To the Editor:
Autologous peripheral blood stem cell (PBSC) transplantation (PBSCT) has been increasingly used and may replace autologous bone marrow transplantation for treatment of cancer patients with high-dose chemotherapy. Granulocyte-macrophage progenitors (colony-forming units–granulocyte-macrophage) and CD34+ cells have been preferentially used to quantitate PBSC contents of apheresis products to ensure rapid engraftment after PBSCT.1-5 However, it is difficult to determine the optimal timing for harvesting the maximum number of PBSC. We have observed that circulating immature cells (CIC), identified morphologically as myeloblasts, promyelocytes, myelocytes, metamyelocytes, and erythroblasts in May-Giemsa–stained peripheral blood (PB) smears, often increase during PBSC mobilization induced by chemotherapy and granulocyte colony-stimulating factor (G-CSF ). We were particularly interested to know whether the measurement of CIC could be used to determine the optimal timing for PBSC harvests because CIC is easily and quickly performed on routine blood counts.
We investigated whether the yield of CD34+ cells in PBSC harvests could be predicted by CIC number on the day of harvest. Between 1994 and 1996, 64 patients undergoing PBSC harvests were entered into this study after informed consent was obtained (Table 1). No patient had any evidence of bone marrow involvement at the time of PBSC harvest. Thirty-seven patients were treated with high-dose etoposide (500 mg/m2 for 3 days or 300 mg/m2 for 3 days plus 300 mg/m2 of carboplatinum on day 1). G-CSF was started on the day of nadir of neutrophils at a dose of 5 μg/kg intravenously or 2 μg/kg subcutaneously. Blood samples were obtained for white blood cells (WBC) counts with leukocyte differentials each day that an increase in WBC counts was documented. Conventional leukocyte differential of 100 WBC was performed on PB smears by technicians who were not informed of this study. CIC counts were calculated by multiplying a percentage of CIC by each corresponding WBC count. PBSC harvests were performed using a Spectra (Cobe Laboratories, Lakewood, CA), as described,3 when WBC and platelet counts were rapidly increasing and greater than 3 × 109/L and 30 × 109/L, respectively. The median processed blood volume per apheresis was 150 mL/kg, ranging from 120 to 200 mL/kg. Flow cytometric analysis for CD34+ cells was performed as described.4
Patients Characteristics
| No. of patients | 64 |
| Median age (range) | 43 (16-70) |
| Sex (M/F) | 33/31 |
| Disease | |
| Non-Hodgkin's lymphoma | 31 |
| Hodgkin's disease | 3 |
| Germ cell tumor | 5 |
| Small cell lung cancer | 6 |
| Breast cancer | 6 |
| Acute myelogenous leukemia | 8 |
| Acute lymphoblastic leukemia | 1 |
| Multiple myeloma | 4 |
| Status at harvest | |
| First complete remission | 16 |
| Second complete remission | 3 |
| Partial remission | 28 |
| Relapse | 8 |
| Primary refractory | 7 |
| Postoperation | 2 |
| Cycles of prior chemotherapy (range) | 4 (0-10) |
| Chemotherapy for PBSC harvest | |
| High-dose etoposide | 37 |
| Intermediate-dose cytosine arabinoside | 9 |
| High-dose cyclophosphamide | 6 |
| High-dose epirubicin | 6 |
| Others | 5 |
| No. of patients | 64 |
| Median age (range) | 43 (16-70) |
| Sex (M/F) | 33/31 |
| Disease | |
| Non-Hodgkin's lymphoma | 31 |
| Hodgkin's disease | 3 |
| Germ cell tumor | 5 |
| Small cell lung cancer | 6 |
| Breast cancer | 6 |
| Acute myelogenous leukemia | 8 |
| Acute lymphoblastic leukemia | 1 |
| Multiple myeloma | 4 |
| Status at harvest | |
| First complete remission | 16 |
| Second complete remission | 3 |
| Partial remission | 28 |
| Relapse | 8 |
| Primary refractory | 7 |
| Postoperation | 2 |
| Cycles of prior chemotherapy (range) | 4 (0-10) |
| Chemotherapy for PBSC harvest | |
| High-dose etoposide | 37 |
| Intermediate-dose cytosine arabinoside | 9 |
| High-dose cyclophosphamide | 6 |
| High-dose epirubicin | 6 |
| Others | 5 |
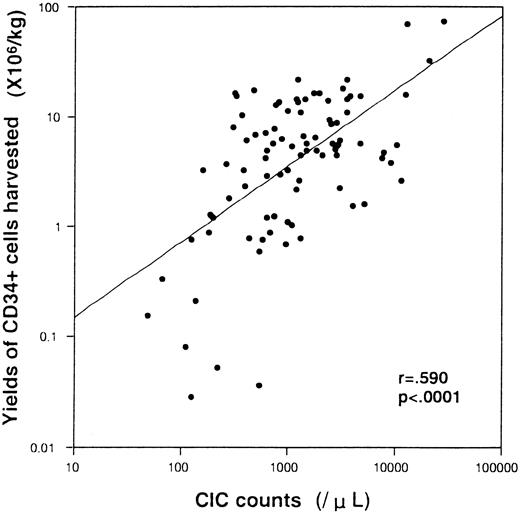
A total of 93 PBSC aphereses were performed in 64 patients (Table 2). The median percentage and count of CIC on the harvest day were 6% (range, 0% to 55%) and 1.1 × 109/L (range, 0 to 28.1 × 109/L), respectively. The median number of CD34+ cells harvested per apheresis was 4.7 × 106/kg (range, 0 to 73.5 × 106/kg). Numbers of CD34+ cells in PBSC products were plotted against the corresponding number of CIC in PB on each harvest day (Fig 1). CIC counts correlated significantly with the numbers of CD34+ cells per kilogram collected (Pearson's rank correlation analysis; r = .59, P < .001). In contrast, numbers of WBC or monocytes in PB were not correlated with the yields of CD34+ cells (data not shown).
Results of PBSC Harvests
| Total no. of apheresis | 93 |
| No. of apheresis per patient | |
| 1 | 42 |
| 2 | 17 |
| 3 | 5 |
| Blood cell counts in the peripheral blood on the day of harvest* | |
| WBC count (×109/L) | 14.7 (3.1-77.2) |
| Percentage of CIC (%) | 6 (0-55) |
| CIC count (×109/L) | 1.1 (0.0-28.1) |
| Platelet count (×109/L) | 82 (30-236) |
| No. of cells harvested* | |
| Mononuclear cells (×108/kg) | 3.3 (1.0-14.3) |
| CD34+ cells (×106/kg) | 4.7 (0.0-73.5) |
| Total no. of apheresis | 93 |
| No. of apheresis per patient | |
| 1 | 42 |
| 2 | 17 |
| 3 | 5 |
| Blood cell counts in the peripheral blood on the day of harvest* | |
| WBC count (×109/L) | 14.7 (3.1-77.2) |
| Percentage of CIC (%) | 6 (0-55) |
| CIC count (×109/L) | 1.1 (0.0-28.1) |
| Platelet count (×109/L) | 82 (30-236) |
| No. of cells harvested* | |
| Mononuclear cells (×108/kg) | 3.3 (1.0-14.3) |
| CD34+ cells (×106/kg) | 4.7 (0.0-73.5) |
Data are expressed as a median (range).
Correlations of CIC count in the PB on harvest day (horizontal axis) and CD34+ cell yield in the apheresis products (vertical axis). A regression line and results of statistical analysis are shown.
Correlations of CIC count in the PB on harvest day (horizontal axis) and CD34+ cell yield in the apheresis products (vertical axis). A regression line and results of statistical analysis are shown.
Our study indicates that measurements of CIC count are useful for predicting the yields of CD34+ cells harvested. Rapid increases in numbers of WBC, monocytes, and platelets are common clinical determinants for the timing of PBSC harvests, but they are less than quantitative and their meaning is uncertain. In fact, our data show that WBC counts and monocytes counts do not predict the yields of CD34+ cells harvested. An increase of WBC count is predictive after G-CSF administration, but the ratio of immature to mature neutrophils varies enormously. Our results suggest that PBSC harvests will not be successful if primarily neutrophils increase; in such a case, CIC can prevent an unnecessary apheresis. It was recently reported that concentrations of CD34+ cells or immature reticulocytes in PB on the day preceding harvest day were correlated well with the numbers of CD34+ cells harvested.4-6 CD34+ cell measurements are greatly affected by small changes in gating because of their low frequency. In contrast, CIC have a relatively high frequency, resulting in a smaller error in quantitation. An increase of CIC count is easily and rapidly recognized with routine blood counts without labor-intensive assays, and PBSC harvests could therefore be timed to coincide with the increases in CIC. Measurements of CIC would clearly be advantageous for financial and operational efficiency of PBSC harvests. This study suggests that an increase of CIC at a level of 2 × 109/L may be a good indicator to harvest greater than 1 × 106/kg of CD34+ cells in a single apheresis.
ACKNOWLEDGMENT
We thank Dr James L.M. Ferrara (Dana-Farber Cancer Institute, Boston, MA) for critical reading of the manuscript. Supported in part by grants-in-aid from the Ministry of Health and Welfare, the Ministry of Education, Science and Culture (06454348), and Uehara Memorial Foundation.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal