Abstract
An in vitro model of folate-deficient erythropoiesis has been developed using proerythroblasts isolated from the spleens of Friend virus-infected mice fed an amino acid-based, folate-free diet. Control proerythroblasts were obtained from Friend virus-infected mice fed the same diet plus 2 mg folic acid/kg diet. Our previous studies showed that, after 20 to 32 hours of culture in folate-deficient medium with 4 U/mL of erythropoietin, the folate-deficient proerythroblasts underwent apoptosis, whereas control erythroblasts survived and differentiated into reticulocytes over a period of 48 hours. The addition of folic acid or thymidine to the folate-deficient medium prevented the apoptosis of the folate-deficient erythroblasts, thereby implicating decreased thymidylate synthesis as the main cause of apoptosis in the folate-deficient erythroblasts. In the study reported here, we examined intracellular folate levels, uracil misincorporation into DNA, p53 and p21 proteins, and reticulocyte formation in erythroblasts cultured in folate-deficient or control medium. In all experiments, the folate-deficient erythroblasts cultured in folate-deficient medium gave results that varied significantly from folate-deficient erythroblasts cultured in control medium or control erythroblasts cultured in either folate-deficient or control media. Folate-deficient erythroblasts cultured in folate-deficient medium had marked decreases in all coenzyme forms of folate that persisted throughout culture, increased uracil misincorporation into DNA, persistent accumulations of p53 and p21, and decreased reticulocyte production but increased size of individual reticulocytes. A model of folate-deficient erythropoiesis based on apoptosis of late stage erythroblasts is presented. This model provides explanations for the clinical findings in megaloblastic anemia.
MEGALOBLASTIC ANEMIA is caused by deficiency of folate or cobalamin (vitamin B12 ). The peripheral blood of patients with megaloblastic anemia is characterized by pancytopenia and an increased erythrocyte mean corpuscular volume (MCV). The bone marrow has a prevalence of large, early stage hematopoietic precursor cells.1-4 Cytogenetic studies have shown increased chromosomal breakage in the bone marrow cells of patients with megaloblastic anemia.5,6 Increased bilirubin and lactate dehydrogenase in the serum results from the destruction of erythroid precursor cells in the bone marrow.1-4 A murine form of megaloblastic anemia can be induced by feeding mice a folate-free, amino acid-based diet.7 An in vitro system of folate-deficient erythropoiesis was developed by culturing proerythroblasts isolated from the spleens of mice that were fed the folate-free diet during the acute erythroblastosis phase of the disease caused by Friend leukemia virus.8 In folate-deficient medium, these erythroblasts undergo apoptosis, whereas in medium containing folic acid, they survive and differentiate into reticulocytes within 2 days.8 Thymidine added to the folate-deficient medium inhibits the apoptosis. Therefore, in the folate-deficient cells, we hypothesize that decreased levels of the coenzyme 5,10-methylene tetrahydrofolate inhibits the conversion of deoxyuridylate (dUMP) to thymidylate (dTMP). The resultant increased ratio of dUMP to dTMP leads to an increased rate of uracil misincorporation into DNA.9,10 This misincorporated uracil can be removed and the DNA repaired. However, the repair of misincorporated uracil residues that are located near each other on opposing strands of DNA has the potential to produce double-stranded breakage of the DNA.11
DNA damage induced by irradiation and chemotherapeutic agents has been associated with accumulation of p53,12-16 a transcriptional factor with tumor-suppressor activity. When p53 is induced by DNA-damaging agents, the protein product of one of its target genes, p21CIP1/WAF1 (p21),17,18 also accumulates.19-21 p21 inhibits cell cycle progression17,22 and is the major effector for the arrest of cell cycle that has been associated with p53 accumulation.22 Although this inhibition of the cell cycle by p53 has been thought to contribute to its tumor-suppressor function, accumulation of p53 in hematopoietic cells has also been associated with death by apoptosis.23 24 We show here that (1) erythroblasts undergoing apoptosis due to intracellular folate deficiency have increased uracil misincorporation into DNA and increased p53 and p21 proteins and (2) the erythroblasts that survive this apoptotic process give rise to larger than normal reticulocytes.
MATERIALS AND METHODS
Mice and diets.Weanling, female CD2F1 hybrid mice were purchased from Harlan-Sprague Dawley (Indianapolis, IN). Experimental (folate-deficient) mice were fed an amino acid-based, folate-free diet7 (Dyets, Bethlehem, PA), whereas control mice were fed the same diet with the addition of 2 mg folic acid per killogram of diet.7 After 2 weeks on the respective diets, the mice were infected with 103 spleen focus-forming units of the anemia-inducing strain of Friend leukemia virus as previously described.8 After 2 weeks of infection and 4 weeks of each respective diet, the mice were killed and their spleens, which had been enlarged by the virus-induced erythroblastosis, were removed. For the in vivo determination of macrocytosis induced by folate deficiency, 8-month-old female CD2F1 mice were fed either control or folate-deficiency diet for a period of 9 weeks. Blood samples taken at 0, 5, 7, and 9 weeks were diluted in phosphate-buffered saline (PBS)25 with 2 mmol/L EDTA as described previously.7 The blood samples were then analyzed with a Technicon H-1 automated blood cell counter (Bayer Corp, Tarrytown, NY) that was programmed with software to count murine cells. After 9 weeks, mice from each group were killed and the total folate content of their livers was determined.
In vitro system of folate-deficient erythropoiesis.Single-cell suspensions of erythroblasts were prepared by straining the splenic contents through nylon mesh. Purified populations of proerythroblasts from both folate-deficient and control mice were separated by velocity sedimentation at unit gravity, as described previously.8,26 The purified proerythroblasts were either immediately used for biochemical measurements or placed in short-term tissue culture. The culture medium was folate-free Iscove's modified Dulbecco's medium (GIBCO-BRL, Grand Island, NY) containing 30% heat-inactivated fetal bovine serum (Hyclone, Logan, UT), 1% deionized bovine serum albumin, 100 μmol/L α-thioglycerol, and 4 U/mL of pure recombinant human erythropoietin (Ortho Biotech, Raritan, NJ). This medium is termed folate-deficient medium because it contained a total of 5 nmol/L folate contributed by the fetal bovine serum.8 To one half of the cultures folic acid was added before incubation. The tissue culture medium of these cultures contained 6.4 μmol/L folic acid and is termed control medium because it contains the folic acid concentration present in normal Iscove's medium. Thus, cultured erythroblasts could be classified into four groups depending on the diet of the mice from which they were isolated and the folate content of their culture medium. These groups were (1) control cells in control medium, (2) control cells in folate-deficient medium, (3) folate-deficient cells in control medium, and (4) folate-deficient cells in folate-deficient medium. At various times of culture, the erythroblasts were collected and analyzed for folate content, uracil misincorporation into DNA, accumulation of wild-type p53 and p21 proteins, and the number and size of newly formed reticulocytes.
Determination of folate content and coenzyme distributions.The harvested cells were washed twice in ice-cold PBS and the number of viable cells was determined by trypan blue dye exclusion. The pelletted erythroblasts were lysed by heating to 100°C for 5 minutes in folate coenzyme extraction buffer [0.2 mol/L β-mercaptoethanol, 2% (wt/vol) sodium ascorbate, 50 mmol/L HEPES, 50 mmol/L 2-(N-cyclohexylamino) ethanesulfonic acid [pH 7.85]) and treated with rat plasma conjugase to hydrolyze folylpolyglutamates.27 The extracted folate coenzymes were separated on a Beckman Ultrasphere (Beckman Instruments Inc, Fullerton, CA) C18 I.P. column. The folate content of unseparated samples of each of the separated fractions was determined with a Lactobacillus casei microbiologic assay as described previously.8 For determination of total folate content in livers, the hepatic tissue was homogenized in the same extraction buffer and assayed with the same microbiologic assay, and results expressed as nanomoles of folate per gram of liver.
Determination of uracil content of DNA.The harvested erythroblasts were pelletted and lysed in DNA extraction buffer (10 mmol/L NaCl, 50 mmol/L Tris-HCl [pH 8.0], 20 mmol/L EDTA, 0.5% sodium dodecyl sulfate) with 200 μg proteinase K/mL. After incubation at 37°C for 24 hours, the DNA was extracted with phenol-chloroform and purified as described previously.28 The purified DNA was precipitated with ethanol and resuspended in 10 mmol/L Tris-HCl (pH 8.0) and 1 mmol/L EDTA. The DNA was quantitated by measuring fluorescence of an aliquot stained with ethidium bromide. The uracil content was determined as described previously.29 Briefly, uracil residues were removed from the DNA with uracil-DNA glycosylase, derivatized with 3,5-bis(trifluoromethyl)benzyl bromide, and analyzed by gas chromatography-mass spectrometry.
Determination of p53 and p21 protein accumulations.The harvested erythroblasts were washed twice with ice-cold PBS and then lysed on ice for 30 minutes in a 0.5% Nonidet p-40 lysis buffer containing 50 mmol/L Tris-HCl (pH 7.5), 100 mmol/L NaCl, and protease inhibitors antipain (10 μg/mL), leupeptin (10 μg/mL), pepstatin A (10 μg/mL), and chymostatin (10 μg/mL; Sigma, St Louis, MO) and 4-(2-aminoethyl)-benzenesulfonyl fluoride (200 μg/mL; Calbiochem-Novabiochem, La Jolla, CA). Cell lysates were centrifuged at 13,000g for 15 minutes. The supernatant was collected and protein concentration determined by the method of Bradford.30 Fifty micrograms of cellular protein was mixed with an equal volume of 2× Laemmli sample buffer31 and boiled at 100°C for 8 minutes. Cellular proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)31 using 10% gels for p53 and 12% gels for p21 and transferred onto polyvinylidene difluoride membranes (Millipore, Bedford, MA). The membranes were incubated with a 1:100 dilution of either anti-p53 Ab-3 or anti-p21 WAF1 Ab-2 (Oncogene Research Products, Calbiochem, Cambridge, MA), followed by incubation with a 1:20,000 dilution of secondary antibody, goat antimouse IgG horseradish peroxidase-conjugate (Pierce, Rockford, IL). Detection was enhanced by chemiluminescence (ECL Western blotting detection system; Amersham, Arlington Heights, IL).
Determination of number and size of reticulocytes formed in vitro.Erythroblasts were harvested at 8-hour intervals beginning at 16 hours of culture. Cells from triplicate cultures were cytocentrifuged onto glass microscope slides, stained with 3,3′-dimethoxybenzidine and hematoxylin, coded, and counted in a blinded manner for reticulocytes, free nuclei, and nucleated cells as described previously.32 All counts were performed on 300 consecutive nuclei encountered in successive 100× microscopic fields. The same slides were then analyzed for sizes of reticulocytes by computer-assisted microscopic analysis of reticulocyte profile areas. Thirty consecutive reticulocytes were analyzed for each slide.
Statistical comparisons were performed using GraphPAD (GraphPAD Software Inc, San Diego, CA) InStat using the Student's t-test or one-way ANOVA with Tukey's Multiple Comparison Test. P < .05 was considered significant.
RESULTS
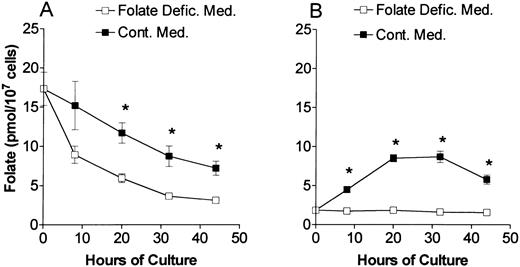
At various times of culture, the total folate content (Fig 1) and the distribution of folate coenzymes (Fig 2) were determined in proerythroblasts from control and folate-deficient mice. At the time of their isolation from the spleens (0 hours in Fig 1A and B), the proerythroblasts from folate-deficient mice had about one-tenth of the total folate content as those proerythroblasts isolated from control mice. During culture in folate-deficient medium, the proerythroblasts from folate-deficient mice had persistently low total folate content (Fig 1B). Culturing the folate-deficient proerythroblasts in control medium repleted the total folate content (Fig 1B) such that at 32 and 44 hours of culture (Fig 1B) the total folate content was similar to erythroblasts from control mice cultured in control medium (Fig 1A). When proerythroblasts from control mice were cultured in folate-deficient medium, they had decreased total folate content as compared with when they were cultured in medium containing folic acid (Fig 1A). The decline over time in the total folate per cell in cultures of control cells in Fig 1A is due to the marked decrease in cell size that occurs during erythroid differentiation.33 34 However, throughout the 44-hour culture period in folate-deficient medium, the control erythroblasts maintained a total folate content that was greater than their counterparts from folate-deficient mice cultured in folate-deficient medium (Fig 1). Also, these control erythroblasts completed their differentiation into reticulocytes in folate-deficient medium without undergoing apoptosis.
Total folate content of erythroblasts in culture. Control (A) and folate-deficient (B) erythroblasts were cultured in either folate-deficient (□) or control medium (▪) and harvested at the times indicated. The total folate concentration was determined by Lactobacillus casei bioassay. Data are the means ± SEM of three experiments. *P < .02 (A) or P < .002 (B) when folate levels are compared between folate-deficient medium and control medium at the respective hours of culture.
Total folate content of erythroblasts in culture. Control (A) and folate-deficient (B) erythroblasts were cultured in either folate-deficient (□) or control medium (▪) and harvested at the times indicated. The total folate concentration was determined by Lactobacillus casei bioassay. Data are the means ± SEM of three experiments. *P < .02 (A) or P < .002 (B) when folate levels are compared between folate-deficient medium and control medium at the respective hours of culture.
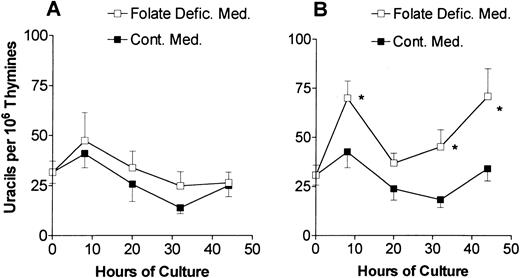
Uracil misincorporation into DNA in cultured erythroblasts. Control (A) or folate-deficient (B) erythroblasts were cultured in folate-deficient (□) or control medium (▪). DNA was extracted from cells collected at various times. The uracils were removed from the DNA with uracil-DNA glycosylase, derivatized with 3,5-bis(trifluoromethyl)benzyl bromide, analyzed by gas chromatography-mass spectrometry, and expressed per 106 thymines in the DNA sample. Results are the means ± 1 SEM of 7 to 17 separate samples. *P < .05 in steady-state levels of uracil in DNA in folate-deficient erythroblasts cultured in folate-deficient as compared with control medium.
Uracil misincorporation into DNA in cultured erythroblasts. Control (A) or folate-deficient (B) erythroblasts were cultured in folate-deficient (□) or control medium (▪). DNA was extracted from cells collected at various times. The uracils were removed from the DNA with uracil-DNA glycosylase, derivatized with 3,5-bis(trifluoromethyl)benzyl bromide, analyzed by gas chromatography-mass spectrometry, and expressed per 106 thymines in the DNA sample. Results are the means ± 1 SEM of 7 to 17 separate samples. *P < .05 in steady-state levels of uracil in DNA in folate-deficient erythroblasts cultured in folate-deficient as compared with control medium.
Because folate-deficient erythroblasts begin to undergo apoptosis at 20 hours of culture in folate deficient-medium,8 the distribution of folate coenzymes was examined at the time of their isolation (0 hours) and after 20 hours of culture. When compared with control erythroblasts, the folate-deficient cells at 0 hours had similar percentages of tetrahydrofolate and 5-formyltetrahydrofolate, an increased percentage of 5-methyltetrahydrofolate (15.3 ± 1.0 v 7.3 ± 0.7 [mean ± 1 SEM]) and a decreased percentage of 10-formyltetrahydrofolate (21.4 ± 2.4 v 34.3 ± 1.9). This pattern of coenzyme distribution in folate-deficient cells persisted after 20 hours of culture in folate-deficient medium. However, after 20 hours of culture in control medium, the folate-deficient erythroblasts had similar distribution of coenzymes as did control erythroblasts in either folate-deficient or control medium. Although no single folate coenzyme form became totally depleted in folate-deficient erythroblasts that were cultured in folate-deficient medium, the persistence of decreased absolute levels of all folate coenzymes in these cells leads to critical intracellular changes that can induce apoptosis.
Although all of the folate coenzymes were decreased in the folate-deficient erythroblasts, the inhibition of their apoptosis by the addition of thymidine to the folate-deficient medium indicated that decreased 5,10-methylenetetrahydrofolate played an important role in the fate of folate-deficient erythroblasts.8 In the conversion of dUMP to dTMP, 5,10-methylenetetrahydrofolate provides a methylene group and reducing equivalents. Decreased levels of 5,10-methylenetetrahydrofolate results in an increased ratio of dUMP/dTMP and ultimately to increased rates of uracil misincorporation into DNA. The steady-state level of misincorporated uracil in DNA was measured in both control and folate-deficient erythroblasts during culture in either control or folate-deficient medium (Fig 2). The freshly isolated proerythroblasts from normal and folate-deficient mice had similar levels of uracil misincorporation in DNA. Erythroblasts from control mice had similar levels of uracil misincorporation throughout their culture in either control medium or folate-deficient medium (Fig 2A). When folate-deficient erythroblasts were cultured in folate-deficient medium, they had significantly increased uracil misincorporation in DNA as compared with when they were cultured in the control medium (Fig 2B). This increased uracil misincorporation was significant at 8 hours of culture and became more prominent at 32 and 44 hours (Fig 2B).
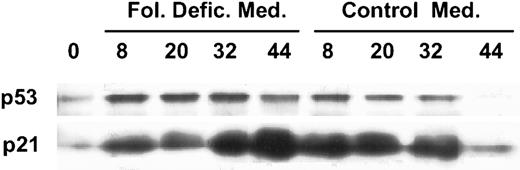
Large amounts of uracil in DNA can lead to DNA damage. Simultaneous repair of uracil residues located near each other on opposing strands of DNA results in double-stranded DNA breaks.10,11 DNA damage, in turn, has been shown to induce the accumulation of the protein product of the tumor-suppressor gene, p53. Therefore, we examined folate-deficient erythroblasts for evidence of p53 protein accumulation during culture in folate-deficient medium. The accumulation of p53 protein was slightly greater at all times examined when folate-deficient erythroblasts were cultured in folate-deficient medium as opposed to control medium (Fig 3). Furthermore, the accumulation persisted through 44 hours in folate-deficient medium, whereas it decreased in control medium. Because p53 is a transcription factor capable of activating several target genes implicated in cell cycle control and DNA repair, we examined the same protein samples from the folate-deficient cells for one of these gene products, p21. p21 protein levels increased markedly in the cells that had persistent p53 protein accumulation as seen in folate-deficient cells at 44 hours of culture in folate-deficient medium (Fig 3). Conversely, p21 levels declined in parallel with p53 in folate-deficient cells after 44 hours of culture in control medium. The patterns of p53 and p21 protein accumulation in control erythroblasts cultured in either folate-deficient medium or control medium were the same as shown for folate-deficient erythroblasts in control medium (data not shown). Thus, the destruction of erythroblasts in folate deficiency appears to be the result of a series of intracellular events that include persistently low folate coenzymes, increased uracil misincorporation into DNA, persistence of intracellular p53 and p21 protein accumulation and, as reported previously,8 apoptosis.
Time courses of p53 and p21 accumulation in cultured, folate-deficient erythroblasts. Folate-deficient erythroblasts were cultured in either control medium or folate-deficient medium. At the hours of culture shown atop each lane, cells were collected and lysed by sonication and boiling in SDS-PAGE sample buffer (Laemmli). The samples were separated by SDS-PAGE. Shown are Western blots of gels that were loaded with 50 μg protein per lane. The blots were probed with antibodies to p53 and p21 and then developed with enhanced chemiluminescence.
Time courses of p53 and p21 accumulation in cultured, folate-deficient erythroblasts. Folate-deficient erythroblasts were cultured in either control medium or folate-deficient medium. At the hours of culture shown atop each lane, cells were collected and lysed by sonication and boiling in SDS-PAGE sample buffer (Laemmli). The samples were separated by SDS-PAGE. Shown are Western blots of gels that were loaded with 50 μg protein per lane. The blots were probed with antibodies to p53 and p21 and then developed with enhanced chemiluminescence.
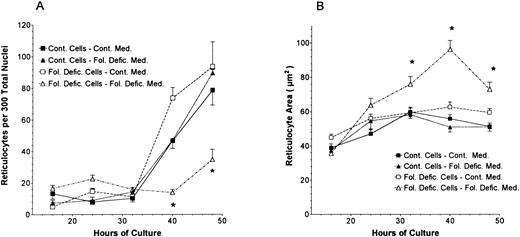
Because megaloblastic anemia is characterized by macrocytosis of the peripheral blood erythrocytes, we examined the size of reticulocytes formed during the culture of folate-deficient and control proerythroblasts. When erythroblasts enucleate in tissue culture they form two-products: free nuclei and reticulocytes. The numbers of reticulocytes that formed in culture are shown in Fig 4A. The number of reticulocytes in cultures of control erythroblasts in either control or folate-deficient media and of folate-deficient cells in control medium were significantly increased at 40 and 48 hours of culture (Fig 4A), as had been previously reported for normal Friend virus-derived erythroblasts in vitro.32 In contrast, the folate-deficient cells cultured in folate-deficient medium produced fewer reticulocytes in vitro (Fig 4A). In addition to this decreased incidence of reticulocyte formation, the total number of cells present in the folate-deficient erythroblast cultures with folate-deficient medium at 40 and 48 hours was reduced by apoptosis to only 30% of the cells found in the other three combinations of erythroblasts and medium culture conditions.8 Thus, total reticulocyte production in vitro by folate-deficient erythroblasts in folate-deficient medium was about 10% to 15% of that found when they were cultured in control medium or that found when control erythroblasts were cultured in either control or folate-deficient medium. Although the number of reticulocytes produced during the culture of folate-deficient proerythroblasts in folate-deficient medium was markedly reduced, those reticulocytes that did form were significantly larger than reticulocytes formed from the same folate-deficient proerythroblasts cultured in control medium or from control proerythroblasts cultured in either control or folate-deficient medium (Fig 4B). Thus, the minority of folate-deficient erythroblasts that were cultured in folate-deficient medium and that were able to survive the in vitro apoptosis produced significantly larger reticulocytes than did their counterparts cultured in control medium or control erythroblasts cultured in either control or folate-deficient medium.
The number of reticulocytes (A) and reticulocyte profile sizes (B) at various times during the in vitro differentiation of control and folate-deficient proerythroblasts. Cytocentrifuge preparations were made of control proerythroblasts cultured in control medium (▪), control proerythroblasts cultured in folate-deficient medium (▴), folate-deficient proerythroblasts cultured in control medium (□), or folate-deficient cells in folate-deficient medium (▵). Reticulocytes were counted in a blinded manner at 100× magnification. Reticulocyte sizes were determined by a computerized calculation of light microscopic images. For each culture, determination of reticulocyte numbers was made by counting consecutive microscopic fields until 300 nuclei (free or intracellular) were counted. For each culture, the determination of reticulocyte size was made from 30 consecutive reticulocytes. All data are the means ± 1 SEM of three separate cultures. *P < .05 when data from folate-deficient cells cultured in folate-deficient medium are compared with all other treatments.
The number of reticulocytes (A) and reticulocyte profile sizes (B) at various times during the in vitro differentiation of control and folate-deficient proerythroblasts. Cytocentrifuge preparations were made of control proerythroblasts cultured in control medium (▪), control proerythroblasts cultured in folate-deficient medium (▴), folate-deficient proerythroblasts cultured in control medium (□), or folate-deficient cells in folate-deficient medium (▵). Reticulocytes were counted in a blinded manner at 100× magnification. Reticulocyte sizes were determined by a computerized calculation of light microscopic images. For each culture, determination of reticulocyte numbers was made by counting consecutive microscopic fields until 300 nuclei (free or intracellular) were counted. For each culture, the determination of reticulocyte size was made from 30 consecutive reticulocytes. All data are the means ± 1 SEM of three separate cultures. *P < .05 when data from folate-deficient cells cultured in folate-deficient medium are compared with all other treatments.
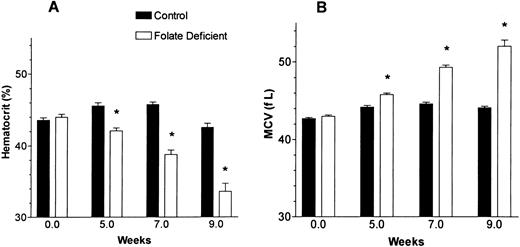
The one characteristic of megaloblastic anemia not noted in our previous in vivo murine study using the folate-deficient diet was erythrocyte macrocytosis. In those studies, weanling mice were fed the folate-free diet for only 6 weeks before being refed folate.7 The weanling mice have a greater body growth than adults and they have a greater folate requirement for survival. Many weanling mice cannot survive 8 weeks on the folate-free diet. However, if the adult mice are fed the folate-free diet, they all survive for more than 10 weeks and their anemia is accompanied by macrocytosis. In Fig 5, adult mice fed the folate-deficient diet for 9 weeks had an increased MCV that accompanied their development of anemia. After 9 weeks, mice fed the folate-free diet had a mean ± 1 SEM of 7.7 ± 0.4 nmol folate/g of liver, whereas mice fed the control diet had 36.6 ± 2.2 nmol folate/g of liver (P < .001).
The hematocrit and MCV changes induced by folate deficiency in adult mice. Mice were fed either control or folate-free diet for 9 weeks. Blood cell counts were determined using an automated counter that was programmed for murine blood cells. All data are the means ± 1 SEM of 10 mice. *P < .001 when comparing folate-deficient mice with control mice at the weeks shown.
The hematocrit and MCV changes induced by folate deficiency in adult mice. Mice were fed either control or folate-free diet for 9 weeks. Blood cell counts were determined using an automated counter that was programmed for murine blood cells. All data are the means ± 1 SEM of 10 mice. *P < .001 when comparing folate-deficient mice with control mice at the weeks shown.
DISCUSSION
Proerythroblasts isolated from folate-deficient mice had about one-tenth the total folate content as did proerythroblasts isolated from control mice. Despite this 10-fold difference in folate levels between folate-deficient and control proerythroblasts, the uracil in DNA, the p53 and p21 protein content, and the percentage of apoptotic cells8 were the same in both populations of proerythroblasts. These results indicate that irreversible changes in the folate-deficient erythroblasts had not yet occurred and that correction of the intracellular folate deficiency could prevent the death of the erythroblasts in culture. Indeed, if folic acid is provided in the medium during the first 8 hours of culture, the folate-deficient cells can be rescued and complete normal differentiation into reticulocytes. If the provision of folic acid is delayed until 20 hours of culture, only two-thirds of the cells that would undergo apoptosis can be rescued. Equal amounts of uracil in DNA are found in folate-deficient and control proerythroblasts when they are first isolated (Fig 2), but when the folate-deficient proerythroblasts are cultured in folate-deficient medium, uracil misincorporation in DNA increased significantly when compared with culture in control medium. At 32 through 44 hours of culture in folate-deficient medium, p53 protein accumulation persisted at high levels in folate-deficient cells and p21 protein progressively accumulated (Fig 3). The most prominent apoptosis in these cells occurs at 32 hours and later times of culture.8 The 32-hour period of culture corresponds to the basophilic and polychromatophilic stages of erythroblast differentiation in the bone marrow.35 Thus, these late stages of erythroid differentiation appear to be especially susceptible to apoptosis due to folate deficiency. At the late basophilic and early polychromatophilic stages of their development, the erythroblasts begin to accumulate hemoglobin and they undergo a dramatic reduction in size.33,34 They have completed one division in vitro and have one remaining division before enucleating. Our previous in vivo results with mice fed the folate-free diet until they developed megaloblastic anemia are consistent with the major proportion of apoptosis occurring at the basophilic-polychromatophilic stages of development. When these folate-deficient mice became pancytopenic after 6 weeks of the folate-free diet, they had (1) increased numbers of colony forming units-erythroid (CFU-E), (2) decreased numbers of reticulocytes, and (3) many immature cells but very few late stage erythroblasts in their bone marrow.7 Ferrokinetic studies were also consistent with the loss of cells at the late stages of erythroid development when the erythroblasts had begun to synthesize hemoglobin.7
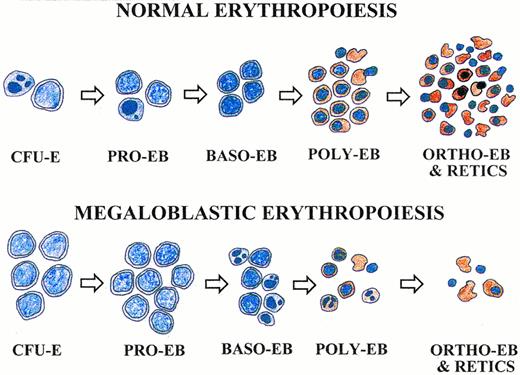
Pathophysiologic model of megaloblastic anemia based on apoptosis at the basophilic and polychromatophilic erythroblast stages that produce large reticulocytes. Cells appear as stained with 3,3′-dimethoxybenzidine and hematoxylin. Apoptotic cells are indicated by condensed and fragmented nuclei. See text for description.
Pathophysiologic model of megaloblastic anemia based on apoptosis at the basophilic and polychromatophilic erythroblast stages that produce large reticulocytes. Cells appear as stained with 3,3′-dimethoxybenzidine and hematoxylin. Apoptotic cells are indicated by condensed and fragmented nuclei. See text for description.
Our results show that nutritional folate deficiency can lead to changes in DNA, increased levels of p53 and p21 proteins, and apoptosis in Friend virus-infected erythroblasts. Partial differentiation of erythroleukemia cells in response to chemical inducers has been associated with p53-independent increases in p21 protein and suggests a role for p21 in erythroid differentiation.21 Our results in Fig 3 are consistent with this differentiation-related function of p21. The late-stage accumulation of p21 protein in folate-deficient erythroblasts, which is most prominent at 44 hours, may be due to altered nucleotide pools,36 increased uracil misincorporation (Fig 2), or relative persistence of undifferentiated cells8 at this time. Alternatively, the increased p21 protein may be due to a p53-dependent mechanism. However, p53-null mice can develop megaloblastic anemia (Koury et al, unpublished data). The p53 accumulation in folate-deficient erythroblasts occurs temporally with the p21 accumulation, which, in turn, may inhibit cell cycle progression. This arrest of cell cycle in folate-deficient erythroblasts may prevent their apoptosis and would be consistent with the previous studies showing an antiapoptotic effect of p21 protein.37
Our in vitro data in Fig 4 indicates that macrocytosis related to folate-deficient erythroblasts is determined during the late stages of erythroblast development. Folate deficiency may lead to increased reticulocyte size by several mechanisms. One is that folate deficiency may select for the erythroblasts that normally produce the largest reticulocytes. If the erythroblast apoptosis of folate deficiency specifically spares the few percent of erythroblasts that normally will produce the largest reticulocytes, then the mean reticulocyte size will be significantly increased. A second mechanism is that folate deficiency inhibits the normal decrease in erythroblast size that occurs during the terminal two divisions of erythropoiesis.33 34 A third mechanism is that, with folate deficiency, the few erythroblasts that do not undergo apoptosis have successfully repaired DNA damage during a p21-mediated delay in cell cycle that, in turn, increases erythroblast size.
A model for the pathophysiology of megaloblastic anemia based on data from this report and our previous studies7,8 is presented in Fig 6. In normal erythropoiesis (upper panel), the number of erythroid progenitor cells increases at each step of differentiation. Normal erythrocyte production results in normal tissue oxygenation and maintains normal erythropoietin levels. Normal erythropoietin levels result in apoptosis of some cells at the CFU-E and proerythroblast stages.38 Apoptotic cells are shown by condensed and fragmented nuclei. In megaloblastic erythropoiesis (lower panel), the CFU-E and proerythroblast population predominate because apoptosis of basophilic and polychromatophilic erythroblasts in response to DNA damage greatly reduces their numbers. Because of this late stage apoptosis, reticulocyte production is decreased. Those few erythroblasts that do complete their differentiation produce reticulocytes that are larger than normal, and the total population of blood erythrocytes eventually develops an increased MCV. The decreased erythrocyte production leads to tissue hypoxia and increased erythropoietin levels. The increased erythropoietin, in turn, expands the populations of its target cells, the CFU-E, and proerythroblasts and thereby further shifts the erythropoietic population toward these earlier stages. This shift in the population toward immature erythropoietic progenitor cells is characteristic of megaloblastic hematopoietic tissue. Although the erythroid lineage is shown in this model, the clinical findings of pancytopenia1-4 and our murine studies showing expansion of myeloid and megakaryocytic progenitor cells in folate-deficient mice7 indicate that nonerythroid hematopoietic lineages are similarly affected during megaloblastic anemia.
ACKNOWLEDGMENT
The authors thank Sarvadaman Rana and Rosalind Holloway for technical assistance and Drs D. Park, S. Brandt, and V. Kravtsov for review of and advice with the manuscript.
Supported by Grant No. 94B80 from the American Institute for Cancer Research (M.J.K.), Merit Review Grants from the Department Of Veterans Affairs (M.J.K. and D.W.H.), NIDDK Grant No. DK-32189 (D.W.H.), National Institutes of Health Institutional Training Grant No. GM07347 (Z.A.B.), Council for Tobbacco Research USA Grant No. 4347 (J.A.P.), National Cancer Institute Outstanding Investigator Grant No. CA-39910 (B.N.A.), NIEHS Center Grant No. ESO-1896 (B.N.A.), and the University of California Toxic Substance Research and Teaching Program (B.C.B. and B.N.A.).
Address reprint requests to Mark J. Koury, MD, 547 MRB II, Vanderbilt University, 2220 Pierce Ave, Nashville, TN 37232-6305.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal