Abstract
Based on initial observations of human CD34+ Thy-1+ cells and long-term culture-initiating cells (LTC-IC) in the bone marrow of some sublethally irradiated severe combined immunodeficient (SCID) mice transplanted intravenously with normal human marrow cells, and the subsequent finding that the NOD/LtSz-scid/scid (NOD/SCID) mouse supports higher levels of human cell engraftment, we undertook a series of time course experiments to examine posttransplant changes in the number, tissue distribution, cycling activity, and in vivo differentiation pattern of various human hematopoietic progenitor cell populations in this latter mouse model. These studies showed typical rapid posttransplant recovery curves for human CD34− CD19+ (B-lineage) cells, CD34+ granulopoietic, erythroid, and multilineage colony-forming cells (CFC), LTC-IC, and CD34+ Thy-1+ cells from a small initial population representing <0.1% of the original transplant. The most primitive human cell populations reached maximum values at 5 weeks posttransplant, after which they declined. More mature cell types peaked after another 5 weeks and then declined. A 2-week course of thrice weekly injections of human Steel factor, interleukin (IL)-3, granulocyte-macrophage colony-stimulating factor (GM-CSF), and erythropoietin (administered just before the mice were killed for analysis) did not alter the pace of regeneration of either primitive or mature human hematopoietic cells, or their predominantly granulopoietic and B-lymphoid pattern of differentiation, although a significant enhancing effect on the level of human cell engraftment sustained after 3 months was noted. Cycling studies showed the human CFC present at 4 to 5 weeks posttransplant to be rapidly proliferating even in mice not given human growth factors. However, by 10 weeks and thereafter, only quiescent human CFC were detected; interestingly, even in mice that were given the 2-week course of growth factor injections. These studies indicate the use of this model for future analysis of the properties and in vivo regulation of primitive human hematopoietic cells that possess in vivo repopulating ability.
THROUGHOUT ADULT LIFE, the bone marrow maintains a population of primitive hematopoietic cells that can permanently regenerate the entire blood-forming system. In mice, this activity is routinely evaluated by transplanting cells into hematologically compromised (usually myeloablated), syngeneic recipients, and this has formed the basis of procedures for quantitating1 and characterizing murine hematopoietic stem cells.2-5 In humans, analysis of the size and composition of clonal populations occasionally seen in recipients of normal marrow allografts has shown that they also contain transplantable totipotent stem cells.6 However, progress in characterizing these human cells and their regulation has been limited by a paucity of models suitable for in vivo experiments. Recently, a number of groups have shown that it may be possible to address this problem through the use of certain xenogeneic recipients that are either ontologically7 or genetically8-11 immunodeficient. In recent studies with transplants of normal human hematopoietic cells, we showed that multilineage human hematopoiesis could be reproducibly obtained in CB17/SCID mice given a near lethal dose of irradiation followed by an intravenous injection of either large numbers of normal human bone marrow cells and subsequent injections of various human hematopoietic growth factors,12 or similar numbers of human cord blood cells without subsequent growth factor administration.13 These studies indicated that at least some human hematopoietic cells can home directly into and be maintained in the microenvironment of the murine bone marrow without the need for specific interactions with stromal cells of nonhematologic human origin (or their products).
Several lines of evidence have suggested that the human hematopoiesis obtainable in the marrow of CB17/SCID mice is due to the reconstituting activity of a minor subpopulation of primitive human cells present in the original transplant (reviewed in Dick14 ). These include the demonstration in the marrow, in occasional mice, of cells with multilineage colony-forming potential in vitro, as well as multiple lineage-restricted and more mature cell types, including those belonging to the erythroid, granulopoietic, and B lymphoid pathways.12,13,15 In addition, it was found that injections of human growth factors enhanced the subsequent production of human hematopoietic cells regardless of whether the growth factor injections were started immediately posttransplant or 3 months later.12 More recently, the gene marking studies of Nolta et al,16 using beige-nude-X-linked immunodeficiency (bg, nu, xid) mice as recipients, have provided definitive evidence that human hematopoietic cells with long-term lymphomyeloid reconstituting potential can engraft murine bone marrow and others reported that a purified subpopulation of CD34+ Thy-1+ Lin− human cells regenerated multilineage hematopoiesis in a fetal sheep.17 However, all of these models share the disadvantage of a low level of engraftment. This has necessitated the injection of input innocula that appear large relative to historical experience with syngeneic mouse transplants1 and has limited systematic studies of human cells with in vivo reconstituting potential.
The present study was originally initiated to determine whether other types of cells with features of very primitive human hematopoietic cells could be found in SCID mice transplanted with normal adult human marrow. During the course of subsequent experiments, in which we were evaluating additional strains of immunodeficient mice as potentially superior hosts, we discovered that much better results could be reproducibly obtained when NOD/LtSz-scid/scid (NOD/SCID) mice were used as recipients.15 A similar finding was also reported recently by Lowry et al18 and Pflumio et al.19 In NOD/SCID mice, the DNA repair gene defect of SCID mice that severely impairs B- and T-cell development20 has been combined with the reduced natural killer cell activity, absence of complement activity and the defect in macrophage function of the NOD mouse.21 As a further test of the use of the NOD/SCID model, we have now compared the regeneration kinetics of various types of human hematopoietic cells subsequently detected in the marrow, spleen, and blood. In addition, we included in these time course studies an analysis of the cell cycle status of the different classes of human myeloid colony-forming cells (CFC) present and evaluated the effect of a 2-week course of human growth factor injections on both the numbers of human cells produced and the cycling activity of the human CFC populations.
MATERIALS AND METHODS
Human cells.Human bone marrow cells were either aspirate samples obtained from normal individuals donating marrow for clinical allogeneic transplantation or were samples of cryopreserved cadaveric marrow obtained from the Northwest Tissue Center (Seattle, WA). In both cases, approved institutional procedures involving written informed consent from each patient or parent were followed. Normal blood was obtained from normal laboratory volunteers. Fresh or thawed cells were centrifuged on Ficoll/Hypaque (Pharmacia, Piscataway, NJ) and the low-density (<1.007 g/mL) cells recovered were then washed twice in Iscove′s medium containing 10% fetal calf serum (FCS; StemCell Technologies Inc, Vancouver, British Columbia, Canada).
Animals.NOD/LtSz-scid/scid (NOD/SCID)21; CB17-scid/scid (CB17/SCID)22; CB17-scid/scid, beige/beige (BEIGE/SCID),23 and RAG-2 knockout (RAG-2 −/−)24 25 mice were bred and maintained in the animal facilities of either the Ontario Cancer Institute (Toronto, Ontario, Canada) and/or the British Columbia Cancer Agency (Vancouver, British Columbia, Canada) from breeding pairs originally from The Jackson Laboratory (Bar Harbor, ME), M. Bosma at the Institute for Cancer Research at the Fox Chase Cancer Center (Philadelphia, PA), D. Mosier, now at The Scripps Clinic and Research Institute (La Jolla, CA), and GenPharm/Taconic (Germantown, NY), respectively. All animals were handled under sterile conditions and maintained under microisolators. Mice to be transplanted were irradiated at 6 to 8 weeks of age with 350 to 400 cGy of total body irradiation from a 137Cs source unless otherwise indicated, and then within 24 hours were given a single intravenous injection of light density normal human bone marrow cells. Some mice were also given intraperitoneal injections of various human recombinant growth factors, as indicated, every other day or three times per week at the following doses: human erythropoietin (Ep; from Ortho BioTech [Don Mills, Ontario, Canada] or StemCell) at 10 or 20 U/mouse/injection, PIXY321 (Immunex Corp, Seattle, WA) at 7 μg/mouse/injection, interleukin-3 (IL-3) and granulocyte-macrophage-CSF (GM-CSF; both from Sandoz International, Basel, Switzerland) and injected at 6 μg each/mouse/injection, and Steel factor (SF; from Immunex [Seattle, WA] or Amgen Corp [Thousand Oaks, CA]) at 10 μg/mouse/injection.
Flow cytometric detection of human cells in murine tissues.Bone marrow cells were flushed from the femurs and tibias of each mouse to be assessed using a syringe and a 26-gauge needle. In a few experiments, marrow cells were similarly removed from the humeri and fibulae. Spleen cells were squeezed out of each end of the splenic capsule into a small volume of medium by applying gentle pressure to the spleen with a curved scissors. Single cell suspensions were then prepared by gentle aspiration. In some cases, aliquots of cells obtained from mice injected and killed in Toronto were suspended in medium containing 10% FCS and then placed in sterile Eppendorf tubes for shipment by overnight courier to Vancouver for analysis the next day. To prepare cells for flow cytometry, contaminating red blood cells were lyzed with 8.3% ammonium chloride and the remaining cells were then washed in Hanks HEPES Buffered Salt solution with 2% FCS, 0.1% sodium azide (HFN), and 5% human serum (to block Fc receptors). The cells were then resuspended at 1 to 2 × 107 cells/mL in medium conditioned by the 2.4 G2 hybridoma, which secretes an antimouse IgG Fc receptor monoclonal antibody (MoAb).26 Cells were then incubated with a MoAb specific for human CD34 (8G12)27 directly labeled with fluorescein isothiocyanate (FITC) at a concentration of 5 μg/mL for 30 minutes at 4°C and, as required, also with an antihuman-Thy–1 MoAb (5E10)28 directly labeled with phycoerythrin (PE) at a concentration of 5 μg/mL to allow assessment of the human CD34+ Thy-1+ population. The total population of human hematopoietic cells was assessed by staining a separate aliquot of each cell suspension with a combination of antihuman-CD45–FITC (HLel, Becton Dickinson, San Jose, CA) and antihuman-CD71–FITC (OKT9). Positive cells are referred to as (CD45/71)+. These cells were simultaneously stained with antihuman-CD34–PE for quantitation of the total human CD34+ population and to allow the isolation of an exclusively human CD34+ cell population for plating in CFC and LTC-IC assays. In some mice, additional aliquots were stained with antihuman-CD19–PE in combination with antihuman-CD34–Cy5 to allow discrimination of an exclusively B-lineage (CD34− CD19+) population.29 Some cells from each suspension were similarly incubated with irrelevant (control) MoAbs labeled with FITC and PE. Cells from a normal CB17/SCID or NOD/SCID mouse were also stained with each of the MoAbs used for detecting positively-stained human cells. Only levels of fluorescence which excluded ≥99.9% of all of these negative controls were considered as specific. After staining, all cells were washed once in HFN alone and then once again in HFN containing 2 μg/mL propidium iodide (PI) to allow dead (PI+) cells to be excluded from both analyses and sorting procedures.
Cells were analyzed and sorted on a FACStar Plus (Becton Dickinson) equipped with 5 W argon and 30 MW helium neon lasers. Specific fluorescence of FITC and PE excited at 488 nm (0.4 W) and 633 nm (30 mW), respectively, as well as known forward and orthogonal light scattering properties of normal human marrow cells were used to establish gates. Data acquisition and analysis were performed using LYSIS II software (Becton Dickinson). For each analysis, a total of 5,000 viable (PI−) cells were assessed and only when ≥5 positive events were recorded were the values used to calculate a number of human cells of a given phenotype present.
Hematopoietic cell cultures.Assays for granulopoietic, erythroid, and multilineage (granulocyte-erythroid–macrophage-megakaryocyte) colony-forming units (CFU-GM, BFU-E, and CFU-GEMM, respectively) were usually performed by plating cells in FCS-containing (30%) methylcellulose media (Stem Cell), to which 3 U/mL Ep (Stem Cell), 50 ng/mL SF (Amgen, Thousand Oaks, CA) and 20 ng/mL each of IL-3, GM-CSF (both from Sandoz), G-CSF (Stem Cell) and IL-6 (Cangene, Mississauga, Ontario, Canada) were added as a source of growth factors. On a few occasions, when CD34+ human cells could not be isolated from the mouse cell suspensions being evaluated (for either practical or quantitative reasons), half of the FCS in the methylcellulose medium was replaced with an equivalent volume of a pretested pool of equivalently supportive normal human serum and the G-CSF and IL-6 were omitted to minimize the stimulation of murine clonogenic cells.12 The different categories of human colonies known to be generated under either of these conditions were scored in situ after 2 to 3 weeks of incubation at 37°C using well established criteria.
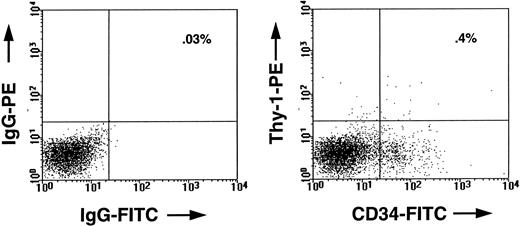
FACS profile of marrow cells from a CB17/SCID mouse transplanted 5 weeks previously with 2 × 107 human marrow cells. In the left panel, the cells were stained with an irrelevant mouse IgG isotope control antibody. In the right panel, the cells were stained with antihuman-CD34–FITC (8G12-FITC) and antihuman-Thy–1-PE (5E10-PE). The human CD34+ Thy-1+ cells seen in the right upper quadrant of this panel comprised approximately 0.4% of all the viable cells in the marrow of that mouse, whereas the total population of human CD34+ cells comprised about 8% of the cells.
FACS profile of marrow cells from a CB17/SCID mouse transplanted 5 weeks previously with 2 × 107 human marrow cells. In the left panel, the cells were stained with an irrelevant mouse IgG isotope control antibody. In the right panel, the cells were stained with antihuman-CD34–FITC (8G12-FITC) and antihuman-Thy–1-PE (5E10-PE). The human CD34+ Thy-1+ cells seen in the right upper quadrant of this panel comprised approximately 0.4% of all the viable cells in the marrow of that mouse, whereas the total population of human CD34+ cells comprised about 8% of the cells.
LTC-IC assays were initiated by suspending initial or sorted CD34+ human cells in myeloid LTC medium (Myelocult, Stem Cell) supplemented with 10−6 mol/L freshly dissolved hydrocortisone (Sigma Chemicals, St Louis, MO) and then seeding the cells onto preformed “feeder” layers consisting of a 1:1 mixture of irradiated M210B4 and S1/S1 mouse fibroblast cell lines, which had been genetically engineered to produce human SF (4 ng/mL), G-CSF (190 ng/mL), and IL-3 (4 ng/mL).30 These LTC-IC assay cultures were then maintained at 37°C for 6 weeks with weekly half media changes, at the end of which, all cells were harvested31 and appropriate aliquots plated for CFC determinations. LTC-IC numbers were calculated from the total CFC numbers detectable in these cultures after 6 weeks assuming that on average, each human LTC-IC produces 18 CFC detectable after 6 weeks using these LTC-IC assay conditions.30 In cases where no CD34+ cells were detected, LTC-IC values were assumed to be zero.
3H-thymidine (3H-Tdr) suicide assay.The cell cycle status of CFC in the CD34+ populations isolated from the bone marrow of transplanted mice was determined as described in detail previously.32 Briefly, aliquots of the cells to be tested were first incubated in serum-free Iscove′s medium for 20 minutes in each of two tubes, one with and one without 20 μCi/mL of high specific activity 3H-Tdr. The cells were then washed and plated in the same methylcellulose media described above to detect surviving CFC. The proportion of CFC in S-phase was then calculated from the difference in colony yields from the two cell suspensions.32 Because the test cell populations were always first selected on the basis of their reactivity with an antihuman CD34 antibody and in some cases also had to be shipped from Toronto to Vancouver for analysis, all cell cycle studies were performed on cells that had been incubated at 4°C overnight after being removed from the mice. To test whether this would affect the results obtained, a series of control experiments were performed. For these, freshly isolated light density normal human blood cells were mixed 1:1 with mouse bone marrow cells and then incubated at 4°C for 24 hours before being sorted and assayed as for test samples. These experiments showed that this had no effect on the cycling status of the human CFC (data not shown) which, in normal blood, are a totally quiescent population.33
DNA extraction and analysis.High molecular weight DNA was isolated from the bone marrow of transplanted mice, EcoRI digests of genomic DNA (1 μg) loaded into each lane and the blots hybridized with a human chromosome 17-specific α-satellite probe (p17H8) as previously described.12 The proportion of human cells in each sample was then inferred from the intensity of the characteristic 2.7 kb band obtained relative to those obtained on the same blot from a series of artificial mixtures of human and mouse DNA (ranging from 0.1% to 50% human DNA).
RESULTS
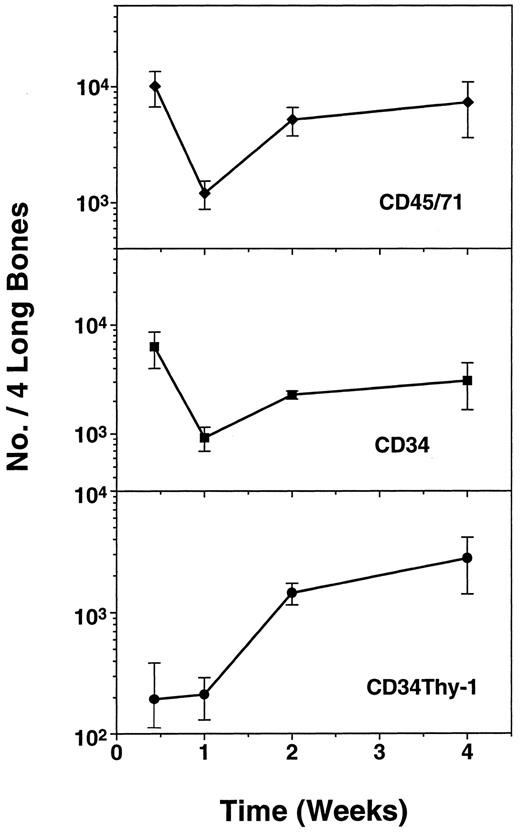
Demonstration of human CD34+ Thy-1+cells and LTC-IC in CB17/SCID mice and the lack of effect of exogenous human growth factor administration on their numbers.In an initial series of experiments, we looked for the presence of very primitive subpopulations of human hematopoietic cells in CB17/SCID mice that had been injected with 2 to 4 × 107 human marrow cells. In experiments with marrow from four different normal individuals, low numbers of CD34+ Thy-1+ human cells (Fig 1) were evident in the marrow of six of nine CB17/SCID mice examined between 4 and 7 weeks posttransplant. CD34+ LTC-IC were also detected, but even less frequently. Time course studies showed that very few of the injected cells (<1% of all (CD45/CD71)+ cells) were detectable in the marrow of the CB17/SCID mice 2 days posttransplant and that plateau numbers of all types monitored appeared to be reached within 2 weeks (Fig 2). In addition, it can be seen that the proportion of CD34+ cells in these mice was disproportionately high, consistent with the existence in these mice of suboptimal conditions for the support of later stages of human hematopoiesis.12
Kinetics of recovery of human (CD45/71)+, CD34+, and CD34+ Thy-1+ cell populations in CB17/SCID mice between 2 days and 2 weeks after transplantation of 4 × 107 normal light density human bone marrow cells. Values shown represent the mean ± standard error of mean (SEM) of data pooled from two experiments in each of which a group of mice were injected with marrow from a different human donor and then one to three animals per experiment killed for individual analysis at the time points shown.
Kinetics of recovery of human (CD45/71)+, CD34+, and CD34+ Thy-1+ cell populations in CB17/SCID mice between 2 days and 2 weeks after transplantation of 4 × 107 normal light density human bone marrow cells. Values shown represent the mean ± standard error of mean (SEM) of data pooled from two experiments in each of which a group of mice were injected with marrow from a different human donor and then one to three animals per experiment killed for individual analysis at the time points shown.
To determine whether repeated exogenous administration of various human growth factors could improve the number of human CD34+ Thy-1+ cells or LTC-IC obtained in these mice, in the two experiments in which 4 × 107 human marrow cells were transplanted, additional CB17/SCID mice were injected with either PIXY321 (7 μg/mouse) and human Ep (20 U/mouse), or human SF (10 μg/mouse), every other day until the mice were killed. However, there was no evidence of a consistent effect of these growth factors on any compartment of human hematopoietic cells evaluated including CD34+ Thy-1+ cells and LTC-IC. Representative results from one experiment are illustrated in Fig 3. In this case, Southern analysis was used to compare the proportion of human cells (DNA) present in the marrow of four mice, one of which had been given PIXY321 plus Ep and the other three, no growth factors.
Lack of effect of injections of PIXY321 and Ep (7 μg and 20 U/mouse, respectively, every other day until killed) on the level of human cell engraftment obtained in the marrow of CB17/SCID mice 5 to 6 weeks after the transplantation of 4 × 107 human marrow cells, as assessed by Southern analysis using a human chromosome 17-specific α-satellite probe. Human:mouse DNA controls are given as % human DNA.
Lack of effect of injections of PIXY321 and Ep (7 μg and 20 U/mouse, respectively, every other day until killed) on the level of human cell engraftment obtained in the marrow of CB17/SCID mice 5 to 6 weeks after the transplantation of 4 × 107 human marrow cells, as assessed by Southern analysis using a human chromosome 17-specific α-satellite probe. Human:mouse DNA controls are given as % human DNA.
Comparison of the extent of human cell engraftment in various strains of immunodeficient mice.In another series of experiments, we compared the ability of CB17/SCID mice to support human hematopoiesis with that of three other strains of immunodeficient mice (BEIGE/SCID, RAG-2 −/−, and NOD/SCID). The BEIGE/SCID and NOD/SCID mice were given 350 to 400 cGy and the RAG-2 −/− mice, a radiobiologically approximately equivalent dose of 1,200 cGy at a low dose rate of 1.5 cGy/minute or 700 to 800 cGy at a dose rate of 100 cGy/minute. A total of 1 to 4 × 107 human marrow cells per mouse were then transplanted into these different strains and the total number of human cells present in the marrow 4 to 10 weeks later assessed either by DNA analysis or flow cytometry. In the four strains compared, more consistent and higher numbers of human cells were routinely detected in the NOD/SCID recipients confirming previous preliminary findings.15 At no time was evidence of engraftment by human cells seen in either the BEIGE/SCID or the RAG-2 −/− mice (Table 1).
Comparison of the Abilities of Different Strains of Sublethally Irradiated Immunodeficient Mice to be Engrafted With Human Marrow Cells
| Recipient Genotype . | No. of Human Cells Injected (per mouse) . | Dose of Radiation Given to Recipients . | Method Used to Detect Engraftment . | No. of Positive Mice/No. of Mice Transplanted (no. of experiments) . |
|---|---|---|---|---|
| NOD/SCID | 107 | 360 | FACS* | 10/10 (4) |
| 2 × 107 | 400 | Southern† | 11/11 (3) | |
| CB17/SCID | 107 | 360 | FACS | 0/10 (4) |
| 2 × 107 | 400 | Southern | 2/3 (1) | |
| BEIGE/SCID | 107 | 360 | FACS | 0/7 (3) |
| RAG-2 −/− | 107 | 1200 | FACS | 0/10 (4) |
| (2-4) × 107 | 700-800 | Southern | 0/5 (2) |
| Recipient Genotype . | No. of Human Cells Injected (per mouse) . | Dose of Radiation Given to Recipients . | Method Used to Detect Engraftment . | No. of Positive Mice/No. of Mice Transplanted (no. of experiments) . |
|---|---|---|---|---|
| NOD/SCID | 107 | 360 | FACS* | 10/10 (4) |
| 2 × 107 | 400 | Southern† | 11/11 (3) | |
| CB17/SCID | 107 | 360 | FACS | 0/10 (4) |
| 2 × 107 | 400 | Southern | 2/3 (1) | |
| BEIGE/SCID | 107 | 360 | FACS | 0/7 (3) |
| RAG-2 −/− | 107 | 1200 | FACS | 0/10 (4) |
| (2-4) × 107 | 700-800 | Southern | 0/5 (2) |
Positivity = >1% of viable cells detected in the recipient BM at either 7 or 10 weeks posttransplant were human CD45+ and/or human CD71+.
Positivity = >0.1% human DNA as indicated by probing the Southern blot with a human-specific α-satellite probe.
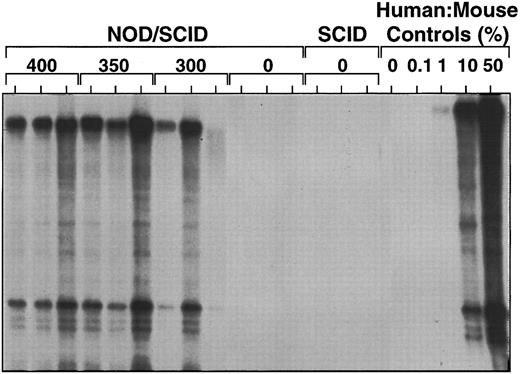
Pretreatment with radiation is required to maximize engraftment of NOD/SCID mice by human marrow cells.We next evaluated the dependence of human cell engraftment of NOD/SCID mice on the dose of irradiation given to the mice. As shown in Fig 4, maximal human cell engraftment was detected in the marrow of mice given between 375 and 400 cGy and no human cells were detectable (within the limits of sensitivity of the procedures used here) in mice that were not irradiated.
Effect of irradiation dose (indicated in cGy) on the level of human cell engraftment seen in the marrow of NOD/SCID or SCID mice transplanted with 2 × 107 human marrow cells and analyzed 5 weeks later by Southern blot analysis using a human chromosome 17-specific α-satellite probe.
Effect of irradiation dose (indicated in cGy) on the level of human cell engraftment seen in the marrow of NOD/SCID or SCID mice transplanted with 2 × 107 human marrow cells and analyzed 5 weeks later by Southern blot analysis using a human chromosome 17-specific α-satellite probe.
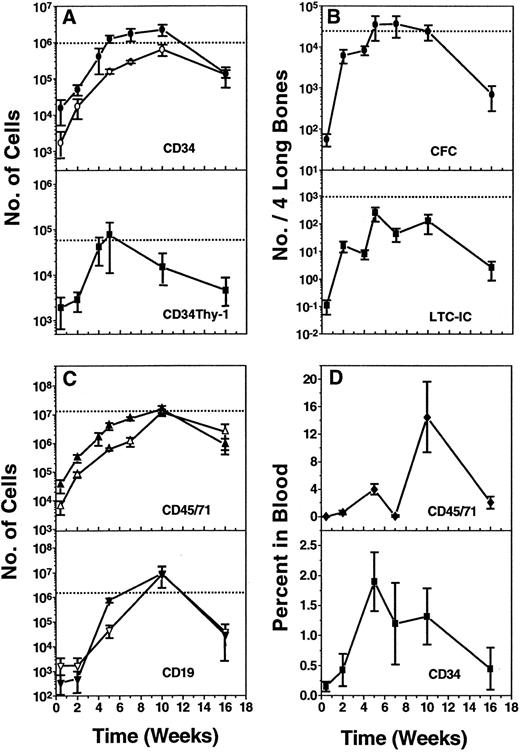
Similar regeneration kinetics of different populations of human hematopoietic cell types in the marrow and spleen of NOD/SCID mice transplanted with normal human marrow.A detailed time course study of the rate and pattern of engraftment of NOD/SCID mice transplanted with a fixed innoculum of 2 × 107 human marrow cells was then undertaken. Figure 5A to C show the pooled results from eight experiments (eight groups of individually analyzed mice, each group transplanted with cells from a different human marrow donor) in which the kinetics of regeneration of the total population of human hematopoietic cells (ie, expressing CD45 and/or CD71, Fig 5C, top) was monitored as well as the total population of human CD34+ cells and various phenotypically and functionally defined subpopulations of human CD34+ cells (ie, total CFC, LTC-IC, and CD34+ Thy-1+ cells). Human CD34−CD19+ (B-lineage) cells were also evaluated, but in a single experiment only. The solid symbols in Fig 5A to C delineate the kinetics of repopulation seen in the marrow. The open symbols show the corresponding results for the spleen, which were determined in four of the same experiments. As seen previously in the time course studies of CB17/SCID repopulation by human cells (Fig 2), <2% of any of the cell types injected could be found in either of the two hind legs (two femurs + two tibias) or in the spleen of the NOD/SCID mice when they were analyzed 2 days posttransplant and in total, these represented <1% of the original transplant (Fig 5C top). After this time, all populations increased rapidly and in parallel. However, in contrast to the results obtained in the CB17/SCID recipients (Fig 2), the numbers of human cells present in the NOD/SCID mice did not plateau after 2 weeks, but rather continued to increase rapidly for another 2 to 3 weeks. Maximum numbers of the most primitive human cell types monitored (CD34 + Thy-1+ cells, CFC, and LTC-IC) were seen at this time (ie, 5 weeks posttransplant). The more mature populations of human hematopoietic cells (total CD34+ cells, total (CD45/71)+ cells and CD34−CD19+ cells continued to increase for another 5 weeks to reach peak values at 10 weeks posttransplant in the marrow and the spleen alike. For each of these cell types, the numbers ultimately attained in the marrow of the two hind legs (which represents ≈ 25% of the total marrow volume in mice34 ) were ≈ 102 to 103 higher than the day 2 values and (except in the case of LTC-IC) also exceeded the number of cells of the corresponding type in the original transplant. By 16 weeks posttransplant (when these experiments were terminated), all populations of human cells had declined significantly. Nevertheless, because of the high levels of human hematopoiesis regenerated within the first 2 1/2 months posttransplant, most types of primitive cells could usually still be detected after 4 months.
Kinetics of regeneration and maintenance of different populations of human cells in NOD/SCID mice between 2 days and 16 weeks after transplantation of 2 × 107 light density human marrow cells. (A and C) show the numbers of human cells (expressing the antigens indicated) that were found to be present in the marrow of two femurs and two tibias (solid symbols) or in the spleen (open symbols). In (C), CD19 refers to cells identified as CD34−CD19+ (most of the CD19+ cells seen were typically CD34−). (B) shows the numbers of human CFC and LTC-IC present in the two femurs and two tibias of the same mice, and (D) shows the percent of all nucleated cells in the blood of these mice that were human CD34+ or human (CD45/CD71)+ cells. In (A to C), the dotted line shows the number of cells of the type evaluated that were present in the original innoculum injected. Values shown are the mean ± SEM of results pooled from individually assessed animals (two to three animals per experiment) killed at the time points shown.
Kinetics of regeneration and maintenance of different populations of human cells in NOD/SCID mice between 2 days and 16 weeks after transplantation of 2 × 107 light density human marrow cells. (A and C) show the numbers of human cells (expressing the antigens indicated) that were found to be present in the marrow of two femurs and two tibias (solid symbols) or in the spleen (open symbols). In (C), CD19 refers to cells identified as CD34−CD19+ (most of the CD19+ cells seen were typically CD34−). (B) shows the numbers of human CFC and LTC-IC present in the two femurs and two tibias of the same mice, and (D) shows the percent of all nucleated cells in the blood of these mice that were human CD34+ or human (CD45/CD71)+ cells. In (A to C), the dotted line shows the number of cells of the type evaluated that were present in the original innoculum injected. Values shown are the mean ± SEM of results pooled from individually assessed animals (two to three animals per experiment) killed at the time points shown.
The relative numbers of different types of human CFC detected also changed with time posttransplant due to the fact that all stages of human erythropoiesis appear to be poorly supported in these mice (Table 2, columns III and IV). This effect was most obvious in the more mature erythroid progenitor compartments and was more pronounced during the latter 2 months of declining human hematopoiesis than during the first 2 months (column I). Thus, by 7 weeks posttransplant, human CFU-E were usually no longer detectable, despite the fact that substantial numbers of primitive BFU-E were measured for up to 16 weeks (≈ 3% of all CFC detected). In contrast, human granulopoiesis appeared to proceed normally with no alteration in the ratio of primitive to mature types of granulopoietic progenitors throughout the entire period of follow-up by comparison to the original human marrow cell suspensions transplanted (column II). Multilineage human progenitors were also detectable at every time point and were maintained at approximately the same frequency (relative to other types of CFC).
Alterations in the Distribution of Different Subclasses of Human CFC at Different Times After the Transplantation of Human Bone Marrow Cells into NOD/SCID Mice
| Time of Assessment (posttransplant) . | I . | II . | III . | IV . |
|---|---|---|---|---|
| . | E:mB:pB . | mGM:pGM . | pB:pGM:GEMM . | total B:total GM:GEMM . |
| SA | 40:26:34 | 86:14 | 79:19:2 | 64:36:0.6 |
| 2 days | 19:40:41 | 94:6 | 95:3:2 | 80:19:1 |
| 4-5 weeks | 27:38:35 | 90:10 | 41:56:3 | 17:83:0.4 |
| 7-10 weeks | 0:37:63 | 82:18 | 22:75:3 | 8:92:0.8 |
| 16 weeks | 0:71:29 | 92:8 | 27:60:13 | 11:87:0.2 |
| Time of Assessment (posttransplant) . | I . | II . | III . | IV . |
|---|---|---|---|---|
| . | E:mB:pB . | mGM:pGM . | pB:pGM:GEMM . | total B:total GM:GEMM . |
| SA | 40:26:34 | 86:14 | 79:19:2 | 64:36:0.6 |
| 2 days | 19:40:41 | 94:6 | 95:3:2 | 80:19:1 |
| 4-5 weeks | 27:38:35 | 90:10 | 41:56:3 | 17:83:0.4 |
| 7-10 weeks | 0:37:63 | 82:18 | 22:75:3 | 8:92:0.8 |
| 16 weeks | 0:71:29 | 92:8 | 27:60:13 | 11:87:0.2 |
Four types of comparisons are shown (I, II, III, IV). Column I shows the relative numbers of CFU-E (E), mature BFU-E (mB), and primitive BFU-E (pB) as a proportion of the total erythroid CFC population (E + mB + pB). Column II shows the ratio of mature (m) to primitive (p) CFU-GM (GM) in the total CFU-GM population detected. Column III shows the relative numbers of different types of primitive CFC, and column IV shows the relative numbers of all erythroid-restricted (E + B), granulopoietic-restricted (GM) and multilineage (GEMM) CFC as a fraction of the total human CFC population detected at each time point. CFC measurements are from the same 8 experiments shown in Fig 5. Criteria used for distinguishing primitive and mature subclasses of BFU-E and CFU-GM are given in Table 3.
In four of these experiments, blood was also collected from animals killed up to 16 weeks posttransplant. Analysis of these samples showed a similar kinetics of appearance and late decline of human cells in the blood. The numbers of circulating human cells were quite variable between mice, but often fell in the range of 5% to 10% of all the WBC present, and ≈ 20% of these human cells, ie, ≈ 1% to 2% of all the nucleated cells in the blood, were CD34+ (Fig 5D).
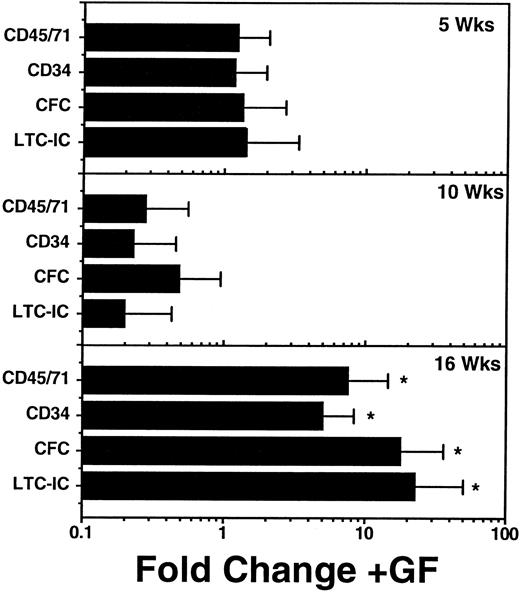
In some of the time course experiments, additional mice were injected with a cocktail of human growth factors 3 times a week for the 2 weeks immediately preceding their assessment. This cocktail consisted of 10 U Ep/mouse/injection, 10 μg/mouse/injection of SF, and 6 μg/mouse/injection each of IL-3 and GM-CSF. Groups of mice treated in this way were killed and assessed at each of the same time points as the nongrowth factor-injected controls described above. These studies failed to show any obvious effect of these growth factor injections on the number of human cells produced during the regenerative phase, ie, before 10 weeks posttransplant in any tissue (data not shown). However, as shown in Fig 6, by 16 weeks, all populations of human myeloid progenitor cells in the marrow were, on average, significantly (P < .05) more numerous in mice that had received the injections of human growth factors than in those that had not. Nevertheless, the magnitude of the increases resulting from growth factor injection (≈ fivefold to 20-fold) were not sufficient to completely counteract the 50-fold to 100-fold decline of all types of human cells that occurred in control mice starting 8 to 10 weeks posttransplant (Fig 5). In addition, although some of the factors injected are known to selectively stimulate erythropoiesis (eg, Ep), there was no evidence of an improved differentiation of human progenitors along this pathway in the growth factor-injected mice. Nevertheless, there was an absolute increase in BFU-E numbers in mice injected with growth factors in the interval between 14 and 16 weeks posttransplant (data not shown).
Effect of a 2-week course of human growth factor (GF) injections on the number of various phenotypically and functionally defined human hematopoietic cells present in the marrow of NOD/SCID mice at 5, 10, and 16 weeks after the transplantation of 2 × 107 light density human marrow cells. Values shown are the mean ± SEM of results pooled from individually assessed mice analyzed in two separate experiments (two or three GF-injected mice and two to three control mice per time point per experiment). Fold change refers to differences seen in the GF-injected mice as compared with control mice that were transplanted with aliquots of the same original human marrow cells and were later injected with saline only. The control mice represent a subset of those used to generate the absolute values shown in Fig 5. Significant increases (P < .05, 1-tailed Student′s t-test), relative to values measured in control mice are indicated by an (*).
Effect of a 2-week course of human growth factor (GF) injections on the number of various phenotypically and functionally defined human hematopoietic cells present in the marrow of NOD/SCID mice at 5, 10, and 16 weeks after the transplantation of 2 × 107 light density human marrow cells. Values shown are the mean ± SEM of results pooled from individually assessed mice analyzed in two separate experiments (two or three GF-injected mice and two to three control mice per time point per experiment). Fold change refers to differences seen in the GF-injected mice as compared with control mice that were transplanted with aliquots of the same original human marrow cells and were later injected with saline only. The control mice represent a subset of those used to generate the absolute values shown in Fig 5. Significant increases (P < .05, 1-tailed Student′s t-test), relative to values measured in control mice are indicated by an (*).
Changes in the cell cycle status of human CFC present in NOD/SCID recipients of human marrow at different times posttransplant, with or without injections of human growth factors.In a first series of experiments, we investigated whether the human CFC populations detectable in the early phase of engraftment (ie, 4 weeks posttransplant) would show the increased cycling characteristics expected of a regenerating population35,36 or alternatively, whether they would still be segregated into proliferating and quiescent compartments according to their proliferative potential (as displayed in methylcellulose assays), which is a characteristic of these cells in steady-state human marrow.32,33 Standard 3H-Tdr suicide studies showed that all CFC detectable in the marrow of the NOD/SCID mice 4 weeks posttransplant were rapidly proliferating (Table 3) and this included both the primitive erythroid and the primitive granulopoietic subsets that, in the marrow of normal adults, are largely quiescent.32 33
Evidence of Increased Proliferative Activity of the Primitive Human CFC Present in NOD/SCID Mice 4 Weeks After Their Transplantation With 2 × 107 Normal Human Marrow Cells
| . | . | % in S-Phase . | |
|---|---|---|---|
| . | . | Human Cells in Mice . | Normal Human Marrow3-150 . |
| CFU-E | (1-2 clusters/colony) | 68 ± 6 | 43 ± 2 |
| Mature BFU-E | (3-8 clusters/colony) | 58 ± 7 | 37 ± 2 |
| Mature CFU-GM | (<500 cells/colony) | 51 ± 2 | 37 ± 3 |
| Primitive BFU-E | (>8 clusters/colony) | 69 ± 4 | 14 ± 2 |
| Primitive CFU-GM | (>500 cells/colony) | 57 ± 5 | 4 ± 2 |
| . | . | % in S-Phase . | |
|---|---|---|---|
| . | . | Human Cells in Mice . | Normal Human Marrow3-150 . |
| CFU-E | (1-2 clusters/colony) | 68 ± 6 | 43 ± 2 |
| Mature BFU-E | (3-8 clusters/colony) | 58 ± 7 | 37 ± 2 |
| Mature CFU-GM | (<500 cells/colony) | 51 ± 2 | 37 ± 3 |
| Primitive BFU-E | (>8 clusters/colony) | 69 ± 4 | 14 ± 2 |
| Primitive CFU-GM | (>500 cells/colony) | 57 ± 5 | 4 ± 2 |
The results of subsequent 3H-Tdr experiments performed in conjunction with the time course studies shown in Fig 5 are presented in Table 4. These results confirmed the previously observed high cycling activity of the primitive human CFC present 4 to 5 weeks posttransplant. However, they also showed that during the next 5 weeks posttransplant, the cycling activity of all the human CFC present changed dramatically such that no cycling (S-phase) CFC could be detected at 10 weeks or later. Moreover, the quiescent status of the CFC present was also not affected by a series of injections of human Ep, SF, GM-CSF, and IL-3 during the 2 preceding weeks.
Lack of Effect of Repeated Growth Factor Injections on the Cell Cycle Status of Human CFC in NOD/SCID Mice Transplanted With Human Marrow Cells
| Time Posttransplant . | Growth Factors . | % in S-Phase . | |
|---|---|---|---|
| . | . | Mature BFU-E/CFU-GM . | Primitive BFU-E/CFU-GM . |
| Week 5 | − | 46 ± 4 | 57 ± 4 |
| + | 43 ± 3 | 70 ± 5 | |
| Week 10 | − | 0 ± 5 | 4 ± 2 |
| + | 14 ± 6 | 0 ± 4 | |
| Week 16 | − | 0 ± 5 | 0 |
| + | 0 ± 9 | 7 ± 3 | |
| Time Posttransplant . | Growth Factors . | % in S-Phase . | |
|---|---|---|---|
| . | . | Mature BFU-E/CFU-GM . | Primitive BFU-E/CFU-GM . |
| Week 5 | − | 46 ± 4 | 57 ± 4 |
| + | 43 ± 3 | 70 ± 5 | |
| Week 10 | − | 0 ± 5 | 4 ± 2 |
| + | 14 ± 6 | 0 ± 4 | |
| Week 16 | − | 0 ± 5 | 0 |
| + | 0 ± 9 | 7 ± 3 | |
Growth factors (10 μg SF, 6 μg IL-3, 6 μg GM-CSF, 10 U Ep) injected 3×/mouse/week for 2 weeks before being killed.
DISCUSSION
The data we report here provide new evidence that the human cells detectable in the hematopoietic tissues of immunodeficient mice transplanted with normal adult human marrow cells are the progeny of a primitive subset. Such cells can clearly home into and respond to signals generated by the hematopoietic microenvironment of the murine marrow and spleen, which then cause a rapid expansion of both primitive and more differentiated types of human hematopoietic cells. This was perhaps best exemplified by the huge increase (up to 1,000-fold) seen during the first 2 months posttransplant in the absolute numbers of human CD34+ cells, CD34+ Thy-1+ cells, CD34− CD19+ cells, CFC, and LTC-IC. Indeed, for many of these populations, the levels attained just in the two hind legs of the transplanted mice (ie, ≈ 25% of the total marrow volume of the mouse34 ) 5 to 10 weeks posttransplant exceeded the total number of such cells in the original innoculum injected. Of particular note was the observation that human hematopoietic cells with a very primitive cell surface phenotype (CD34+ Thy-1+ ), as well as those that could be functionally identified as CFU-GEMM and as LTC-IC, showed the same dramatic regrowth kinetics as later cell types, but reached peak values approximately 1 month earlier.
The continuing presence of these primitive human cells at readily detectable levels even 4 months posttransplant provides additional strong evidence that these mice are initially engrafted by totipotent human stem cells with long-term in vivo repopulating potential, which then undergo extensive self-renewal to regenerate and sustain these related populations. Some evidence of human hematopoietic stem cell self-renewal in NOD/SCID mice reconstituted with human cord blood cells has recently been obtained from experiments with secondary transplants.37 The initially high proliferative activity in vivo of both primitive and mature types of CFC, as well as the fact that large numbers of more mature CD34− human cells expressing CD45 and/or CD71 could be maintained in the mice 4 months after injection, would also indicate a continuous process of hematopoietic cell proliferation and differentiation, ultimately from a very primitive cell population present in the original transplant.
Interestingly, separate analysis of progenitors restricted to different lineages indicated that human granulopoiesis was well supported and appeared to proceed relatively normally (as compared with the original human marrow cell suspensions). However, the generation of human erythropoietic progenitors was clearly compromised with extensive suppression or failure of the terminal stages of this pathway by 7 weeks, even though primitive human erythroid-restricted progenitors could still be detected 16 weeks posttransplant. Moreover, the inability of repeated injections of multiple growth factors that are known to stimulate erythropoiesis both in vitro and in vivo to correct this deficiency in erythropoiesis suggests that other factors may limit the differentiation of primitive human erythroid progenitors in this model.
The data presented here also confirm previous reports15,18,19 that NOD/SCID mice are superior to CB17/SCID mice as recipients of human hematopoietic cells. The ability of NOD/SCID mice to become engrafted after the transplantation of lower cell doses and to regenerate higher numbers of very primitive human cells may be due, at least in part to a reduced ability of NOD/SCID mice to eliminate foreign (xenogeneic) cells, particularly when low numbers of cells are injected because of the additional defects that are characteristic of these mice as compared to CB17/SCID mice.21 It is also possible that there are other differences between NOD/SCID and CB17/SCID mice that contribute to the different levels and patterns of engraftment seen in the two strains; for example, in terms of potential differences in the supportive properties of the hematopoietic microenvironments present in their marrow and spleen. Higher initial levels of human cell engraftment would involve a more rapid production of mature human hematopoietic cells, whose ability to secrete various species-specific growth factors might then further enhance the extent of human hematopoietic cell recovery stimulated by endogenous murine factors. Detection of a stimulating effect of exogenously supplied growth factors might then be restricted to situations where the number of human cells transplanted was relatively reduced, a possibility consistent with the present observation of no growth factor effect on the first 3 months of engraftment in mice transplanted with relatively high numbers of cells.
In summary, we have demonstrated the feasibility of using the NOD/SCID mouse as a recipient for routine experimental studies of human hematopoiesis in an in vivo setting. The observed kinetics of human cell engraftment, the extensive range of primitive and mature cell types produced including both lymphoid and myeloid lineages, and characteristic changes in the cycling activity of early clonogenic cell types obtained within the hematopoietic tissues of these mice are all hallmarks of the regeneration pattern expected from transplantable hematopoietic stem cells of syngeneic origin. This model should therefore serve as a useful starting point for the further characterization of such cells in human tissues, as well as opening up new opportunities for investigating their regulation and responses to novel manipulations or treatments in vivo.
ACKNOWLEDGMENT
The authors thank J. Maltman, M. Sinclaire, and G. Thornbury for technical assistance, S. Clegg for manuscript preparation and Amgen, Immunex, Stem Cell, and Sandoz for generous gifts of growth factors and culture reagents.
J.D.C. and T.L. contributed equally to this work.
Supported by operating grants from the Medical Research Council (MRC) of Canada, the Canadian Genetic Diseases Network of the National Centers of Excellence, Sandoz International and the National Cancer Institute of Canada (NCIC) with funds from the Terry Fox Foundation, and National Institutes of Health (NIH) (Grant No. HL55435 to C.J.E. and Grant No. A130389 to L.D.S.), in addition to postdoctoral fellowships from the NCIC (to T.L.), the MRC of Canada (to J.W.) and the Leukemia Research Fund of Canada (to J.W.), an MRC Scientist award and an NCIC Research Scientist award (to J.E.D.), and an NCIC Terry Fox Cancer Research Scientist award (to C.J.E.).
Address reprint requests to Connie J. Eaves, PhD, Terry Fox Laboratory, 601 W 10th Ave, Vancouver, BC V5Z 1L3 Canada.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal