Abstract
Interleukin-13 (IL-13) is a novel T-cell–derived cytokine with IL-4–like effects on many cell types. In human B lymphocytes, IL-13 induces activation, stimulates proliferation in combination with anti-IgM or anti-CD40 antibodies, and directs Ig isotype switching towards IgE and IgG4 isotypes. We show here that IL-13 also regulates human B-cell apoptosis. IL-13 reduced spontaneous apoptosis of peripheral blood B cells in vitro, as shown by measurement of DNA fragmentation using the TUNEL and Nicoletti assays. The inhibition of cell death by IL-13 alone was significant but modest, but was potently enhanced in combination with CD40 ligand (CD40L), a survival stimulus for B cells by itself. Interestingly, IL-13 increased the expression of CD40 on peripheral blood B cells, providing a possible mechanism for the observed synergy. IL-13 alone was a less potent inhibitor of apoptosis than IL-4. Moreover, there was no additive effect of combining IL-4 and IL-13 at supraoptimal concentrations, which is consistent with the notion that the IL-4 and IL-13 binding sites share a common signaling subunit. The combination of IL-13 with CD40L augmented the expression of the Bcl-2 homologues Bcl-xL and Mcl-1, suggesting this as a possible intracellular mechanism of induced survival. By contrast, levels of Bcl-2, and two other Bcl-2 family members, Bax and Bak, remained unaltered. Given the importance of the CD40-CD40L interaction in B-cell responses, these results suggest a significant role of IL-13 in the regulation of B-cell apoptosis.
APOPTOSIS, OR PROGRAMMED cell death, has been shown to play an important role for several stages of B-cell development and maturation. First, the vast majority of B-cell precursors in the bone marrow undergo apoptosis, probably caused by faulty rearrangement of Ig genes.1,2 Second, apoptosis is the mechanism by which self-reactive IgM+/IgD− clones are deleted, thus contributing to the induction of B-cell tolerance.3 Third, widespread programmed cell death of activated B cells occurs in the germinal centers of secondary lymphoid tissue and is instrumental in the selection of B cells expressing antibodies with a high affinity for antigen.4 Recently, we and others have shown that mature resting B lymphocytes also undergo apoptosis, as shown by their propensity to die spontaneously when cultured in vitro.5-7 Furthermore, apoptosis of these cells is regulated. For example, various B-cell growth stimuli, including anti-IgM antibodies and interleukin-4 (IL-4), inhibit cell death, whereas the B-cell growth inhibitors transforming growth factor (TGF) β and cyclic adenosine monophosphate (cAMP)-inducing stimuli accelerate apoptosis.5-7 This suggests that apoptosis could play a regulatory role not only for activated B cells, as previously documented, but for resting B cells as well.
IL-13 is a recently identified cytokine produced by activated T cells.8 IL-13 displays many of the biological activities of IL-4, and recent reports indicate that the IL-13 receptor and IL-4 receptor share a common subunit involved in signal transduction.9,10 IL-13 has IL-4–like effects on human B cells, in which it induces the expression of CD23 and major histocompatibility complex (MHC) class II antigens, stimulates proliferation in combination with anti-IgM and CD40 antibodies, and directs Ig class switching to produce IgE and IgG4.11-13 IL-13 also regulates mast cell function in a parallel way to IL-4.8,14 Thus, like IL-4, IL-13 appears to play a role in the allergic response. This notion is underscored by the finding that IL-13 is highly expressed in asthmatic patients at sites of allergen challenge.15 IL-13 also regulates monocyte morphology, phenotype, function, and cytokine production in a similar way to IL-4.8 However, IL-13 and IL-4 also show distinct differences. Unlike IL-4, IL-13 has no activity on T cells, because these cells lack receptors for IL-13.8,16 Furthermore, IL-4 and IL-13 are produced by different T helper subsets and with different kinetics. Whereas IL-4 is produced mainly by the TH2 subset, IL-13 is secreted by all three subsets, TH0, TH1, and TH2,16 and typically for longer times after stimulation. Finally, the signaling pathways for IL-4 and IL-13 are partly different in that IL-4 activates both Janus kinases JAK1 and JAK3, whereas IL-13 only activates JAK1.17 Thus, IL-13 is a unique cytokine with a distinct role in the regulation of cells of the immune system in vivo.
In the present study, we have investigated the effect of IL-13 on apoptosis of resting B lymphocytes from peripheral blood (PB). Like IL-4, IL-13 inhibited the cell death of PB B cells, although less potently. However, the effect was greatly potentiated by CD40 ligand (CD40L), suggesting that IL-13 may play a significant role in the regulation of B-cell apoptosis in vivo. We also studied the possible involvement of several members of the Bcl-2 family, which are important intracellular regulators of apoptosis. Whereas Bcl-2, Bax, and Bak protein levels remained unchanged, the expression of the survival proteins Mcl-1 and Bcl-xL was markedly upregulated, suggesting a possible mechanistic role for these Bcl-2 homologues in IL-13–mediated inhibition of apoptosis.
MATERIALS AND METHODS
Reagents.Recombinant human (rhu) IL-13 was kindly provided by Dr A. Minty (Sanofi Elf Bio Recherches, Labège, France), and rhuIL-4 was a gift from Dr Paul P. Trotta (Schering-Plough Research, Bloomfield, NJ). Both cytokines were used at 40 ng/mL, unless stated otherwise. Soluble human trimeric CD40L expressed in COS cells was a generous gift of Immunex Corp (Seattle, WA)18 and was used at 1:10 dilution. Alternatively, rhuCD40L (trimeric) from the same source was used; a concentration of 10 ng/mL gave identical results. Antihuman IL-13 was purchased from Pepro Tech Inc (Rocky Hill, NJ). F(ab′)2 fragments of rabbit polyclonal antibodies to human IgM heavy chain (anti-μ) were from Dako (Copenhagen, Denmark). O-tetradecanoylphorbol-13-acetate (TPA) was purchased from Sigma Chemical Co (St Louis, MO). Staphylococcus aureus Cowan I (SAC; Calbiochem-Behring, Cambridge, UK) was used at 1:40,000 dilution.
Purification of B cells from PB.Normal human B cells were isolated from PB buffy coats by positive selection using anti-CD19–coated magnetic beads (Dynabeads M-450 Pan B; Dynal, Oslo, Norway) as previously described.19 Briefly, buffy coats were incubated for 30 minutes at 4°C with Dynabeads using a target-to-bead ratio of 1:10. Rosetted cells were captured using a samarium cobalt magnet and nonrosetted cells were removed by suction. To obtain a pure B-cell population, the cell rosettes were washed seven times. After resuspending rosettes in 100 μL RPMI with 1% fetal bovine serum (FBS), beads were removed from positively selected cells by incubating with 10 U (100 μL) of anti-Fab antiserum (DETACHaBEAD; Dynal) for 45 minutes at room temperature and subsequently placing the suspension on magnet. The resulting B cells are more than 99% pure.19
Cell culture and determination of cell death.PB B cells (1 × 106/mL) were cultured in RPMI 1640 (GIBCO, Grand Island, NY) supplemented with penicillin, streptomycin, and 1% FBS. Viability (or cell death) was measured by vital dye exclusion test using propidium iodide (PI). Cells were stained directly with 5 μg/mL PI (Calbiochem Corp, La Jolla, CA) for 5 to 30 minutes on ice before running at least 5,000 cells per sample on a FACScan flow cytometer (Becton Dickinson, Lincoln Park, NJ) and analyzing the data using Lysys II software (Becton Dickinson). The percentage of apoptotic cells was measured by analyzing forward scatter (FSC) versus side scatter (SCC), as described.20
IL-13 inhibits cell death of normal PB B cells in vitro. (A) PB B cells were cultured in medium or in the presence or absence of rhu IL-4 or rhu IL-13, both at 40 ng/mL. Viability was measured at the indicated time points by PI-exclusion using flow cytometry. Results (mean ± standard error of mean [SEM]) are from five experiments. (B) Cells were cultured for 3 days with IL-13 or IL-4 at the indicated concentrations, and the proportion of dead cells was measured by PI staining (vital dye exclusion). Results are presented as means ± SEM (n = 8). * Significantly different from control values according to the paired Wilcoxon test (P < .05).
IL-13 inhibits cell death of normal PB B cells in vitro. (A) PB B cells were cultured in medium or in the presence or absence of rhu IL-4 or rhu IL-13, both at 40 ng/mL. Viability was measured at the indicated time points by PI-exclusion using flow cytometry. Results (mean ± standard error of mean [SEM]) are from five experiments. (B) Cells were cultured for 3 days with IL-13 or IL-4 at the indicated concentrations, and the proportion of dead cells was measured by PI staining (vital dye exclusion). Results are presented as means ± SEM (n = 8). * Significantly different from control values according to the paired Wilcoxon test (P < .05).
DNA fragmentation.DNA fragmentation was quantitated by terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick end labeling (TUNEL) as described previously.5 21 Briefly, cultured cells (2 × 106) were washed in phosphate-buffered saline (PBS) and fixed in 1% paraformaldehyde in PBS (pH 7.4) for 10 minutes at 4°C. Then, after washing in PBS, cells were resuspended in 100% methanol and stored at −20°C for up to 3 weeks. After rehydration in PBS, cells were incubated for 30 minutes at 37°C in 50 μL of a solution containing 0.2 mol/L potassium cacodylate, 0.1 mmol/L dithiotreitol, 25 mmol/L Tris-HCl, 25 mg/mL bovine serum albumin, 1.5 mmol/L CoCl2, 5 U of TdT, and 0.5 nmol/L biotin-16-dUTP (Boeringer Mannheim Biochemica, Mannheim, Germany). Cells were washed once in PBS, washed twice in PBS plus 0.1% triton X-100, and preincubated for 5 minutes at 4°C in PBS/Triton X-100 containing 5% dried milk. This was followed by 1 hour incubation at 4°C in fluorescein isothiocyanate (FITC)-streptavidin (5 μg/mL) dissolved in the same preincubation solution. Cells were then washed in PBS/Triton X-100 buffer and resuspended in 500 μL of PBS/Triton X-100 containing PI (10 μg/mL) and RNase (100 μg/mL). Green (biotin-dUTP) and red (PI) fluorescence from individual cells were measured with a FACScan flow cytometer. Five thousand cells were collected and doublets were excluded from the analysis by gating on PI fluorescence pulse width versus area.
The percentage of cells displaying DNA fragmentation was also determined by the Nicoletti technique, as previously described.22 Briefly, 1 × 106 cells were fixed in 70% ethanol, washed once in Hanks' buffered salt solution (HBSS), resuspended in 1 mL HBSS and 1 mL phosphate citric acid buffer (0.2 mol/L Na2PO4 and 0.004 mol/L citric acid, pH 7.8), and incubated at room temperature for 5 minutes. After centrifugation, cells were resuspended in 1 mL HBSS containing 20 μg/mL PI and 10 Kunitz units of DNase free RNase A and incubated at room temperature for 30 minutes. Finally, cells were analyzed on a FACScan flow cytometer. Because of the fragmentation of DNA, apoptotic cells appear as hypodiploid in the DNA histogram.
Expression of activation antigens.Cultured B cells were stained with the monoclonal antibodies (MoAbs) BI-3C5 (anti-CD34, isotype-matched control antibody) and G28-5 (anti-CD40), followed by second layer staining with FITC-conjugated goat-antimouse antibodies (Dakopatts, Glostrup, Denmark).
Western blot analysis.A total of 10,000,000 cells were washed twice in PBS, resuspended in 50 μL of sample buffer (10% glycerol, 5% mercaptoethanol, 0.0625 mol/L Tris-HCl (pH 6.8), 2.5% sodium dodecyl sulfate [SDS]), and boiled for 15 minutes. Ten micrograms of total protein from each sample was run on 12% SDS-polyacrylamide gels and blotted onto nitrocellulose filters. The filters were pretreated with TBS containing 0.1% Tween-20 and 5% dry milk and incubated at room temperature overnight in TBS buffer (TBS plus 0.1% Tween-20, 0.5% dry milk, 0.1% FBS) containing 0.1% (vol/vol) anti–Bcl-2 MoAb (Santa Cruz Biotechnology, Heidelberg, Germany), or 0.1% rabbit antiserum against Bax, Bcl-x, Bak (Santa Cruz Biotechnology), or Mcl-123 proteins. After washing, the blots were incubated for 1 hour with 0.033% (vol/vol) goat antimouse IgG (Bcl-2) or 0.025% goat antirabbit IgG (Mcl-1, Bax, Bcl-x, and Bak) antibodies conjugated to horseradish peroxidase (Biorad, Hercules, CA), and the respective proteins were visualized using enhanced chemiluminescence (ECL) Western blotting kit (RPN 2106; Amersham, Buckinghamshire, UK) according to the manufacturer's protocol. To control protein loading of the gels, Western blots were also hybridized with the MoAb 34C1,24 which detects a protein of 83 kD. p83 is distributed diffusely and abundantly throughout the nucleoplasm of all human cells tested, and the expression per cell does not appear to vary with growth rate or tissue origin.24
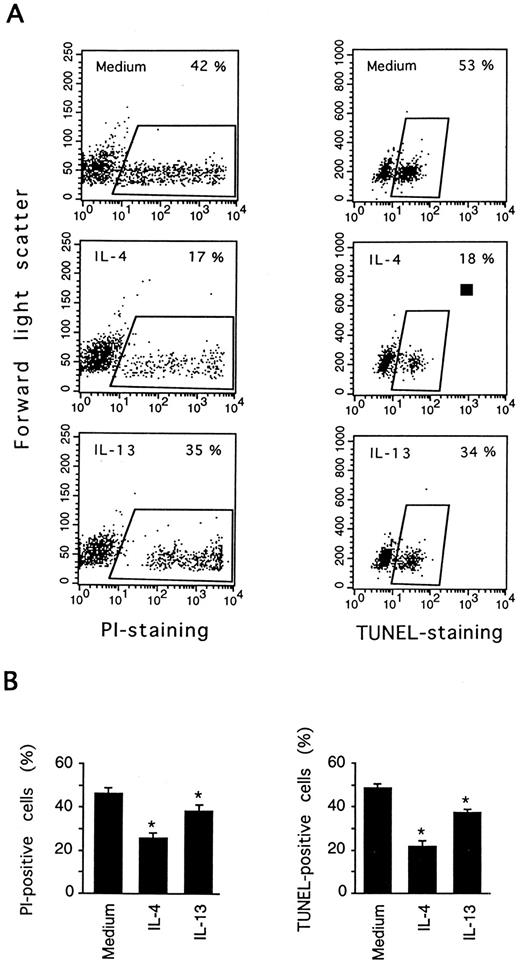
IL-13 inhibits DNA fragmentation in PB B cells. PB B cells were cultured for 3 days in medium, or in the presence or absence of IL-4 or IL-13. Cell death was measured by PI staining and the TUNEL assay. In (A) the results of one representative experiment are shown to indicate how cell death was estimated using the two methods. (B) shows the results (mean ± SEM) of eight (PI staining) and six (TUNEL staining) experiments. *Significantly different from the medium control values according to the paired Wilcoxon test (P < .05).
IL-13 inhibits DNA fragmentation in PB B cells. PB B cells were cultured for 3 days in medium, or in the presence or absence of IL-4 or IL-13. Cell death was measured by PI staining and the TUNEL assay. In (A) the results of one representative experiment are shown to indicate how cell death was estimated using the two methods. (B) shows the results (mean ± SEM) of eight (PI staining) and six (TUNEL staining) experiments. *Significantly different from the medium control values according to the paired Wilcoxon test (P < .05).
RESULTS
IL-13 inhibits apoptosis in human PB B cells.B cells were isolated from human PB as described in Materials and Methods. The resulting cells have a resting phenotype, as judged by several criteria, including a low cellular volume and low expression of activation antigens such as MHC class II, CD23, and CD69.19 We have previously shown that these cells gradually undergo spontaneous cell death when cultured in vitro.5 As shown in Fig 1A, rhu IL-13 (40 ng/mL) delayed cell death throughout an 8-day culture period. The death of ≈50% of the B cells cultured with IL-13 occurred at 4 days after initiation of cultures compared with 3 days for control cells, and ≈15% of the IL-13–treated cells remained viable after 8 days compared with less than 1% of control B cells. The effect on cell death was concentration dependent, with maximum effect observed at 0.4 ng/mL (Fig 1B), and was blocked by an IL-13 neutralizing antiserum (data not shown). Comparisons with rhuIL-4 showed that IL-13 was a less potent viability stimulus for PB B cells. Treatment with IL-4 resulted in 45% viability at day 8 (Fig 1A), with a maximum response obtained in the concentration range of 0.4 to 4 ng/mL (Fig 1B). As shown in Fig 1B, there was no additive effect of combining IL-13 with IL-4 at supraoptimal concentrations (40 ng/mL).
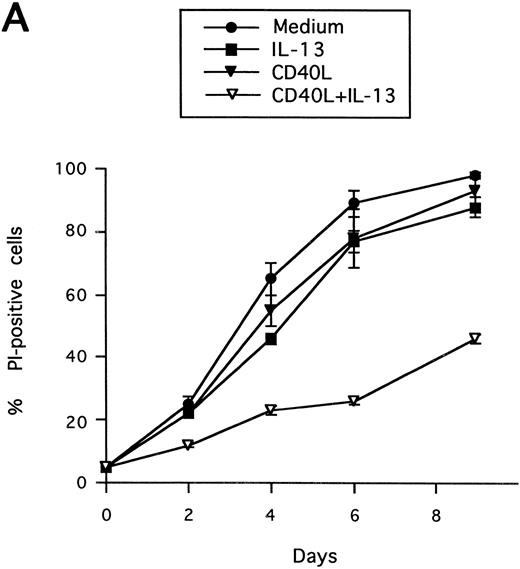
Synergistic effect of IL-13 with CD40L on PB B-cell apoptosis. B cells were cultured with IL-13 (A) or IL-4 (B), alone or in combination with CD40L (1:10 dilution), and cell death was measured by PI staining at the indicated time points. Results are presented as mean ± SEM of three experiments.
Synergistic effect of IL-13 with CD40L on PB B-cell apoptosis. B cells were cultured with IL-13 (A) or IL-4 (B), alone or in combination with CD40L (1:10 dilution), and cell death was measured by PI staining at the indicated time points. Results are presented as mean ± SEM of three experiments.
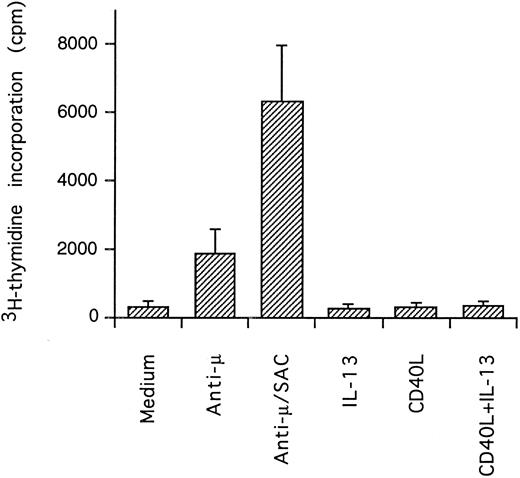
Effect of IL-13 and CD40L on DNA synthesis. PB B cells were cultured in triplicates for 3 days and DNA synthesis was measured by pulsing with 3H-thymidine the last 24 hours of culture (mean ± SEM of 3 separate experiments).
Effect of IL-13 and CD40L on DNA synthesis. PB B cells were cultured in triplicates for 3 days and DNA synthesis was measured by pulsing with 3H-thymidine the last 24 hours of culture (mean ± SEM of 3 separate experiments).
Spontaneous PB B-cell death in vitro occurs by apoptosis, as confirmed previously by showing the characteristic morphological changes and internucleosomal DNA fragmentation.5 To verify that IL-13 influenced apoptosis, cultured cells were examined by DNA nick end labeling (TUNEL-assay) to detect single-strand DNA fragmentation. As shown in Fig 2, IL-13 significantly reduced the percentage of cells with fragmented DNA (TUNEL-positive) from 49% (medium control) to 37% at day 3, whereas IL-4 reduced the proportion of TUNEL-positive cells more markedly (to 22%). In accordance with previous studies, there was a good correlation of PI staining and TUNEL estimates of apoptosis. Cell death was also quantitated by analysis of FSC versus SSC, by exploiting the observation that FSC decreases and SSC increases in apoptotic lymphocytes,20 and similar cell death values were obtained (data not shown).
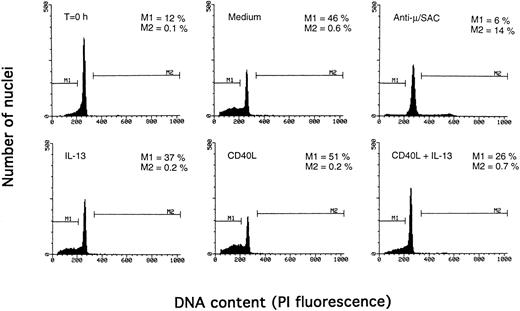
Determination of apoptosis and cell cycle distribution by the Nicoletti technique. PB B cells were cultured with the indicated agents for 3 days, and DNA content was determined by the Nicoletti technique as described in Materials and Methods. The percentage of apoptotic cells in the subdiploid peak is indicated by M1, and the percentage of cells in the S/G2/M phase of the cell cycle by M2. The results are from one representative experiment of three.
Determination of apoptosis and cell cycle distribution by the Nicoletti technique. PB B cells were cultured with the indicated agents for 3 days, and DNA content was determined by the Nicoletti technique as described in Materials and Methods. The percentage of apoptotic cells in the subdiploid peak is indicated by M1, and the percentage of cells in the S/G2/M phase of the cell cycle by M2. The results are from one representative experiment of three.
IL-13 enhances CD40 expression on PB B cells. Cells were cultured for 3 days in medium (thin line) or with IL-4 (thick line) or IL-13 (dotted line), and cells were stained with the anti-CD40 MoAb G28-5 (lower panel) or an irrelevant isotype-matched control MoAb against CD34 (upper panel). The results are from one representative experiment of three.
IL-13 enhances CD40 expression on PB B cells. Cells were cultured for 3 days in medium (thin line) or with IL-4 (thick line) or IL-13 (dotted line), and cells were stained with the anti-CD40 MoAb G28-5 (lower panel) or an irrelevant isotype-matched control MoAb against CD34 (upper panel). The results are from one representative experiment of three.
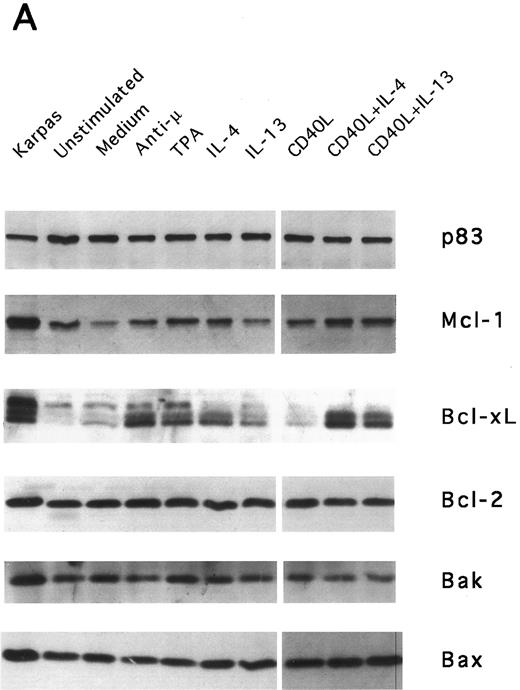
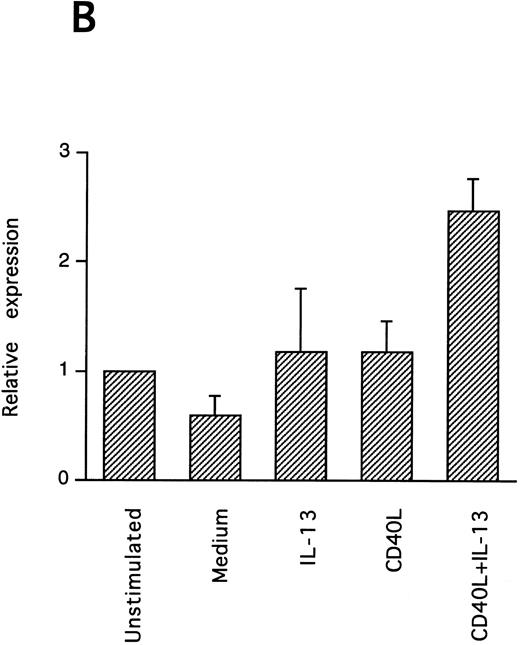
IL-13–induced PB B-cell survival and expression of Bcl-2 family members. PB B cells were cultured in vitro for 48 hours with the indicated stimuli, and relative protein expression of Mcl-1, Bcl-xL, Bcl-2, Bak, and Bax was determined by Western blotting. Each lane was loaded with 10 μg of total protein, and as a control for equal loading the expression of the nuclear antigen p83 was used. For comparison, levels of Bcl-2 homologues in the B-cell line Karpas 422 are shown. The following concentrations were used: anti-μ (37.5 μg/mL), TPA (10 nmol/L), IL-4 (40 ng/mL), IL-13 (40 ng/mL), and rhuCD40L (10 ng/mL). In (A), one representative blot is shown, whereas the expression of Mcl-1 and Bcl-xL relative to expression in unstimulated cells determined by densitometry is shown in (B) and (C), respectively (mean ± SEM from 3 separate experiments).
IL-13–induced PB B-cell survival and expression of Bcl-2 family members. PB B cells were cultured in vitro for 48 hours with the indicated stimuli, and relative protein expression of Mcl-1, Bcl-xL, Bcl-2, Bak, and Bax was determined by Western blotting. Each lane was loaded with 10 μg of total protein, and as a control for equal loading the expression of the nuclear antigen p83 was used. For comparison, levels of Bcl-2 homologues in the B-cell line Karpas 422 are shown. The following concentrations were used: anti-μ (37.5 μg/mL), TPA (10 nmol/L), IL-4 (40 ng/mL), IL-13 (40 ng/mL), and rhuCD40L (10 ng/mL). In (A), one representative blot is shown, whereas the expression of Mcl-1 and Bcl-xL relative to expression in unstimulated cells determined by densitometry is shown in (B) and (C), respectively (mean ± SEM from 3 separate experiments).
Synergistic effect of IL-13 with CD40L on PB B cell apoptosis.Ligation of CD40 with MoAb leads to inhibition of cell death of both germinal center (GC) B cells25 and immature B-cell lines,26 and partly also of human PB B cells.5 Because CD40-stimulating agents and IL-13 are known to cooperate with respect to B-cell proliferation and Ig production, we tested the effect of this combination on B-cell death. Soluble human trimeric CD40L (COS cell supernatant) was used, and by itself this inhibited cell death in a manner similar to the anti-CD40 antibody G28-5 (data not shown). Moreover, using a 1:10 dilution of CD40L, a clear synergy between CD40L and IL-13 was shown (Fig 3A). Thus, the combination of CD40L with IL-13 resulted in a viability of ≈50% at day 9, whereas only ≈10% of the cells were viable when cultured with CD40L or IL-13 alone, respectively. This result was similar to that obtained by stimulating cells with the combination of CD40L and IL-4 (≈60%; Fig 3B). The synergistic effect observed was primarily caused by inhibition of cell death and not induction of cell proliferation, because no concomittant increase in DNA synthesis was seen, neither after 3 days (Fig 4) nor at later time points (data not shown). Although it is possible that some cells may still be slowly proliferating, this population is very small and most likely insignificant. The lack of effect on cell proliferation was not caused by any general unresponsiveness of PB B cells in our system because the B-cell activators anti-μ antibodies and SAC potently stimulated DNA synthesis. TUNEL analysis and evaluation of FSC versus SSC by flow cytometry confirmed that the enhancement of viability was caused by the inhibition of apoptosis (data not shown). This was also verified by the apoptosis-specific Nicoletti assay (Fig 5), which additionally confirmed that CD40L plus IL-13 did not drive the cells into the G1/G2/M phases of the cell cycle. The finding that the combination of IL-13 and CD40L does not induce PB B-cell proliferation is in contrast to previous findings for tonsillar11,12 and splenic B cells.27 However, PB B cells generally require stronger stimuli to proliferate than tonsillar and splenic B cells.28 In addition, two of these studies12,27 used anti-CD40 antibodies or CD40L bound to membranes, which delivers a stronger signal than the corresponding soluble agent.18
To explore the mechanisms behind the observed synergy between CD40L and IL-13, we tested whether IL-13 induced CD40 expression. Although CD40 is abundantly expressed on unstimulated cells, IL-13 significantly increased the expression of this receptor (Fig 6). Again, the effect of IL-13 was less potent than that of IL-4.
Role of the Bcl-2 family in IL-13–induced B-cell survival.Bcl-2 is an antiapoptotic gene that was first isolated from the chromosomal breakpoint t(14; 18) of human follicular B-cell lymphoma.29 Its 26-kD protein product is localized to the mitochondrial, nuclear, and endoplasmic reticulum membranes and inhibits apoptosis by a still unknown mechanism. Recently, several proteins with homology to Bcl-2 have been identified, which share many of the essential characteristics of Bcl-2, such as intracellular localization and the ability to modulate apoptosis. The Bcl-2 family members may be divided functionally into two groups. Bcl-2, Bcl-xL (long splice variant),30 Mcl-1,31 and A132 inhibit apoptosis, whereas Bax,33 Bak,34-36 Bcl-xS (short form),30 Bik,37 and Bad38 promote cell death. A complex pattern of dimerization between the different members has been shown.
Bcl-2 is an important determinant of B-cell life span, as shown in studies with Bcl-2 knockout mice.39 However, when PB B cells are treated with both apoptosis-promoting (cAMP and TGFβ) and apoptosis-inhibitory (IL-4, anti-μ, phorbol ester TPA) stimuli, Bcl-2 protein levels are unaltered, suggesting a Bcl-2–independent mechanism of cell death regulation.5 This is in contrast to germinal center B cells, in which the rescue from apoptosis by survival stimuli in vitro is often, but not always, accompanied by the induction of Bcl-2 expression.4,40 In PB B cells, we have previously shown the possible involvement of Mcl-1 instead of Bcl-2, because Mcl-1 levels rapidly decline in spontaneously dying cells, but are maintained by survival stimuli.41 To determine possible mechanisms of IL-13–induced cell survival, we studied the expression of both Bcl-2 and Mcl-1 in addition to Bcl-x, Bax, and Bak by Western blotting (Fig 7). IL-13 by itself weakly prevented the decline in Mcl-1 levels and together with CD40L gave a marked increase relative to cells cultured in medium, as shown by using densitometry (Fig 7B and C). More strikingly, IL-13 strongly synergized with CD40L with respect to induction of Bcl-x expression. In contrast to Mcl-1 and Bcl-xL, the protein levels of Bcl-2, Bax, and Bak remained unaltered. Bcl-xS could not be detected under any of the treatments.
DISCUSSION
The central role of apoptosis in the regulation of B-cell responses and B-cell homeostasis is now widely acknowledged. One important task is to define the physiologically relevant factors that regulate B-cell apoptosis. In the present study we have investigated the role of the recently discovered cytokine IL-13. We found that IL-13 significantly inhibited cell death in vitro of human B lymphocytes from PB, as measured by PI staining. This was consistent with the suppression of apoptosis, becasue IL-13 also reduced DNA fragmentation estimated with the TUNEL and Nicoletti assays. IL-13 was considerably less potent than IL-4. However, the potency increased markedly in combination with CD40L to almost the same level as IL-4 in combination with CD40L. Because CD40L seems to play a central role in the regulation of B-cell responses and is present in many settings of B-cell life in the periphery,42 IL-13 may therefore play an equally significant part as IL-4 in the control of B-cell survival in vivo. In agreement with our finding, IL-13 has recently been found to inhibit apoptosis of neoplastic B lymphocytes from chronic lymphocytic leukemia patients.43 Whether IL-13 also inhibits apoptosis of other subpopulations of B lymphocytes, such as activated B cells of the germinal center, remains to be determined.
The observation that CD40 expression was upregulated by IL-13 provides a possible mechanism for the observed synergy between CD40L and IL-13. Another possibility, although not tested because of the lack of suitable reagents, is that stimulation with CD40L could lead to an increase in IL-13R expression. Similar to the results on apoptosis, IL-13 had less effect on CD40 expression than IL-4. This difference between IL-4 and IL-13 responses could be consistent with fewer PB B cells expressing IL-13 than IL-4 binding sites, and/or with a lower level of expression per cell of IL-13R than of IL-4R. When combining IL-13 and IL-4 at supraoptimal concentrations, no additive effect on apoptosis was observed, which concurs with the recent finding that the IL-4 and IL-13 binding sites probably share a common subunit involved in signal transduction.9,10 This subunit is not identical to the IL-2Rγc chain, a component of the IL-4R as well as of the IL-7R, IL-9R, and IL-15R.44,45 Rather, new evidence suggests that the IL-13–binding subunit IL-13Rα is a component of a novel IL-4R complex, containing the IL-4Rα chain but lacking the γc subunit.44-46
To explore the intracellular mechanisms of IL-13–induced PB B-cell survival, we also studied the expression of several Bcl-2 family proteins after IL-13 stimulation. The finding that IL-13 in combination with CD40L potently and selectively upregulated the expression of Mcl-1 and Bcl-xL suggests the possible involvement of these Bcl-2 homologues. This capacity of IL-13/CD40L to alter Mcl-1 and Bcl-xL protein levels is shared with several other survival stimuli for PB B cells (anti-μ, IL-4, and TPA), indicating that it may be a general survival mechanism in PB B lymphocytes. Although we have previously reported that Bcl-xL is not appreciably altered by anti-μ, TPA, and IL-4, upregulation of Bcl-xL by these stimuli was consistently observed in the present study, in which we used a new antibody that more sensitively detected Bcl-x (Fig 7A). In agreement with this result, Bcl-xL has recently also been shown to be upregulated in murine peripheral B lymphocytes by both IgM cross-linking, CD40 stimulation, and lipopolysaccharides (LPS).47,48 A role for Bcl-xL in the regulation of mature B-lymphocyte survival is supported by a recent study of Bcl-xL transgenic mice in which the bcl-x gene was expressed in B-lymphoid cells by placing it under the control of the IgH enhancer. In these mice peripheral B cells accumulate in lymphoid organs and B-cell survival in vitro is enhanced.47
Bcl-xL may not only play a role for the survival of resting B cells. The fact that Bcl-xL is highly expressed in germinal centers49,50 and is induced by various activation stimuli47,48 suggests that it plays a role in maintaining the survival also of activated B cells. Paradoxically, such cells are susceptible to apoptosis (activation-induced cell death), and the modulation of Bcl-xL levels could influence the outcome when cells are exposed to Fas ligand or other cell death triggers. In keeping with this, Choi et al48 have shown that CD40/sIg-activated B cells, which have high Bcl-xL levels, are less susceptible to apoptosis induced by the Ca2+ mobilizing drug thapsigargin than unstimulated cells. Bcl-xL seems to play a similar role in activated T cells.51
Mcl-1 is a much less studied Bcl-2 homologue than Bcl-x. The gene was originally cloned by differential screening of cDNA libraries derived from a human myeloid leukemia cell line induced to undergo differentiation by phorbol ester. Studies have shown Mcl-1 overexpression inhibits cell death in yeast and human cell lines, although less efficiently than Bcl-2.31,52,53 Mcl-1 is typically expressed in differentiated cells, as opposed to Bcl-2 which in many tissues is high in undifferentiated cells, suggesting a role in the regulation of cell death during differentiation.54 As indicated by our previous studies,41 and further strengthened by the present findings, Mcl-1 may play a role in the regulation of resting B-cell survival. Interestingly, Mcl-1 displays a similar pattern of expression in follicles as Bcl-xL: high levels in the germinal centers and a low level in the surrounding mantle zone.23 Thus, Mcl-1 may also play a role in the regulation of cell death in activated B cells. Whether or not the upregulation of Mcl-1 and Bcl-xL by IL-13 /CD40L is necessary for the prevention of PB B-cell apoptosis, must be determined by a knockout strategy directed against these proteins, eg, using antisense oligonucleotides or ribozymes.
In summary, this study has shown that IL-13, in the context of CD40L, is a potent inhibitor of B-cell apoptosis in vitro. Thus, IL-13 may regulate normal B-cell responses not only by promoting proliferation and differentiation, but also by preventing apoptosis.
ACKNOWLEDGMENT
We thank Dr Adrian Minty for providing rhuIL-13 and Immunex Corp for the gift of rhuCD40L.
Supported by The Norwegian Cancer Society and National Institutes of Health Grants No. NCI-CA60181 and CA69381.
Address reprint requests to Jon Lømo, MD, Department of Immunology, Institute for Cancer Research, The Norwegian Radium Hospital, N-0310 Oslo, Norway.

![Fig. 1. IL-13 inhibits cell death of normal PB B cells in vitro. (A) PB B cells were cultured in medium or in the presence or absence of rhu IL-4 or rhu IL-13, both at 40 ng/mL. Viability was measured at the indicated time points by PI-exclusion using flow cytometry. Results (mean ± standard error of mean [SEM]) are from five experiments. (B) Cells were cultured for 3 days with IL-13 or IL-4 at the indicated concentrations, and the proportion of dead cells was measured by PI staining (vital dye exclusion). Results are presented as means ± SEM (n = 8). * Significantly different from control values according to the paired Wilcoxon test (P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/12/10.1182_blood.v89.12.4415/3/m_bl_0012f1a.jpeg?Expires=1763462996&Signature=WXd1j-lT~m4o0oxOh-yoIccRX2qK6CVy26q2bxZiym5QF87TVFZnv8QXuokFdnRfjxQe-FNN6RN0p7QjycoU31zDs9SrrcbS1L3EJ3rloFttJ7IwZM54fyrBMyLCcGe18iphREkqDQNNzdQ7CqnKFTblGEq817JC2O0Kdlr6Lq~zLpzybkhnwqUk-ARTxPyLQm9eQQDvhFqsBKV6YfnveCaKLsRCL1ly8qWl23A-8jRXQS1tRxto6gd-y0qbtmtItD-pyqCVzBfpCpUFHolHkAwSDOq7m~7DtFgbS7rvEEckUd1kWlz1mi7fmtogddvXkoX1L8bkDhtwmu9b4K5R~Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. IL-13 inhibits cell death of normal PB B cells in vitro. (A) PB B cells were cultured in medium or in the presence or absence of rhu IL-4 or rhu IL-13, both at 40 ng/mL. Viability was measured at the indicated time points by PI-exclusion using flow cytometry. Results (mean ± standard error of mean [SEM]) are from five experiments. (B) Cells were cultured for 3 days with IL-13 or IL-4 at the indicated concentrations, and the proportion of dead cells was measured by PI staining (vital dye exclusion). Results are presented as means ± SEM (n = 8). * Significantly different from control values according to the paired Wilcoxon test (P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/12/10.1182_blood.v89.12.4415/3/m_bl_0012f1b.jpeg?Expires=1763462996&Signature=HibwdkemdUdJx9B3mvKfjXxxkVk6ao3Ehbb~qV-oB56lH2ZgQueP2z7oIBwL~RPFnTXY7855I4vNXFRkfNduDZz6sMfzFbqPZcz3d3ZTHkGjoAPI4ERlymhSl8Mg3D3sVTf1sbQS8OFNXdVUw2W8yK-rQXMrL72i-WXMeI3UfmlI3428UX2QPIS6yQdFsr52rWhLl8jsLbSt~0lo-tKfIqS3xDIuLz8Bl1BnZB-LCugF2ROq06dj4M2czu~iLNNPrcC18cMJGAbp69JYzyghKHFBLEmlV2xKJ4VK2-da423NatcPmyoM~Jj9gsRbdpkl9XT4poh1CjoEr75bSPkJew__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal