a relative late event (2 days), suggesting that multiple preceding events, presumably protein synthesis, are required in response to the initial PMA stimulation for the induction of cortactin expression. Finally, cortactin expression in bone marrow cells was markedly induced by Tpo, dramatically induced by the combination of Tpo and IL-11, and moderately induced by IL-3. All three cytokines have been shown for megakaryopoietic activities.
It is noteworthy that the average size of cortactin-containing bone marrow cells in the presence of IL-3 alone was considerably smaller than that in the Tpo-stimulated culture. This observation agrees with the previous reports that Tpo and IL-3 induce formation of megakaryocytes that differ in size and morphology.30,42 The combination of IL-3 and Tpo did not significantly increase in the cortactin-positive cells compared with Tpo alone. This finding suggests that IL-3 stimulation is not a prerequisite for Tpo-mediated stimulation. Our data support the view that IL-3 may be a factor targeting a different population of megakaryocyte progenitors or acting at an intermediate state of megakaryocyte maturation.30,31,41 In contrast, the combination of Tpo and IL-11 resulted in a strong synergistic enhancement of the cortactin staining, whereas IL-11 alone did not induce significant cortactin expression. Because IL-11 functions primarily on the progenitors at the early phase in megakaryocytopoiesis,30,32 33 cortactin expression thus represents a specific phase of megakaryocyte maturation posterior to that targeted by IL-11. It should be noticed that none of these cytokines at the concentrations used for bone marrow cells has significant effects on cortactin expression in CMK cells. The possible reasons for their discrepancy with bone marrow cells, such as low expression levels of receptors for those cytokines, remain to be determined.
The precise role of cortactin in megakaryocytopoiesis is not known. Cortactin has been shown to act as one of the major substrates of Src kinases.1 2 In CMK cells, expression of Src kinases was also regulated by PMA treatment, although the time course for the expression of Src-related kinases precedes that of cortactin. Src-related proteins were evident as early as 2 hours and peaked about 1 day after PMA treatment. In contrast, cortactin can be only weakly detected after 1 day, and it reaches maximum levels after 4 or 5 days. Thus, the expression of Src kinases could be a prerequisite for the cortactin expression. Because the dramatic increase in the tyrosine phosphorylation of cortactin was detected at day 4 after PMA treatment and decreased immediately afterwards, cortactin may act in concert with the function of Src kinases only at a specific differentiation period such as the late phase of megakaryocyte maturation.
It is also possible that cortactin is synthesized abundantly during megakaryocyte maturation to make platelets competent for their specific functions after their release into the circulation. One of the primary functions of platelets is to change their shape in response to agonists, a response that requires an extensive and rapid reorganization of the platelet cytoskeleton. Cortactin has been demonstrated as a potent F-actin binding protein and is associated with cytoskeleton structures. Thus, cortactin may participate in a signaling to enable cells to remodel their shape rapidly, such as forming pseudopodia. Indeed, our recent studies show that cortactin is a potent F-actin cross-linking protein (C. Huang, manuscript submitted). In response to thrombin, tyrosine phosphorylation of cortactin is immediately enhanced before aggregation and subsequently translocated into the cytoskeleton.10 11 Future investigation of megakaryocytes with altered cortactin genes should shed new lights on the physiologic roles of cortactin in megakaryocytopoiesis and platelet function.
ACKNOWLEDGMENT
We thank Drs Y. Shi for assistance with the harvesting of bone marrow cells and D. Scott for his critical reading of the manuscript. We also thank D. Norman for the expert photographic assistance.
Supported by National Institutes of Health Grant No. R29 HL52753 (to X.Z.).
Address reprint requests to Xi Zhan, PhD, Department of Experimental Pathology, The Jeremy H. Holland Laboratory, American Red Cross, 15601 Crabbs Branch Way, Rockville, MD 20855.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal