Abstract
The primary function of polymorphonuclear neutrophils (PMN) in the immune response appears to be acute phagocytic clearance of foreign pathogens and release of inflammatory mediators. Consistent with their assumed lack of major histocompatibility complex (MHC) class II expression, PMN have not been considered to play a role in antigen presentation and T-cell activation. However, recent reports have shown that human PMN can express MHC class II molecules both in vitro and in vivo after stimulation with either granulocyte-macrophage colony-stimulating factor (GM-CSF ) or interferon-γ (IFN-γ). Thus, under appropriate conditions, PMN could play a significant role in immune regulation, including T-cell activation. In this report, we demonstrate that human class II–expressing PMN can serve as accessory cells in superantigen (SAg)-mediated T-cell activation. This accessory activity for SAg presentation was present only after induction of MHC class II expression, and was especially pronounced following culture of PMN with GM-CSF plus IFN-γ, which acted synergistically to induce MHC class II molecules on PMN. Moreover, the level of MHC class II expression and the magnitude of SAg-induced T-cell responses were found to be highly correlated and distinctly donor dependent, with PMN from some donors repeatedly showing fivefold higher responses than PMN from other donors. On the other hand, culture of PMN with GM-CSF plus IFN-γ under conditions that resulted in optimal MHC class II expression did not enable them to function as antigen-presenting cells for either intact tetanus toxoid (TT) or for a TT peptide. These results delineate a new pathway for T-cell activation by SAg that may play an important role in the severity of SAg-induced inflammatory responses. They also identify a donor-specific polymorphism for induction of PMN MHC class II expression which may be of significance for therapies involving GM-CSF and IFN-γ.
POLYMORPHONUCLEAR neutrophils (PMN) play an important role in the effector arm of host immune defense through the clearance of immune complexes, the phagocytosis of opsonized particles, and the release of inflammatory mediators.1-3 PMN can synthesize a number of cytokines, such as interferon-α (IFN-α), interleukin-1 (IL-1), IL-6, IL-8, and tumor necrosis factor-α, as well as constituitively express various surface proteins, including Fc receptors, CR1, CR3, and major histocompatibility complex (MHC) class I molecules.2-9 However, PMN do not normally express MHC class II and, therefore, are believed not to play a direct role in CD4+ T-cell activation.
Presentation of antigen (Ag) to CD4+ T cells is most frequently associated with professional antigen-presenting cells (APC) that constituitively express MHC class II such as B cells, macrophages, and dendritic cells. However, several other cell types have been shown to present Ag to CD4+ T cells when MHC class II expression is induced.10 We have recently shown that human PMN can be induced to express MHC class II molecules both in vitro and in vivo. Specifically, in vitro incubation of human PMN from healthy donors with either granulocyte-macrophage colony-stimulating factor (GM-CSF ), IFN-γ, or IL-3 results in low-level expression of HLA-DR.11,12 In addition, significant HLA-DR expression has been observed on PMN from patients treated with either GM-CSF or IFN-γ.13,14 Given that PMN are the predominant cell type in many inflammatory reactions, and that HLA-DR expression, and subsequent Ag presentation, is key in initiating or suppressing most cellular immune responses, we examined the ability of HLA-DR–expressing PMN to present two major types of Ag, each with significantly different requirements for initiation of T-cell activation. Tetanus toxoid (TT), a soluble protein Ag which requires processing before presentation, was not presented by GM-CSF–treated (HLA-DR+) PMN. This is despite the fact that T cells from these individuals exhibited a potent response to TT in the presence of professional APC. PMN treated with a combination of GM-CSF and IFN-γ, which we show in these studies to significantly increase MHC class II expression above that of PMN treated with either cytokine alone, also failed to support activation of TT-specific T cells. In contrast, T-cell activation was observed in the presence of superantigen (SAg) and GM-CSF/IFN-γ–treated PMN. SAg normally does not require processing, and under some circumstances appears not to require second signal molecules be present on the APC.15
These studies suggest that PMN may play an active and heretofore unappreciated role in immune and inflammatory responses involving SAg.
MATERIALS AND METHODS
Media and reagents.Cells were cultured with either AIM-V (GIBCO, Grand Island, NY), a defined serum free medium, or RPMI-1640 (Hazelton Biologies, Inc, Lenexa, KS). The cytokines G-CSF, GM-CSF, and IL-2 were generously donated by Immunex Corp (Seattle, WA). IFN-γ was a gift from Genentech, Inc (San Francisco, CA). One unit of GM-CSF is equivalent to 0.025 ng of protein. One unit of IFN-γ is equivalent to 0.25 ng of protein. Antibodies used in these studies included: IVA12, an IgG1 anti–HLA-DR,-DP,-DQ; L243, an IgG2a anti–HLA-DR; OKT3, an IgG2a anti-CD3; OKT8, an IgG2a anti-CD8 (all from ATCC, Rockville, MD); FMC63, an IgG2a anti-CD19 (generous gift of Dr Heddy Zola, Child Health Research Institute, Adelaide Medical Centre for Women and Children, North Adelaide, South Australia); P3, an IgG1 isotype control; AML223, an IgG2b anti-CD14; 22.2, an IgG1 anti-CD64; IV.3, an IgG2b anti-CD32; 3G8, an IgG1 anti-CD16 (all provided by Medarex, Inc, Annandale, NJ); anti–B7-1 and anti–B7-2 (Ancell Corp, Bayport, MN), and fluorescein isothiocyanate (FITC)-labeled 531, an IgG1 anti-CD66b (AMAC, Inc, Westbrook, ME). FITC-labeled F(ab′)2 goat-anti-mouse Ig and FITC-labeled goat-anti-rabbit Ig secondary antibodies used in these studies were obtained from Caltag Laboratories, Inc (San Francisco, CA). Anti-human HLA-DR monoclonal antibody (MoAb) used in Western blot analysis was purchased from DAKO Corp (Carpinteria, CA). Alkaline phosphatase conjugated goat-anti-mouse IgG antibody and fetal bovine serum (FBS) were purchased from HyClone Laboratories, Inc (Logan, UT). Rabbit polyclonal anti-SEA IgG and the SAg SEA, SEB, SEC1, SEE, were all purchased from Toxin Technologies (Sarasota, FL), while the TSST-1 was graciously donated by Dr J. Parsonnet (Dartmouth Medical School, Lebanon, NH). The Ag (TT) was purchased from Accurate Chemical Corp (Westbury, NY). The TT peptide TT830-844 (QYIKANSKFIGITEL) was synthesized and purified to greater than 95% purity by Peptidogenic Corporation (Livermore, CA), and is a universal T-cell epitope for TT-specific T cells.16
Cell isolation.Peripheral blood mononuclear cells (PBMC) were obtained from healthy donors and were separated into mononuclear cell and granulocyte fractions using HISTOPAQUE 1077 (Sigma, St Louis, MO) layered over HISTOPAQUE 1119 (Sigma) according to the manufacturer's instructions. To further enrich the PMN population used in TT presentation studies, residual monocytes were removed by adherence and subsequent treatment with L-Leucine Methyl Ester (LME) (Sigma). Both histochemical and flow cytometric analysis demonstrated that the resulting PMN population was 95% to 98% pure. In the case of studies involving SAg, the granulocyte fraction was further enriched by immunodepletion. Cells were incubated for 30 minutes at 4°C with the mouse MoAbs L243, FMC63, and OKT3 (10 μg/mL) for 30 minutes at 4°C, washed three times with phosphate-buffered saline (PBS) supplemented with 2.5% FBS (PBS-FBS), and eluted through goat-anti-mouse Ig and anti-human Ig columns. Resulting PMN purity was monitored by FACScan analysis to confirm that no MHC class II+ APC were present in the PMN population before incubation with cytokines. The PMN population was greater than 99.9% pure after negative selection, with the contaminating cells being eosinophils. Monocytes were used as control APC and were isolated via adherence. Specifically, PBMC were resuspended in AIM-V to a concentration of 5 × 106 cells/mL, and 100 μL added/well to a 96-well plate for 1 hour at 37°C. Nonadherent cells were then removed by three washes with HEPES-buffered RPMI containing 0.1% human albumin (Sigma). TT-specific and TT peptide-specific T cells were isolated using a modification of methods previously described.17 Briefly, fresh PBMC (30 × 106) were stimulated with 5 μg/mL TT or TT peptide in 50 mL AIM-V medium for 3 days, at which point 10 U/mL recombinant human (rh) IL-2 and 1.25 mL of autologous serum (2.5%) were added for an additional 7 to 11 days at, which time T cells were harvested, washed, and stored in liquid nitrogen until needed. Before use, Ag-specific T cells were adhered for 1 hour at 37°C and subsequently treated with LME to remove residual monocytes. Normal T lymphocytes used in SAg studies were enriched using CD4+ T-cell isolation columns (Pierce, Rockford, IL). Briefly, mononuclear cells from the HISTOPAQUE 1077 layer were then incubated for 30 minutes at 4°C with the mouse MoAbs OKT8, AML223, FMC63, 3G8, IV.3, and 22 (10 μg/mL). The cells were washed three times with PBS supplemented with 2.5% FBS (PBS-FBS) and eluted through the goat-anti-mouse Ig and goat-anti-human Ig column to obtain a population of CD4+ T lymphocytes that were identified by flow cytometric analysis to be greater than 94% CD4+, with the contaminating cells being mainly CD8+ T lymphocytes. CD4+ T cells were either used immediately in assays or cryopreserved for future use.
Flow cytometry.For analysis of FcγRI/CD64 and class II upregulation by cytokines, PMN were isolated as described above and resuspended in AIM-V containing 2.5% pooled human serum (PHS) and 250 U/mL G-CSF. PMN (5 × 105) were added to each well of a 96-well plate (Costar, Cambridge, MA) followed by addition of various combinations of IL-3, GM-CSF, and IFN-γ. Concentrations of each cytokine are presented in the figure legends. The final volume for each well was adjusted to 200 μL, and the PMN were incubated for 44 to 45 hours at 37°C in 5% CO2 before staining. The viability of PMN following 2-day incubation in 2.5% PHS and 250 U/mL of G-CSF was consistently greater than 95%. Analysis was performed on a standard FACScan flow cytometer (Becton Dickinson, San Jose, CA) with 5,000 cells analyzed per sample unless otherwise indicated. For staining, 20 μL of cells were combined in a 96-well flat bottom plate with 20 μL of human IgG (12 mg/mL; Sigma) to block Fc binding of the MoAbs and 20 μL of primary MoAb (60 μg/mL). Cells were then incubated on a rotator at 4°C for 1 hour. After three washes with 200 μL PBS containing 2 mg/mL of bovine serum albumin (BSA) (PBS-BSA), 40 μL of FITC-labeled F(ab′)2 goat-anti-mouse Ig (20 μg/mL) was added for an additional 1 hour. Samples were then washed three times, resuspended in PBS-BSA for immediate analysis, or PBS-BSA supplemented with 1% paraformaldehyde for analysis 1 to 7 days later, depending on availability of the flow cytometer. Although most samples were analyzed within 48 hours of fixation, for those samples analyzed at later time points, background fluorescence remained relatively low, and our ability to measure class II expression was unaffected by the added storage time. SEA binding studies were performed by incubating the PMN with 25 nmol/L SEA at 4°C for 1 hour. PMN were washed three times with PBS-BSA, then incubated with human adsorbed rabbit polyclonal anti-SEA (60 μg/mL) for an additional 1 hour. After three washes with PBS-BSA, PMN were stained with 40 μL of FITC-labeled F(ab′)2 goat-anti-rabbit Ig (20 μg/mL) for another 1 hour, washed three times with PBS-BSA, then resuspended in PBS-BSA supplemented with 1% paraformaldehyde until flow cytometric analysis. Relative binding was measured by the mean fluorescence intensity (MFI) of positive cells over that of the isotype control for each treatment.
Ag presentation assays.Presentation of TT or TT peptide to Ag-specific T-cell lines was performed in a manner similar to that previously described.17 PMN were incubated with or without MHC class II–inducing cytokines for approximately 24 hours, then cytokines were washed away and cells were resuspended in AIM V containing 2.5% autologous serum and 250 U/mL G-CSF. Concentrations of each cytokine are indicated in individual figures. However, the concentrations usually ranged between 50 and 100 U/mL, which results in maximal MHC class II induction after 44 hours of incubation time11 (and data not shown). In addition, pulsing PMN for 24 hours with cytokines is sufficient to induce maximal MHC class II expression by 44 hours (data not shown). PMN were then added to individual wells at concentrations ranging from 1.25 to 5 × 105/well. To eliminate the possibility that proliferation observed might be due to replication of contaminating lymphocytes within the PMN population, PMN were irradiated with 2,000 rad in all experiments presented in this report. However, similar results were also obtained when using nonirradiated PMN (data not shown). In some instances irradiated monocytes or PBMC were substituted for PMN as control APC. After addition of Ag and T cells (1 × 105/well), plates were incubated for 72 hours, followed by a 24-hour [3H]TdR pulse. Cells were then obtained using a Skatron harvester (Sterling, VA), and counted on a Beckman scintillation counter (Wallac Inc, Gaithersburg, MD).
SAg assays were performed similarly, in AIM-V medium containing G-CSF supplemented with 2.5% PHS. PMN were isolated as described above and cultured in 6-well plates (Costar) at a concentration of 1 × 106/mL for 44 to 45 hours in either the presence or absence of 100 U/mL GM-CSF plus 100 U/mL IFN-γ. The PMN were harvested, washed once, and then 1 × 105 PMN were placed in 96-well plates (Costar) with 5 × 104 CD4+ T lymphocytes in either the absence or presence of SAg. The final concentration of SAg used in each assay was determined by titrating each of the SAg tested, and selecting the lowest SAg concentration which elicited the greatest T-cell proliferative response. In the case of SEA, SEB, SEC1, and SEE, 1 nmol/L SAg was selected. A concentration of 10 nmol/L was required to induce a maximal T-cell response when using TSST-1. After 66 hours of incubation with SAg, the wells were pulsed for 18 to 20 hours with [3H]TdR to measure T-cell proliferation. For antibody blocking assays, each MoAb was incubated with the PMN and T lymphocytes 30 minutes before SAg addition.
RESULTS
PMN HLA-DR induction by GM-CSF/IFN-γ.Initial attempts directed at demonstrating TT presentation by HLA-DR+ PMN focused on the use of human PMN treated with GM-CSF, because previous studies showed that GM-CSF was the optimal cytokine for inducing HLA-DR expression on cultured human PMN.11 Therefore, we performed several experiments with cells from three different donors examining the ability of GM-CSF–treated PMN to stimulate TT-specific T-cell proliferation in response to TT. However, in each instance GM-CSF–treated PMN failed to generate a TT-specific T-cell proliferative response significantly above that of untreated PMN, or GM-CSF–treated PMN incubated with TT-specific T cells in the absence of TT (data not shown). In contrast, a twofold to threefold increase in 3H-thymidine incorporation was observed in response to TT when the same TT-specific T cells used above were combined with TT and professional APC (ie, irradiated PBMC or monocytes). Therefore, we considered the possibility that HLA-DR expression might still be limiting, and that a further increase in HLA-DR expression might enhance the ability of these cells to function as APC.
Preliminary studies in our laboratory using combinations of cytokines had suggested that the level of MHC class II on human PMN could be further increased by treating PMN with a combination of GM-CSF and IFN-γ. Therefore, we conducted additional studies to confirm this observation. In fact, GM-CSF and IFN-γ were synergistic for upregulation of MHC class II expression on human PMN, when compared with MHC class II induction by either GM-CSF or IFN-γ alone (Fig 1). Analysis using anti–HLA-DR-, DP-, and DQ-specific antibodies showed that this synergistic upregulation of PMN MHC class II was predominantly of the HLA-DR isotype (data not shown), as is the case when using GM-CSF alone.11 Induction of HLA-DR expression by GM-CSF/IFN-γ was dose-dependent, with a maximum induction being observed at 50 U/mL of GM-CSF combined with 50 U/mL IFN-γ for all donors examined. In addition, as observed with GM-CSF treatment alone,11 donors could still be distinguished based on the relative amounts of MHC class II they expressed after GM-CSF/IFN-γ treatment (Table 1). To confirm that the donor-dependent expression of HLA-DR was in fact specific for HLA-DR, we compared HLA-DR induction on PMN to that of CD64 (FcγRI), which is modulated on PMN by both GM-CSF and IFN-γ. When treated with either GM-CSF, IFN-γ, or the combination of these cytokines, there appeared to be no correlation between the expression of HLA-DR and CD64, in that both HLA-DRlow and HLA-DRhigh donors showed similar levels of CD64 expression (Fig 1). This suggests that the difference in HLA-DR expression between donors resides at a stage downstream from cytokine-receptor signaling. Although IL-3 also induces low levels of MHC class II expression on human PMN, addition of IL-3 to PMN GM-CSF/IFN-γ cultures resulted in no significant enhancement of PMN HLA-DR expression (data not shown).
Enhanced induction of PMN class II expression by GM-CSF plus IFN-γ. PMN from both an HLA-DRlow responder and an HLA-DRhigh responder were cultured in the presence of various concentrations of GM-CSF (♦), IFN-γ (▪), or GM-CSF/IFN-γ (•). After 44 hours of culture, the PMN were stained with either MoAb IVA12 (anti-MHC class II) or MoAb 22 (anti-CD64) before flow cytometric analysis. The level of PMN class II induction differed markedly for HLA-DRlow responder (A) and the HLA-DRhigh responder (B) but was synergistically enhanced for both donors when compared to stimulation with either GM-CSF or IFN-γ alone. Regulation of PMN CD64 expression was similar for both donors (C and D). Surface expression levels of both class II and CD64 are presented as mean fluorescence intensity and represent the average of duplicate samples ± SD.
Enhanced induction of PMN class II expression by GM-CSF plus IFN-γ. PMN from both an HLA-DRlow responder and an HLA-DRhigh responder were cultured in the presence of various concentrations of GM-CSF (♦), IFN-γ (▪), or GM-CSF/IFN-γ (•). After 44 hours of culture, the PMN were stained with either MoAb IVA12 (anti-MHC class II) or MoAb 22 (anti-CD64) before flow cytometric analysis. The level of PMN class II induction differed markedly for HLA-DRlow responder (A) and the HLA-DRhigh responder (B) but was synergistically enhanced for both donors when compared to stimulation with either GM-CSF or IFN-γ alone. Regulation of PMN CD64 expression was similar for both donors (C and D). Surface expression levels of both class II and CD64 are presented as mean fluorescence intensity and represent the average of duplicate samples ± SD.
Donor-Dependence of PMN MHC Class II Expression
| Donor . | No. of Samples . | Sampling Period (d)* . | % Positive Cells† . | Average (MFI)‡ . |
|---|---|---|---|---|
| 1 | 4 | 174 | 87.3 ± 1.1 | 255 ± 35 |
| 2 | 3 | 181 | 87.3 ± 3.4 | 189 ± 34 |
| 3 | 3 | 29 | 83.3 ± 0.4 | 193 ± 30 |
| 7 | 5 | 219 | 60.3 ± 1.5 | 47 ± 20 |
| 8 | 3 | 28 | 71.1 ± 0.4 | 29 ± 3 |
| 9 | 3 | 57 | 37.0 ± 2.3 | 29 ± 4 |
| Donor . | No. of Samples . | Sampling Period (d)* . | % Positive Cells† . | Average (MFI)‡ . |
|---|---|---|---|---|
| 1 | 4 | 174 | 87.3 ± 1.1 | 255 ± 35 |
| 2 | 3 | 181 | 87.3 ± 3.4 | 189 ± 34 |
| 3 | 3 | 29 | 83.3 ± 0.4 | 193 ± 30 |
| 7 | 5 | 219 | 60.3 ± 1.5 | 47 ± 20 |
| 8 | 3 | 28 | 71.1 ± 0.4 | 29 ± 3 |
| 9 | 3 | 57 | 37.0 ± 2.3 | 29 ± 4 |
PMN class II induction after 44 hours of culture in the presence of 100 U/mL GM-CSF/IFN-γ is presented for three HLA-DRhigh responders and three HLA-DRlow responders.
The sampling period is the number of days between the first and last sample tested for each donor.
The number of PMN demonstrating class II expression levels above the isotype control are presented as the % positive cells ± SEM.
The MFI represents the level of PMN class II expression above isotype control and is the average for all the samples examined ± SEM.
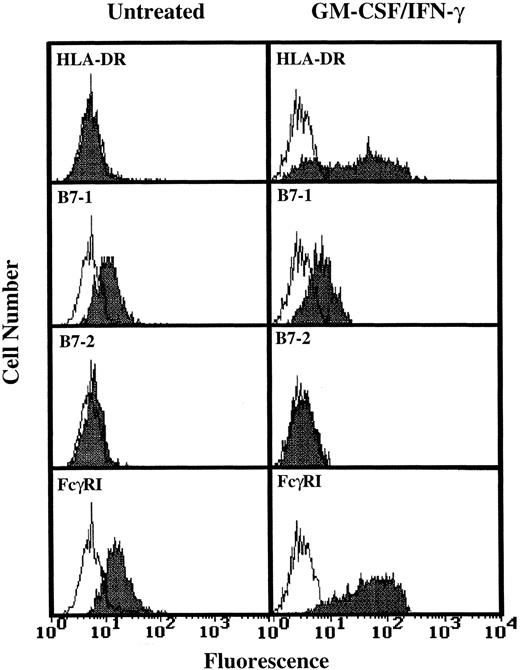
Analysis of TT and TT peptide presentation by GM-CSF/IFN-γ–treated PMN.Having determined that higher levels of HLA-DR expression by human PMN could be achieved (Fig 1), three additional experiments monitoring T-cell activation in response to TT were conducted using GM-CSF/IFN-γ–treated PMN from HLA-DRhigh responding donors. As shown by data from one of these three experiments, the HLA-DR+ PMN again failed to stimulate TT-specific T cells while at the same time autologous monocytes supported TT-induced T-cell proliferation (Fig 2). To determine if the problem was at the level of Ag processing, further experiments were conducted which examined the ability of HLA-DR+ PMN to present preprocessed TT, in the form of TT peptide, to peptide-specific T cells. Again, no significant T-cell response was observed (Fig 3), despite relatively high levels of HLA-DR expresssion by PMN (Fig 3, inset), and the ability of these same T cells to respond to TT peptide presented by adhered monocytes (Fig 3). To determine if PMN lack the necessary second signals to activate T cells, GM-CSF/IFN-γ–treated and untreated PMN from two donors whose PMN had previously failed to stimulate T-cell proliferation, as well as PMN from two additional donors, were analyzed for the presence of B7-1 and B7-2 using flow cytometry. Little or no expression of either B7-1 or B7-2 was observed on human PMN regardless of whether the above PMN were incubated with GM-CSF and IFN-γ (Fig 4). Addition of IL-2 to compensate for this apparent lack of second signal molecules also failed to facilitate Ag presentation by HLA-DR+ PMN (data not shown).
Inability of GM-CSF/IFN-γ–treated (HLA-DR+) PMN to stimulate TT-specific T cells. Purified monocytes (Mφ; 5 × 104/well) were used as control APC. PMN were first incubated for 24 hours in the presence of GM-CSF/IFN-γ (50 U/mL), and then added to wells of a 96-well plate at concentrations of 0, 1.25, or 2.5 × 105 cells/well. TT-specific T cells were than added, and the Ag presentation assay performed as described in Materials and Methods. [3H]TdR uptake was measured at 72 to 96 hours. Data are expressed as cpm ± SD of duplicate samples.
Inability of GM-CSF/IFN-γ–treated (HLA-DR+) PMN to stimulate TT-specific T cells. Purified monocytes (Mφ; 5 × 104/well) were used as control APC. PMN were first incubated for 24 hours in the presence of GM-CSF/IFN-γ (50 U/mL), and then added to wells of a 96-well plate at concentrations of 0, 1.25, or 2.5 × 105 cells/well. TT-specific T cells were than added, and the Ag presentation assay performed as described in Materials and Methods. [3H]TdR uptake was measured at 72 to 96 hours. Data are expressed as cpm ± SD of duplicate samples.
Inability of GM-CSF/IFN-γ–treated (HLA-DR+) PMN to stimulate TT peptide-specific T cells. Flow cytometric analysis of PMN treated with GM-CSF/IFN-γ (50 U/mL) for the presence of HLA-DR (inset) was conducted as described in Materials and Methods. The open peak represents staining with the isotype control. The closed peak represents staining with HLA-DR–specific antibody. T-cell stimulation was conducted as described in Fig 2, but using 5 μg/mL TT peptide instead of whole TT. [3H]TdR uptake was measured at 72 to 96 hours. Data are expressed as cpm ± SD of triplicate samples.
Inability of GM-CSF/IFN-γ–treated (HLA-DR+) PMN to stimulate TT peptide-specific T cells. Flow cytometric analysis of PMN treated with GM-CSF/IFN-γ (50 U/mL) for the presence of HLA-DR (inset) was conducted as described in Materials and Methods. The open peak represents staining with the isotype control. The closed peak represents staining with HLA-DR–specific antibody. T-cell stimulation was conducted as described in Fig 2, but using 5 μg/mL TT peptide instead of whole TT. [3H]TdR uptake was measured at 72 to 96 hours. Data are expressed as cpm ± SD of triplicate samples.
Flow cytometric analysis of B7-1 and B7-2 expression on GM-CSF/IFN-γ–treated PMN. PMN were cultured for 44 hours in the absence or presence of 100 U/mL GM-CSF/IFN-γ before staining. Open peaks represent staining with isotype controls. Closed peaks represent staining with HLA-DR, B7-1, B7-2, or FcγRI-specific antibodies. Antibody binding to PMN cultured in the absence of GM-CSF/IFN-γ had the same MFI as the isotype control. Flow cytometric histograms represent 5,000 to 10,000 gated PMN.
Flow cytometric analysis of B7-1 and B7-2 expression on GM-CSF/IFN-γ–treated PMN. PMN were cultured for 44 hours in the absence or presence of 100 U/mL GM-CSF/IFN-γ before staining. Open peaks represent staining with isotype controls. Closed peaks represent staining with HLA-DR, B7-1, B7-2, or FcγRI-specific antibodies. Antibody binding to PMN cultured in the absence of GM-CSF/IFN-γ had the same MFI as the isotype control. Flow cytometric histograms represent 5,000 to 10,000 gated PMN.
SAg-induced T-cell activation by HLA-DR+ PMN.As a result of our failure to demonstrate presentation of TT or TT peptide by HLA-DR+ PMN, we examined the ability of HLA-DR+ PMN to stimulate T cells using SAg. Use of SAg bypasses the requirement for Ag processing, as well as the need for second signal molecules in some instances.15 We initially determined, using flow cytometry, whether PMN treated with 100 U/mL GM-CSF/IFN-γ could bind the bacterial SAg staphylococcal enterotoxin A (SEA). PMN from an HLA-DRlow responder (Fig 5A and B) and an HLA-DRhigh responder (Fig 5C and D) were cultured for 44 hours in the presence of GM-CSF/IFN-γ , and then incubated with SEA. SEA did bind, and SEA binding correlated with the level of HLA-DR expression by PMN from the individual donors (Fig 5). GM-CSF/IFN-γ–treated (HLA-DR+) PMN were also examined for their ability to elicit SAg-induced T-cell proliferation. In four separate experiments, SAg-induced T-cell proliferation was consistently greater for HLA-DRhigh responders than HLA-DRlow responders, although overall T-cell proliferation varied among individual experiments (Table 2). This difference in the ability of the HLA-DRlow responders and HLA-DRhigh responders to elicit SAg-induced T-cell proliferation was observed for a number of different SAg. As presented in Fig 6, T-cell proliferative responses induced by either SEA, SEB, SEC1, SEE (each at 1 nmol/L), or TSST-1 (10 nmol/L) were consistently greater for the HLA-DRhigh responder then the HLA-DRlow responder. SEA-induced T-cell proliferation from these same two donors was decreased considerably in the presence of an anti–HLA-DR MoAb (L243), but not control antibodies specific for either CD14 (AML223) or CD64 (22.2) (Fig 7). Thus, despite the relative lack of second signal molecules, and inability of HLA-DR+ PMN to present TT or TT peptide, HLA-DR+ PMN are capable of initiating HLA-DR–restricted, SAg-dependent T-cell proliferation.
Flow cytometric analysis of SEA binding to GM-CSF/IFN-γ–treated PMN. Open peaks represent staining of PMN when SEA is not present. Closed peaks represent staining of PMN after incubation with SEA. PMN were cultured for 44 hours in the absence or presence of 100 U/mL GM-CSF/IFN-γ before staining with SEA. HLA-DR expression by an HLA-DRlow responder (A) and an HLA-DRhigh responder (C) correlated with the level of SEA binding to the HLA-DRlow responder (B) and HLA-DRhigh responder (D). SEA binding to PMN cultured in the absence of GM-CSF/IFN-γ had the same MFI as the untreated control. Flow cytometric histograms represent 10,000 gated PMN.
Flow cytometric analysis of SEA binding to GM-CSF/IFN-γ–treated PMN. Open peaks represent staining of PMN when SEA is not present. Closed peaks represent staining of PMN after incubation with SEA. PMN were cultured for 44 hours in the absence or presence of 100 U/mL GM-CSF/IFN-γ before staining with SEA. HLA-DR expression by an HLA-DRlow responder (A) and an HLA-DRhigh responder (C) correlated with the level of SEA binding to the HLA-DRlow responder (B) and HLA-DRhigh responder (D). SEA binding to PMN cultured in the absence of GM-CSF/IFN-γ had the same MFI as the untreated control. Flow cytometric histograms represent 10,000 gated PMN.
Correlation Between SAg-Induced T-Cell Activation and PMN Class II Expression
| Experiment . | Donor . | MHC Class II Expression (MFI)* . | [3H]TdR Incorporation cPM)† . | ||
|---|---|---|---|---|---|
| . | . | Control . | GM-CSF/IFN-γ . | Control . | GM-CSF/IFN-γ . |
| 1 | 1 | 14 ± 0 | 278 ± 7 | 1,813 ± 764 | 10,986 ± 515 |
| 8 | 9 ± 1 | 34 ± 1 | 941 ± 253 | 1,980 ± 344 | |
| 2 | 4 | 18 ± 1 | 185 ± 4 | 823 ± 305 | 21,863 ± 5,378 |
| 9 | 17 ± 1 | 28 ± 2 | 458 ± 250 | 6,663 ± 847 | |
| 3 | 5 | 10 ± 1 | 149 ± 3 | 1,220 ± 489 | 14,367 ± 3,452 |
| 10 | 10 ± 0 | 32 ± 1 | 730 ± 104 | 4,101 ± 1,283 | |
| 4 | 2 | 18 ± 0 | 180 ± 3 | 2,283 ± 814 | 27,760 ± 3,364 |
| 7 | 13 ± 0 | 55 ± 2 | 1,156 ± 234 | 5,377 ± 1,155 | |
| Experiment . | Donor . | MHC Class II Expression (MFI)* . | [3H]TdR Incorporation cPM)† . | ||
|---|---|---|---|---|---|
| . | . | Control . | GM-CSF/IFN-γ . | Control . | GM-CSF/IFN-γ . |
| 1 | 1 | 14 ± 0 | 278 ± 7 | 1,813 ± 764 | 10,986 ± 515 |
| 8 | 9 ± 1 | 34 ± 1 | 941 ± 253 | 1,980 ± 344 | |
| 2 | 4 | 18 ± 1 | 185 ± 4 | 823 ± 305 | 21,863 ± 5,378 |
| 9 | 17 ± 1 | 28 ± 2 | 458 ± 250 | 6,663 ± 847 | |
| 3 | 5 | 10 ± 1 | 149 ± 3 | 1,220 ± 489 | 14,367 ± 3,452 |
| 10 | 10 ± 0 | 32 ± 1 | 730 ± 104 | 4,101 ± 1,283 | |
| 4 | 2 | 18 ± 0 | 180 ± 3 | 2,283 ± 814 | 27,760 ± 3,364 |
| 7 | 13 ± 0 | 55 ± 2 | 1,156 ± 234 | 5,377 ± 1,155 | |
PMN SAg-induced T-cell proliferation is presented for four separate experiments, each using one HLA-DRhigh and HLA-DRlow responding pair. As described in Materials and Methods, 1 × 105 PMN cultured in the presence or absence of 100 U/mL of GM-CSF/IFN-γ for 44 hours were incubated with 5 × 104 CD4+ T lymphocytes and 10 nmol/L SEA.
The level of class II induction for both the unstimulated and GM-CSF/IFN-γ–stimulated PMN was determined by FACScan analysis and is presented as the MFI.
PMN SAg-induced T-cell proliferation is presented as counts per minute (cpm). Values reflect the amount of [3H]TdR incorporated following an 18 to 20-hour pulse and are the mean of triplicate samples ± SD.
SAg-induced T-cell proliferation by HLA-DR+ PMN. PMN were cultured for 44 hours in either the absence or presence of 100 U/mL GM-CSF/IFN-γ from a known HLA-DRlow responder (donor 7; , unstimulated; □, GM-CSF/IFN-γ stimulated) and HLA-DRhigh responder (donor 2; ▨, unstimulated; ▪, GM-CSF/IFN-γ stimulated). SAg presentation assays were performed by incubating 1 × 105 PMN with 5 × 104 CD4+ T lymphocytes in the presence of 1 nmol/L SEA, SEB, SEC1, SEE, or 10 nmol/L TSST-1 for 66 hours. PMN SAg-induced T-cell proliferation is presented as counts per minute (cpm). Values reflect the amount of [3H]TdR incorporated after an 18- to 20-hour pulse and are the mean of triplicate samples ± SD.
SAg-induced T-cell proliferation by HLA-DR+ PMN. PMN were cultured for 44 hours in either the absence or presence of 100 U/mL GM-CSF/IFN-γ from a known HLA-DRlow responder (donor 7; , unstimulated; □, GM-CSF/IFN-γ stimulated) and HLA-DRhigh responder (donor 2; ▨, unstimulated; ▪, GM-CSF/IFN-γ stimulated). SAg presentation assays were performed by incubating 1 × 105 PMN with 5 × 104 CD4+ T lymphocytes in the presence of 1 nmol/L SEA, SEB, SEC1, SEE, or 10 nmol/L TSST-1 for 66 hours. PMN SAg-induced T-cell proliferation is presented as counts per minute (cpm). Values reflect the amount of [3H]TdR incorporated after an 18- to 20-hour pulse and are the mean of triplicate samples ± SD.
PMN SAg-induced T-cell proliferation is HLA-DR–restricted. PMN from both an HLA-DRlow responder (donor 7; , unstimulated; □, GM-CSF/IFN-γ) and HLA-DRhigh responder (donor 2; ▨, unstimulated; ▪, GM-CSF/IFN-γ) were cultured for 44 hours in the absence or presence of 100 U/mL GM-CSF/IFN-γ, and 1 × 105 PMN from each condition were incubated with 5 × 104 CD4+ T lymphocytes in the presence of 1 nmol/L SEA. The MoAbs L243 (anti–HLA-DR), 197 (anti-CD64), and AML223 (anti-CD14) were added to the appropriate wells 30 minutes before the addition of SAg. T-cell proliferation was measured at 66 hours with an 18- to 20-hour [3H]TdR pulse and is presented as counts per minute (cpm). Values represent the mean of triplicate samples ± SD.
PMN SAg-induced T-cell proliferation is HLA-DR–restricted. PMN from both an HLA-DRlow responder (donor 7; , unstimulated; □, GM-CSF/IFN-γ) and HLA-DRhigh responder (donor 2; ▨, unstimulated; ▪, GM-CSF/IFN-γ) were cultured for 44 hours in the absence or presence of 100 U/mL GM-CSF/IFN-γ, and 1 × 105 PMN from each condition were incubated with 5 × 104 CD4+ T lymphocytes in the presence of 1 nmol/L SEA. The MoAbs L243 (anti–HLA-DR), 197 (anti-CD64), and AML223 (anti-CD14) were added to the appropriate wells 30 minutes before the addition of SAg. T-cell proliferation was measured at 66 hours with an 18- to 20-hour [3H]TdR pulse and is presented as counts per minute (cpm). Values represent the mean of triplicate samples ± SD.
DISCUSSION
Previous reports have shown that PMN can synthesize and express HLA-DR both in vitro and in vivo, when exposed to GM-CSF or IFN-γ.11-14 However, it has not been determined whether PMN can use this HLA-DR to present Ag. Our data indicate that HLA-DR expressing PMN cannot effectively process and present TT. Neither GM-CSF, nor GM-CSF/IFN-γ–treated PMN stimulated TT-specific T-cell lines to proliferate (Fig 2 and data not shown). This result is apparently not due to an overall inability of the PMN to function, because we found that PMN cultured under identical conditions for 44 to 48 hours exhibited normal oxidative burst activity, as well as the ability to phagocytose IgG-coated red blood cells, confirming prior studies in which PMN cultured for up to 48 hours with IFN-γ without the other cytokines functioned normally.18 Another potential explanation for the failure of HLA-DR expressing PMN to present TT could be that PMN normally engulf particulate Ag and may not be able to effectively take up and process soluble Ag such as TT. Therefore, we provided preprocessed Ag in the form of TT peptide, and examined the ability of TT peptide-specific T cells to proliferate in the presence of TT peptide and HLA-DR–expressing PMN. Despite multiple attempts, the use of multiple donors, and relatively high levels of HLA-DR expression by PMN, TT peptide-specific T cells also failed to respond, unless monocytes were provided as APC (Fig 3). Therefore, we considered two additional explanations for the lack of TT presentation by MHC class II expressing PMN: (1) that second signal molecules required for T-cell activation are absent on PMN, and (2) that differences might exist between HLA-DR on PMN and that on monocytes. It is now generally recognized that in order for T cells to proliferate in response to Ag they usually require that second signal molecules such as B7-1 and B7-2 be present on APC with which they interact.19 20 In the absence of such accessory molecules, the autocrine growth factor IL-2 is not produced and T cells are thus unable to proliferate. Flow cytometry analysis of PMN confirmed that little or no B7.1 or B7.2 is present on PMN whether or not they are treated with cytokines. However, it appears that the absence of B7-1 and B7-2 by itself is not sufficient to explain the lack of TT presentation, because addition of exogenous IL-2 also did not result in a T-cell proliferative response to TT (data not shown). Another possibility is that PMN express aberrant forms of HLA-DR molecules. Western blot analysis of HLA-DR derived from whole cell extracts of PMN in vitro and in vivo did show apparent differences in the structure of PMN HLA-DR as compared to that of monocytes (data not shown). Even a slight change in MHC class II structure has the potential to alter the production and trafficking of HLA-DR, as well as peptide loading, possibly explaining why both TT peptides, and the addition of exogenous IL-2 to TT presentation assays, failed to bring about T-cell proliferation. Further studies to determine the precise nature of these apparent differences in the structure of PMN HLA-DR are in progress.
In contrast to TT, it is clear that HLA-DR expressing PMN are capable of supporting T-cell activation by bacterial SAg. The level of SAg-induced T-cell proliferation correlated with the level of HLA-DR expression (Fig 6 and Table 2) and was inhibited in the presence of an anti–HLA-DR MoAb (Fig 7). The ability of PMN to stimulate SAg-induced T-cell proliferation suggests that required accessory molecules, such as intracellular adhesion molecule-1 and vascular cell adhesion molecule-1,21,22 are expressed by PMN in sufficient amounts to stimulate T-cell activation in the absence of B7-1 and B7-2 . Furthermore, these results are similar to those obtained in studies using human eosinophils. Upregulation of eosinophil HLA-DR expression has been observed in the presence of either IL-3, GM-CSF, or IFN-γ.23-25 Functionally, these HLA-DR+ eosinophils are capable of stimulating SAg-induced, but not Ag-induced, CD4+ T-cell proliferation.26
In contrast to conventional Ag presentation in which peptides of the Ag are presented in the cleft of the HLA-DR molecule, bacterial SAg bind to various external sites of the HLA-DR molecule, depending on the SAg.27-30 These SAg also bind to the β chain variable (Vβ) region of the T-cell Ag receptor (TCR), causing association of the class II molecule with the TCR, ultimately leading to T-cell activation.31,32 Given this, and the number of potential clinical applications which use GM-CSF and IFN-γ, it will be important to better understand the full consequences of HLA-DR induction on PMN in vivo in response to these cytokines.13,14 SAg such as Staphylococcus aureus enterotoxins, toxic shock syndrome toxin (TSST-1), and exfoliative toxin are a group of well-characterized proteins implicated in the pathogenesis of food poisoning,33 toxic shock syndrome,34 and scalded skin syndrome, respectively.35 The exact mechanisms through which these SAg initiate such pathophysiological conditions remain incompletely understood. However, it is thought that such symptoms are primarily related to the binding of SAg to MHC class II molecules on monocytes/macrophages, followed by T-cell activation and cytokine release. In addition, due to the fact that there are multiple Vβ specificities, a given SAg can stimulate more than 10% of all T cells. This is in contrast to the possible one per thousand to one per million T cells capable of being stimulated by a particular Ag through conventional Ag presentation. Thus, significantly increasing the number of SAg-presenting cells through induction of HLA-DR on PMN could have serious consequences for patient outcome, particularly where there is risk of exposure to SAg-expressing bacteria. Moreover, the threshold for a significant T-cell response to SAg by different individuals may in some instances be related to their capacity to express class II on PMN. Although doses of SAg were purposely chosen to elicit T-cell responses with PMN from both high- and low-responder individuals (Figs 6 and 7), SAg could be titrated to lower doses which stimulated significant T-cell proliferation only in the presence of high-responder PMN.
This difference in threshold for a T-cell response would be expected to result in significantly different SAg-induced responses in vivo. In addition, it has been proposed SAg may play a significant role in a number of disease processes in which GM-CSF and IFN-γ may be present. These include rheumatic fever, lupus erythematosis, and rheumatoid arthritis.36 Given also that PMN enter inflammatory sites in very large numbers, expression of HLA-DR on PMN and their subsequent interaction with SAg could significantly exacerbate such diseases, dependent on the level of donor expression. Although these studies suggest presentation of soluble protein Ag, such as TT, may not represent a serious concern, further studies using other Ag are needed.
This work represents the first delineation of the functional capabilities of HLA-DR+ PMN, and represents the first significant step in understanding what impact these cells may have on immune regulation. Our data document a polymorphism among individuals with respect to induction of MHC class II on PMN and consequently on the capacity of an individual's PMN to support SAg-mediated T-cell activation. These observations may have particular relevance for patients treated with IFN-γ and/or GM-CSF, or for patients with SAg-induced inflammatory responses.
ACKNOWLEDGMENT
We acknowledge Alice Given and Gary Ward for their expertise in flow cytometric analysis, Sharon Swink and Lorraine Pfefferkorn for help with superoxide assays, Michelle Lennartz and Kambiz Karimi for assistance in conducting phagocytosis assays, Brian Whalen for his analysis of PMN HLA-DR expression following a 24- and 48-hour pulse with cytokines, and Dorit Voigtlaender for her assistance in preparation of this manuscript. We also thank Dr J. Parsonnet for providing TSST-1 and for helpful advice.
Supported by National Institute of Health Grants No. AI34478, AI35327, AI37212, and AI34478. Flow cytometry analysis was performed either at Dartmouth-Hitchcock Medical Center in the Herbert C. Englert Cell Analysis Laboratory, which was established with a grant from the Fannie E. Rippel Foundation, and is supported in part by the core grant of the Norris Cotton Cancer Center (CA-23108), or at Albany Medical Center in the Cellular Immunology Laboratory.
Address reprint requests to Edmund J. Gosselin, PhD, Department of Microbiology, Immunology and Molecular Genetics, Albany Medical College, Albany, NY 12208.


![Fig. 2. Inability of GM-CSF/IFN-γ–treated (HLA-DR+) PMN to stimulate TT-specific T cells. Purified monocytes (Mφ; 5 × 104/well) were used as control APC. PMN were first incubated for 24 hours in the presence of GM-CSF/IFN-γ (50 U/mL), and then added to wells of a 96-well plate at concentrations of 0, 1.25, or 2.5 × 105 cells/well. TT-specific T cells were than added, and the Ag presentation assay performed as described in Materials and Methods. [3H]TdR uptake was measured at 72 to 96 hours. Data are expressed as cpm ± SD of duplicate samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.4128/4/m_bl_0007f2.jpeg?Expires=1767751826&Signature=wP6toxT9wR~V2CZ3cIqIdBXkp4azFCPfYjXIE0mopvS~0n~uPqzGkrp7w2XJlROlrGHQucGyFS1cTFN~X651ccwFXc0avvB4hkn-wx7DOFGCYiTtFPqd101zf4YOQHMcGwxO7fzSkL0~I~lB3ZbIXdba~Ihv~kPq9vKOHCxf40737sIYAbWO1~JnqtSuGCChslnbeaDa4cqnkvhb6JYn2iTLHu5YbKIbeLJ1mtbJEc5mrs7-rx0uZrfTbY2Bm3wjKBmE88gqV5e-fUhFkmdaTYHSuqMLkBOOnEXY6hbwWEC84GK6-RuyxZyabUi-1C7A5Ca3LfW876~oSLmIUgdSWQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Inability of GM-CSF/IFN-γ–treated (HLA-DR+) PMN to stimulate TT peptide-specific T cells. Flow cytometric analysis of PMN treated with GM-CSF/IFN-γ (50 U/mL) for the presence of HLA-DR (inset) was conducted as described in Materials and Methods. The open peak represents staining with the isotype control. The closed peak represents staining with HLA-DR–specific antibody. T-cell stimulation was conducted as described in Fig 2, but using 5 μg/mL TT peptide instead of whole TT. [3H]TdR uptake was measured at 72 to 96 hours. Data are expressed as cpm ± SD of triplicate samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.4128/4/m_bl_0007f3.jpeg?Expires=1767751826&Signature=lZ0OcZfWlglk8HOu-hVNixqVeSrTvzOmUOzZUnjMjymh5xoiIdeMfe5~Yj~fPhSSvu6G79cvQ1-nef1p1npPJgJdajHVMA8yR0HmpiZm01pDxayJVugN8ZrLjU6lLMhtbB9zNOgu1ESXj~xpooQMK6p5yT8Rw0~IKwjP3IYQ-BNdDHRHW~RgiivWRyA6NYoxEmQIQmyrIcqWSdleppJr-Di-IKQTEDvmiOwuvUTFLutS-Qbm00WNEFG~E7iMBZnzdRD7308o27Hgvw4tkbsJmwBU39Afr4w3WrRvrTdFRFlNTFN-FUTLCokoWkGfoz~p60h-GRt8p6qr~VMkDqGYLg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 6. SAg-induced T-cell proliferation by HLA-DR+ PMN. PMN were cultured for 44 hours in either the absence or presence of 100 U/mL GM-CSF/IFN-γ from a known HLA-DRlow responder (donor 7; , unstimulated; □, GM-CSF/IFN-γ stimulated) and HLA-DRhigh responder (donor 2; ▨, unstimulated; ▪, GM-CSF/IFN-γ stimulated). SAg presentation assays were performed by incubating 1 × 105 PMN with 5 × 104 CD4+ T lymphocytes in the presence of 1 nmol/L SEA, SEB, SEC1, SEE, or 10 nmol/L TSST-1 for 66 hours. PMN SAg-induced T-cell proliferation is presented as counts per minute (cpm). Values reflect the amount of [3H]TdR incorporated after an 18- to 20-hour pulse and are the mean of triplicate samples ± SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.4128/4/m_bl_0007f6.jpeg?Expires=1767751826&Signature=wN6dSzdYwqQdiJfnTzcCsZj0ibsj~hwS78yXG68paz1SsN9ezTipvCDc0gCVT71KdJ5IczfAdZIoi-IxMplGpOv3iGKruRNnu3T45MKpwlY8zRWYgHUOCLFKQONi2w3gThGwWv0LiPZRi-T0dqUkx6lu6aRfulSuMbjthwVjPI0EGsb3DzRp1Q78N44VvKuNXSNc0VUXKkWWfHUw-G4tQvkjtxIHjM6Bc7vjNaGOPN6sRH10HKSqy59YB-3c3paEHE4UBPcaXcSmQFo3MZVIl2pTAgJB3wgTsUWkPCPHupTjwdhum6HbGQioQKzxP2saQ4Yb1Z9ntciMykh8IlrrsA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. PMN SAg-induced T-cell proliferation is HLA-DR–restricted. PMN from both an HLA-DRlow responder (donor 7; , unstimulated; □, GM-CSF/IFN-γ) and HLA-DRhigh responder (donor 2; ▨, unstimulated; ▪, GM-CSF/IFN-γ) were cultured for 44 hours in the absence or presence of 100 U/mL GM-CSF/IFN-γ, and 1 × 105 PMN from each condition were incubated with 5 × 104 CD4+ T lymphocytes in the presence of 1 nmol/L SEA. The MoAbs L243 (anti–HLA-DR), 197 (anti-CD64), and AML223 (anti-CD14) were added to the appropriate wells 30 minutes before the addition of SAg. T-cell proliferation was measured at 66 hours with an 18- to 20-hour [3H]TdR pulse and is presented as counts per minute (cpm). Values represent the mean of triplicate samples ± SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.4128/4/m_bl_0007f7.jpeg?Expires=1767751826&Signature=ZPiLdNvVg6-EoEtQRQXJ6IjL1lp0GhYzzyMkUmgtYrF3-Ll9NEDr45BxUT97jb1w6R~Fm5tZiaBj1azzFDv0mnjfMz1crxxnQrxD-xrcDa9KuTxhQEzoDczwuBzSeHYQeQVXjCwAAOftbi72HEj81MxTJtHfboVsYc2BDYNjb3f7rTv2xRnJn8bm8XjE3p5xeH6pQV-9Y7JhafacipllZ3iX7h-puIlh3zmxSSg~rpNsi6Y8WyzdPNz0SbGrrbNxlfndud0jelHV4cKAu6AI~bNKm2WbmTH8acJvgEBDuL06qtxQQrjrgdDJ4sHrNwX2pXklgc4djYcO3PBNLcfRzQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal