Abstract
RANTES and related molecules, constitute the C-C class of chemokine supergene family and a group of cytokines produced by hematopoietic cells constitute the MCP-1 or C-X-C class. The roles of most of these chemokines are not well known, although members of the C-X-C family are inflammatory agents. Here, we report that intradermal injection of RANTES 10 ng/50 μL subcutaneously in the abdominal skin produced a strong inflammatory reaction, as evidenced by Evans blue dye, greater than FMLP (10−6 mol/L) (∼57%); while MCP-1, 10 ng/50 μL was less effective than FMLP (10−6 mol/L) (∼54%). Moreover, the histologic analysis of the cells stained with Toluidine blue (0.1%) were analyzed at a magnification of ×40). RANTES 10 ng/50 μL and LPS produced higher numbers (142 ± 11 and 193 ± 21 of cells/200 mm2, respectively) of basophilic cell accumulation in the skin injection sites compared with FMLP (10−6 mol/L) (127 ± 14/200 mm2), while MCP-1 10 ng/50 μL was less effective (88 ± 10/200 mm2). Electron microscopy (×13,800) studies of skin injection sites revealed that RANTES was chemoattractant for mast cells. In a Northern blot analysis from homogeneous tissue biopsy from the intradermal injection sites, RANTES was more potent than MCP-1 in increasing histidine decarboxylase (HDC) mRNA, the sole enzyme responsible for the production of histamine from histidine. Since PGD2 is formed by mast cells on cell activation, we also studied the effect of RANTES and MCP-1 on PGD2 production in inflamed tissue in vivo. RANTES (20, 10, and 5 ng) and MCP-1 (20, 10, and 5 ng) strongly stimulated PGD2 , in a dose-dependent manner, with a potency rank order of RANTES (10 ng/mL) approximately two times greater than MCP-1 (10 ng/mL).
THE CHEMOKINE SUPERGENE family are 8- to 10-kD proteins inducible in a number of pathophysiologic processes.1-3 These chemotactic cytokines have been subdivided according to the position of two conserved cysteine residues in the conserved motif: CXC chemokines, separated by one amino acid, and C-C chemokines, with two adjacent cysteines.2 Members of the C-C subfamily include monocyte chemotactic proteins (MCP-1, MCP-2, MCP-3), macrophage inflammatory proteins (MIP-1α, MIP-1β), and regulated on activation, normal T-cell expressed and presumably secreted (RANTES), which generally mediate monocyte/macrophage activation and recruitment.2-4 Members of the C-X-C chemokine subfamily include IL-8, which is produced by stimulated mononuclear phagocytes and other cells, and MIP-2,5,6 which is chemokinetic for both neutrophils and macrophages; IL-8 is an activating and chemotactic factor for neutrophils and, to a lesser extent, for eosinophils, basophils, and lymphocytes.2,7 RANTES, a protein of 8 to 10 kD, chemoattracts eosinophils, monocytes, and certain T lymphocyte subsets and is coded for by a gene cluster located on human chromosome 17.1 RANTES is a human T-cell specific molecule first characterized by Schall et al.8 This molecule increased the adherence and recruitment of monocytes to endothelial cells, playing a major role in inflammation. RANTES is a member of the β intercrine subfamily, reported to be a selective chemoattractant for human monocytes rather than neutrophils, and is also a chemoattractant for memory T lymphocytes, CD4+ cells. RANTES-induced migration of CD4+ T lymphocytes in the dog resulted in rapid formation of eosinophilic infiltrate in the site of inflammation.8-10 Recent experiments indicate that RANTES does not contribute to the accumulation of PMN in the lung after the intraperitoneal (ip) administration of endotoxin.11
MCP-1 is the prototype of the C-C chemokine subfamily, purified from different sources with chemoattractant and activator properties.1-3 MCP-1 is a potent proinflammatory protein, as effective as the complement component C5a in allergic diseases, induces high levels of histamine release from human basophils, and stimulates exocytosis.12,13 However, little is known about the factors that induced the migration, activation, and mediator release of basophils/mast cells at the site of inflammation.14 15 A rat in vivo model has been established to determine whether RANTES (which induces and activates eosinophils, mast cells, lymphocytes, macrophages) and MCP-1 can recruit and activate basophils to the site of inflammation. In this study, a single intradermal injection of RANTES or MCP-1 in the rat resulted in basophilic cell-rich inflammatory exudate within 4 hours. RANTES (human recombinant) resulted in a more potent chemoattractant compound than human recombinant MCP-1. We also determined the impact of these two cytokines on histidine decarboxylase (HDC) activation and mRNA levels, an important basophilic cell biochemical and functional marker responsible for the production of histamine from histidine.
MATERIALS AND METHODS
Animals and materials.Sprague-Dawley rats obtained from Tacomic (Germantown, NY), were kept in virus-free sections of a modern animal facility (Tufts University) and were allowed access to food and water ad libitum. Male rats 250 to 300 g in weight were anesthetized with Ketamine HCl (1 mL/1 lb body weight) 0.2-mL injection (USP Fort Dodge Lab Inc, Fort Dodge, IA) plus xylazine HCl (100 mg/mL) 0.05-mL injection (Fermenta Animal Health Co, Kansas City, MO). The injection was made in the lower right quadrant of the abdomen. After 5 minutes, the abdomens of the rats under anesthesia were shaved in four places and were injected with a 50-μL intradermal injection of chemokines (RANTES and MCP-1), PBS (negative control), and lipopolysaccharide (LPS) from Escherichia coli serotype 0111:B4 TCA extract (Sigma, St Louis, MO; catalogue or stock no. L-4130) or N-formylmethionine-leucyl-phenylalanine (FMLP, Sigma, positive control) in different concentrations. In another set of experiments, after the injections of the chemokines, PBS and the positive control, a 0.6-mL solution of Evan's blue dye (1% in saline) was injected into the tail vein of the rats attaining a blue color in the areas where the compounds were injected in the positive controls; in the injected areas of the negative controls there was no discoloration. As for other intravital dyes the intensity for Evan's blue dye accumulation at the site of inflammation is correlated to plasma exudation and cellular extravasation events. After 4 hours from the intradermic injections, the animals were killed by inhalation of CO2 and then decapitated. The areas colored with the dye (Sigma E2129) were measured, enucleated, and immerged in a fixative solution (5% formaldehyde, Polyscience Inc, Warrington, PA; plus 0.2 m PBS for at least 10-15 minutes before use). Slides were prepared with sections of the tissue and colored with Toluidine blue (0.1% in 1% sodium borate for 2 minutes) and analyzed under an optic microscope (× 10, × 20, or × 40, Nikon Diaphore THD microscope, Tokyo, Japan). The basophilic cells were counted in the optic field using a grating size 5 × 5 mm.
Recombinant human monocyte chemotactic protein-1 (MCP-1) and RANTES were purchased from R&D systems, Minneapolis, MN. Preparation of RANTES and MCP-1 contained less than 1 endotoxin U/mg of protein, as determined by the Limulus amaebocyte lysate reaction.
Electromicroscopy studies.Other tissue samples of injected compounds were removed for electron microscopy analysis.12 16 The samples were enucleated and fixed for 1 hour at room temperature with 3% glutaraldehyde in 0.1 mol/L cacodylate-HCl buffer (pH 7.2). The tissues were then infiltrated with two 10-minute changes of 100% propylene oxide followed by overnight exposure to a 1:1 mixture of propylene oxide and DMP-30. The next day the skin tissues were embedded in Epon with DMP-30. Embedded tissues were placed in a 56°C oven to polymerize for 48 hours.
Thick and thin sections were cut on a Sorvall MT-1 and MT-2B Ultramicrotomes equipped with glass and diamond knives, respectively. Sections (1,000 Å) were picked up on 300 mesh copper grids and stained with both uranium and lead salts. The sections were then examined and photographed using a JEOL JEM-100s transmission electron microscope operated at an accelerating voltage of 80 kV.
Preparation of the histidine decarboxylase (HDC) probe.We used a probe made from a reverse transcribed rat brain polyA+ mRNA. Total cellular RNA was extracted from New England Deaconess Hospital rat brain. PolyA mRNA was purified by one-step chromatography on oligodT column. A sample of 2 μg polyA+ mRNA was reverse transcribed at 42°C for 40 minutes in a 20 mL mixture containing 4 mL of 5× reverse transcriptase buffer (250 mmol/L TRI HCl pH 8.3 at 42°C, 50 mmol/L MgCl2 , 250 mmol/L KCl, 15 mmol/L DTT, 10 U placental RNase inhibitor, 0.5 mmol/L each dNTP, 50 pmol oligodT primer and 20 U Avian myeloblastosis virus (AMV) reverse transcriptase. After reverse transcription the HDC cDNA was amplified by polymerase chain reaction using two specific primers synthesized on a gene assembler plus (Pharmacia LKB): 5′ primer, 5′-ATGATGG-AGCCCAGTGAATACC and 3′ primer, 5′CCAGAATTCGCATGT-CTGAGG-TAG. Four milliliters of single-stranded cDNA mixture was supplemented with 50 pmol of each 5′ and 3′ primers in a volume of 50 mL denatured for 2 minutes in a boiling bath and added to a 50-mL mix prewarmed at 72°C containing 0.25 mmol/L each dNTP, 10 mL 10× Taq polymerase buffer (500 mmol/L KCl, 100 mmol/L TRIS-HCl pH 8.3 at 25°C, 15 mmol/L MgCl2 and 0.1% gelatin) and 1.5 U of Taq polymerase (Perkin-Elmer Cetus). The PCR program consisted of one cycle of 1 minute at 94°C and 15 min at 72°C followed by 35 cycles of 30 seconds at 94°C, 30 seconds at 55°C, and 4 minutes at 72°C, and was completed by an additional annealing at 55°C for 30 seconds and a final elongation at 72°C for 15 minutes. PCR was performed in a Techne PHC-2 programmable heating block. Amplified products were purified by glass-milk procedure (Geneclean BIO 101), blunt-ended with the Klenow fragment and cut by EcoRI. The resulting blunt-end EcoRI fragments were cloned in the p-MAL vector (Biolabs) cut by both Stu I and EcoRI restriction enzymes. The resulting recombinant plasmid was amplified in the TB1 E coli strain. Plasmid DNA was sequenced by the double stranded protocol of the sequenase kit (USB Cleveland). Plasmid containing amplified HDC cDNA was prepared according to the alkaline lysis method and purified on a CL4B column.
Northern blot analysis.Northern blot analysis was performed on enucleated tissue from the intradermal injection sites tested with RANTES and MCP-1, the negative control (PBS) and the positive control (LPS or FMLP).
Total RNA was isolated by guanidine hydrochloride as previously described.17 18 Total RNA (10 mg/lane) was fractioned by electrophoresis on a 2% agarose gel, transferred to nylon membranes (Hybond N, Amersham), and hybridized with 32P (2 × 108 cpm/mg). It was then washed four times at room temperature for 15 minutes in 2× SSC and 0.1% SDS, heated to 48°C for 30 minutes, then washed twice in 0.1× SSC and 0.1% SDS. Membranes were finally exposed to Kodak XAR5 for 3 days at −70°C. Signals were compared with ribosomal RNA to evaluate an equal quantity of RNA for each lane.
Histamine measurement.Histamine in tissue supernatants was estimated by a radioenzymatic method, essentially a modification of the method described by Kaplan et al and others.19-21 Tissues were minced in cold PBS then resuspended in water (1 g/0.2 mL). This suspension was sonicated in a Branson 1200 ultrasound devise for 10 minutes vortexed for 5 minutes, and pelleted by 10-minute centrifugation at top speed in an Eppendorf microcentrifuge. Tissue histamine taken from the injection sites was determined after centrifugation at 400g for 5 minutes. To 20 μL of supernatant taken from disrupted tissue, 2 μL 20% perchloric acid, 6 μL 1 N NaOH, and 100 μL 0.05 mol/L sodium phosphate pH 7.4 was added. The precipitate was pelleted by 5-minute centrifugation at top speed in an Eppendorf microfuge and aliquots were taken from the supernatant for further histamine assay. In 50 μL of reaction mixture for histamine assay 5 to 25 μL sample, 5 μL rat kidney histamine methyl transferase, and S-(methyl-3H) adenosyl-L methionine (73.8 Ci/mmol/L, Amersham) were present.
Isotope and enzyme were diluted with 0.05 mol/L sodium phosphate pH 7.4 for optimal conditions. The reaction mixture was incubated for 1 hour at 37°C, following which 20 μL 1.5 mol/L perchloric acid, 20 μL 10 mol/L NaOH, and 500 μL freshly prepared toluene-isoamylalcohol (4:1 vol/vol) was added and the mixture was shaken for 10 minutes. After 10-minute centrifugation at 200g, 0.3 mL was taken from the upper phase and 4 mL of Aquasol (New England Nuclear, Boston, MA) scintillation fluid was added. Radioactivity was measured in a beta counter and the concentration of histamine was computed by using a standard curve. Samples were always performed in duplicate from duplicate cultures. Histamine methyltransferase was a gift from the laboratory of Dr David Cochrane (Medford, MA) and dinitrophenyl-bovine serum albumin (DNP-BSA) was provided by Dr Fu-Tong Liu, Scripps Clinic, La Jolla, CA.
Quantitation and characterization of prostaglandin D2 by radioimmunoassay method.After 4 hours of intradermal injection of RANTES, MCP-I (at different concentrations 20-10-5 ng/50 μL), LPS (10 ng/50 μL), and PBS (control, 50 μL) the tissues from intradermal injection sites were enucleated, minced in cold PBS, and vortexed at 4°C for 3 minutes. The minced tissue was subsequently centrifuged at 300 rpm and the tissue-free supernatants were stored at −70°C before RIA. Immunoreactive PGD2 was measured by RIA carried out in triplicate and determined in pg/mL.22 Release of PGD2 was performed with a kit from New England Nuclear. Antisera to PGD2 cross-reacts with thromboxane B2 (TxB2 ) 0.022% and with 6-keto-PGF1α 0.01%.
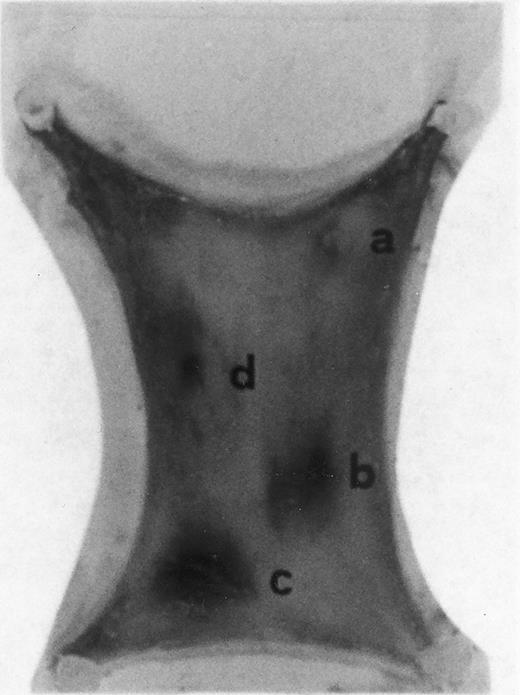
Evan's blue-stained intradermal injection sites showing the kinetics of the dermal response to exogenous human RANTES 10 ng/50 μL (c) and MCP-1 10 ng/50 μL (d); while (a) is a negative control (PBS 50 μL) and (b) is a positive control (FMLP 10−6 mol/L in 50 μL). This representative experiment was found to be reproducible and has been performed at least three times.
Evan's blue-stained intradermal injection sites showing the kinetics of the dermal response to exogenous human RANTES 10 ng/50 μL (c) and MCP-1 10 ng/50 μL (d); while (a) is a negative control (PBS 50 μL) and (b) is a positive control (FMLP 10−6 mol/L in 50 μL). This representative experiment was found to be reproducible and has been performed at least three times.
Mean Measured Diameters (in mm) of the Inflamed Area After Injection With PBS, FMLP, LPS, RANTES, and MCP-1
| Treatment 50 μL . | Mean of Major Diameters of 4 Samples (mm) . | P< . |
|---|---|---|
| PBS (control) | ND | — |
| FMLP 10−6 mol/L | 37.16 ± 8 | .05 |
| LPS 10 ng | 84.66 ± 10 | — |
| RANTES 20 ng | 70.52 ± 12 | (*) |
| RANTES 10 ng | 58.45 ± 11 | (**) |
| RANTES 5 ng | 10 ± 10 | — |
| MCP-1 20 ng | 22.1 ± 5 | .01 |
| MCP-1 10 ng | 20.3 ± 5 | .01 |
| MCP-1 5 ng | ND | — |
| Treatment 50 μL . | Mean of Major Diameters of 4 Samples (mm) . | P< . |
|---|---|---|
| PBS (control) | ND | — |
| FMLP 10−6 mol/L | 37.16 ± 8 | .05 |
| LPS 10 ng | 84.66 ± 10 | — |
| RANTES 20 ng | 70.52 ± 12 | (*) |
| RANTES 10 ng | 58.45 ± 11 | (**) |
| RANTES 5 ng | 10 ± 10 | — |
| MCP-1 20 ng | 22.1 ± 5 | .01 |
| MCP-1 10 ng | 20.3 ± 5 | .01 |
| MCP-1 5 ng | ND | — |
These values represent the mean ± SD of the major diameters of inflamed injection sites in four separate experiments. Each animal received a 50 μL intradermal injection and then was killed after 4 hours. The P values (Student's t-test) are calculated by comparing RANTES (20-10 ng)-treated animals, respectively.
Abbreviation: ND, not determined.
Statistical analyses.Data from different experiments were combined and reported as the mean ± SD. Student's t-test for independent means was used to provide a statistical analyses (P > .05 was considered as not significant).
RESULTS
Intradermal injection sites of chemokines RANTES and MCP-1.In order to assess the inflammatory activities of RANTES and MCP-1 in an in vivo model we have injected 50 μL of these chemokines in four distinct sites of the abdomen of rats for the duration of 4 hours. At this time point animals were killed in accordance with previous data indicating that 4 hours is the optimal time for chemokine-induced chemoattraction.23-26 RANTES and MCP-1 were used at 10 ng/50 μL; while the chemoattractant peptide FMLP was used at 10−6 mol/L.8 In Fig 1 PBS (a, negative control) was not effective in promoting inflammation; RANTES at 10 ng/50 μL (c) enhanced dye extravasation at a greater extent than FMLP 10−6 mol/L (b, positive control); while MCP-1 at 10 ng/50 μL (d) was less effective than FMLP. In Table 1 we report a representative experiment with 12 treated rats. After chemokine challenge (4 hours) we calculated the mean ± S.D. of the major diameters (in mm) of dye extravasation at the site of injections (4 rats). Reported P values were obtained by comparing the effect of RANTES to MCP-1.
The size of the inflamed areas treated with RANTES (10-20 ng/50 μL) were significantly larger when compared with MCP-1 (10-20 ng/50 μL) and FMLP (10−6 mol/L) (50 μL)-treated areas (Table 1). However, in LPS treatment, larger dye extravasation areas were produced than those observed with RANTES at either concentrations of 10 or 20 ng/50 μL. Maximum dye extravasation was obtained with LPS (84.6 mm ± 10, 10 ng/50 μL) and RANTES treatments (70.52 ± 12, 20 ng/50 μL). When H1 or H2 blockers were used to exclude the possible involvement of basophilic cell degranulation, the inflammatory effect of RANTES and MCP-1 was not changed.31
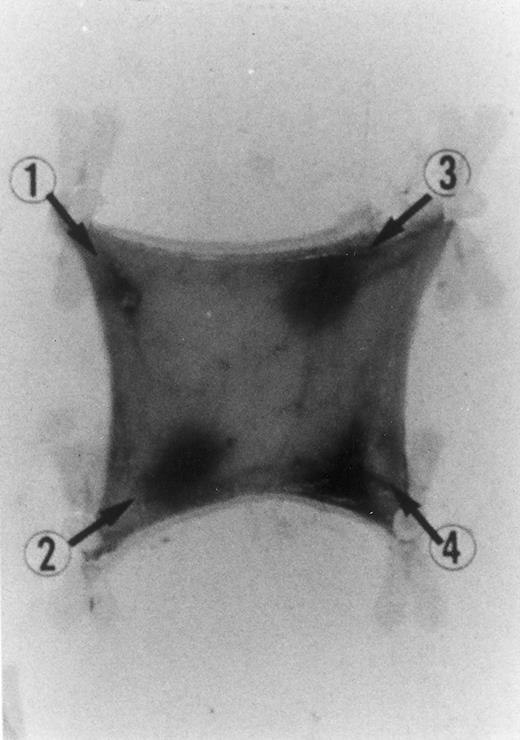
Evan's blue-stained intradermal injection sites showing the kinetics of the dermal inflammatory response to exogenous human recombinant RANTES 20 ng/50 μL (no. 2) and 10 ng/50 μL (no. 3); while PBS 50 μL was a negative control (no. 1) and LPS 10 ng/50 μL is a positive control (no. 4). This representative experiment was found to be reproducible and has been performed at least three times.
Evan's blue-stained intradermal injection sites showing the kinetics of the dermal inflammatory response to exogenous human recombinant RANTES 20 ng/50 μL (no. 2) and 10 ng/50 μL (no. 3); while PBS 50 μL was a negative control (no. 1) and LPS 10 ng/50 μL is a positive control (no. 4). This representative experiment was found to be reproducible and has been performed at least three times.
In Fig 2 we show the histologic results of a representative experiment (N = 12 rats) 4 hours after intradermal injection (50 μL) of LPS (10 ng), RANTES 20 and 10 ng, and PBS (control). This figure shows that while PBS (injection site no. 1) does not produce any appreciable effect, LPS (10 ng/50 μL, injection site no. 4) and RANTES (20 ng, site no. 2; and 10 ng, site no. 3) produced a marked enlargement of dye effusion.
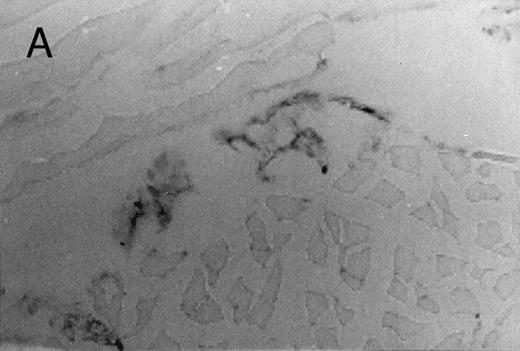
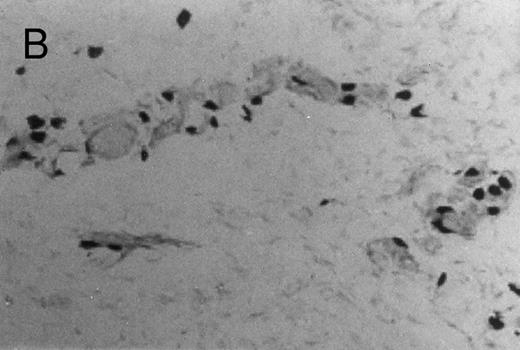
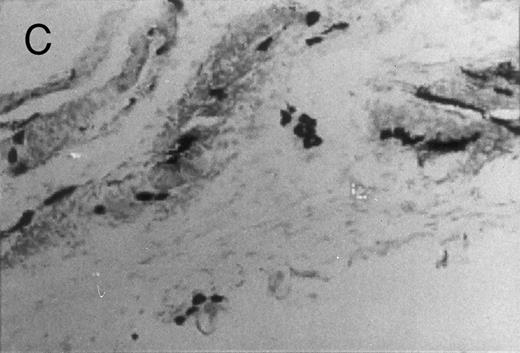
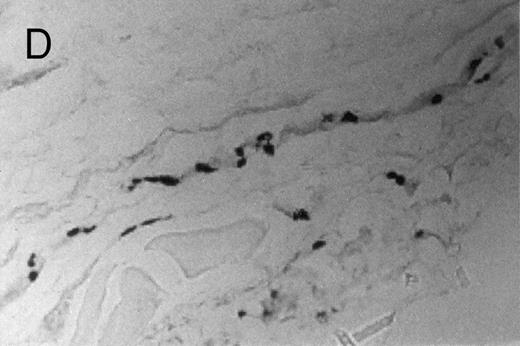
Chemotactic effect of human recombinant RANTES and MCP-1 on basophilic cells.Recently it has been reported that RANTES and MCP-1, -2, and -3 are chemotactic agents for human T lymphocytes.23 26 In this study we evaluated the selective chemotactic effects of RANTES and MCP-1 on basophilic cells. Figure 3 shows an increased number of basophilic cells after a 50 μL intradermal injection of FMLP (10−6 mol/L), RANTES 10 ng and MCP-1 10 ng, while PBS (vehicle) did not produce any appreciable effect. The infiltrated basophilic cells were colored with Toluidine blue (0.1%) and counted in the optic field using a grating of size 5 × 5 mm, under an optic microscope (× 40). In Table 2 results indicate that RANTES at 20 and 10 ng is more potent than equal doses of MCP-1 (<0.05 and 0.01, respectively). The highest number of basophilic cell accumulation in the skin injection sites was obtained with LPS (10 ng/50 μL) (193 ± 21), while FMLP (10−6 mol/L) (127 ± 14) was found to be less effective.
Basophilic cell migration induced by human recombinant RANTES 10 ng (C) and MCP-1 10 ng (D) into sites of injection. The sections were stained with Toluidine blue (0.1%) and analyzed at a magnification of ×40 (Nikon Diaphore). (A) represents a control and (B) is a positive control where FMLP was used at 10−6 mol/L. This representative experiment was found to be reproducible and has been performed at least three times.
Basophilic cell migration induced by human recombinant RANTES 10 ng (C) and MCP-1 10 ng (D) into sites of injection. The sections were stained with Toluidine blue (0.1%) and analyzed at a magnification of ×40 (Nikon Diaphore). (A) represents a control and (B) is a positive control where FMLP was used at 10−6 mol/L. This representative experiment was found to be reproducible and has been performed at least three times.
Number of Basophils Chemoattracted to the Inflamed Area After Injection With PBS, FMLP, LPS, RANTES, and MCP-1
| Treatment . | No. of Cells in 200 mm2 . | P< . |
|---|---|---|
| PBS (control) | 32 ± 12 | — |
| LPS 10 ng | 193 ± 21 | — |
| FMLP 10−6 mol/L | 127 ± 14 | — |
| RANTES 20 ng | 150 ± 10 | (*) |
| RANTES 10 ng | 142 ± 11 | (**) |
| RANTES 5 ng | 80 ± 16 | — |
| MCP-1 20 ng | 110 ± 15 | .05 |
| MCP-1 10 ng | 88 ± 10 | .01 |
| MCP-1 5 ng | 49 ± 13 | — |
| Treatment . | No. of Cells in 200 mm2 . | P< . |
|---|---|---|
| PBS (control) | 32 ± 12 | — |
| LPS 10 ng | 193 ± 21 | — |
| FMLP 10−6 mol/L | 127 ± 14 | — |
| RANTES 20 ng | 150 ± 10 | (*) |
| RANTES 10 ng | 142 ± 11 | (**) |
| RANTES 5 ng | 80 ± 16 | — |
| MCP-1 20 ng | 110 ± 15 | .05 |
| MCP-1 10 ng | 88 ± 10 | .01 |
| MCP-1 5 ng | 49 ± 13 | — |
Number of basophilic cells responding to exogenous human RANTES and MCP-1 at various doses in 50 μL intradermal injection in the rat. The sections were stained with Toluidine blue at 0.1% and counted in an optic field using a grating size 5 × 5 mm under an optic microscope × 40. This representative experiment was found to be reproducible and has been performed at least three times.
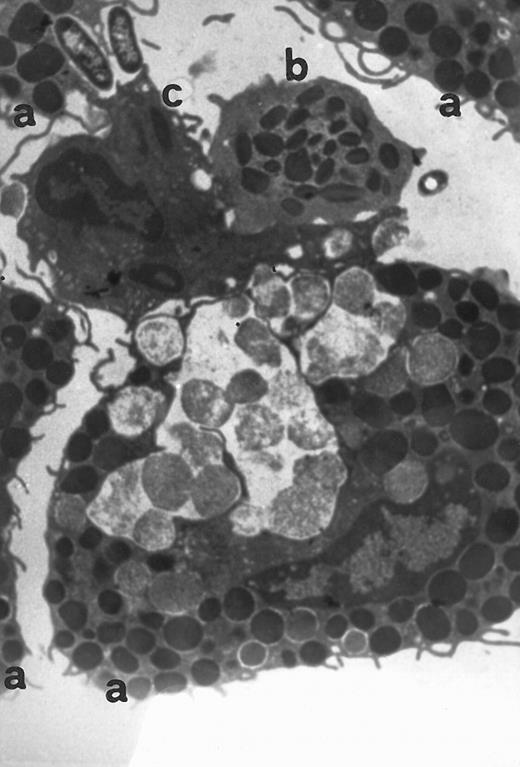
Electron microscopy of skin injection sites.To better analyze the skin injection sites exposed to RANTES, an electron microscopic study was performed. Here we show a representative experiment of four (Fig 4). This electron micrograph (×13,800) depicts accumulation of four basophilic cells (a), one eosinophil (b), and a macrophage (c), 4 hours from injection, with a pattern describing a typical inflammatory event and showing chemoattraction activities toward several cell types.
Transmission electromicrography of a representative experiment of rat inflamed injection site produced by RANTES 10 ng. The section showing the kinetics of the dermal response to exogenous RANTES 4 hours after injection. Evidence for intravascular activation of basophilic cells (a), eosinophil (b), and macrophage (c). Original magnification × 13,800.
Transmission electromicrography of a representative experiment of rat inflamed injection site produced by RANTES 10 ng. The section showing the kinetics of the dermal response to exogenous RANTES 4 hours after injection. Evidence for intravascular activation of basophilic cells (a), eosinophil (b), and macrophage (c). Original magnification × 13,800.
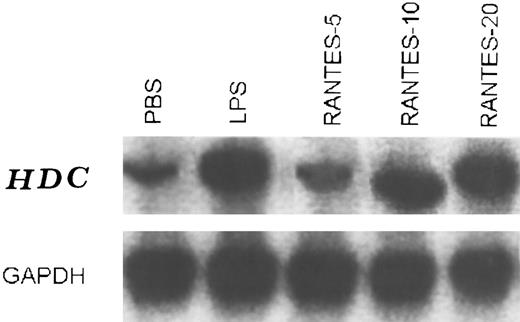
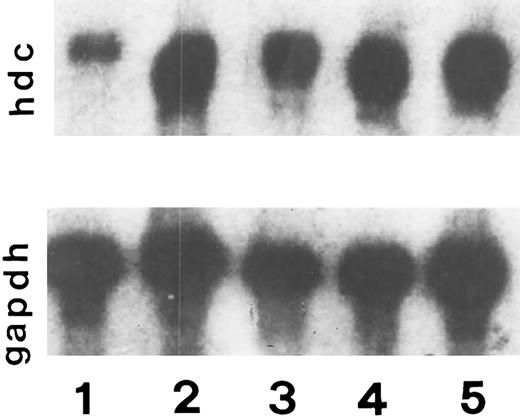
Stimulation of HDC mRNA levels by RANTES and MCP-1.A probe was prepared to detect mRNA encoding the HDC gene. Oligonucleotide primers specific for HDC sequences were used to amplify a product from rat brain cDNA and this yielded a 1,019-bp fragment of DNA following digestion with EcoRI restriction enzyme. After cloning into the p-Mal plasmid, this was used to detect HDC mRNA by Northern blot hybridization.12 Since RANTES and MCP-1 have been presented as chemoattractants for mast cells, in this study we analyzed the generation of HDC mRNA in skin injection sites. Steady-state levels of HDC mRNA in control (PBS) were low (Fig 5, lane 1). In the presence of RANTES, HDC mRNA was induced in a dose-dependent manner compared with the controls. HDC mRNA reached maximum levels when the animals were injected with RANTES (10-20 ng/50 μL) (lane 3 and 4) or LPS (20 ng/50 μL) (lane 5) (positive control). When the animals were exposed to RANTES 5 ng/50 μL there was a small increase in HDC mRNA (lane 2), while the injection of RANTES 10 ng/50 μL was more effective (lane 3) compared with the control.
Steady-state levels of HDC mRNA in the intradermal injection site tissue in the presence of RANTES (5, 10, and 20 ng/50 μL) lane 2, 3, and 4, respectively, and PBS (control) lane 1, and LPS 20 ng/50 μL (lane 5) after 4 hours of treatments. This is a representative experiment of four.
Steady-state levels of HDC mRNA in the intradermal injection site tissue in the presence of RANTES (5, 10, and 20 ng/50 μL) lane 2, 3, and 4, respectively, and PBS (control) lane 1, and LPS 20 ng/50 μL (lane 5) after 4 hours of treatments. This is a representative experiment of four.
In Fig 6 we show a Northern blot analysis in the skin injection site in rats exposed to PBS (50 μL) control (lane 1), LPS (10 ng/50 μL), (lane 2), FMLP 10−6 mol/L (lane 3), RANTES (10 ng/50 μL) (lane 4), and MCP-1 (10 ng/50 μL) (lane 5). Steady state levels of HDC mRNA in control (PBS) were low compared with the other treatments. In the presence of LPS (lane 2) and FMLP (lane 3), HDC mRNA was strongly induced compared with the controls; however, LPS was more potent than FMLP. HDC mRNA reached maximum levels when RANTES (10 ng/50 μL) was injected (lane 4). When the animals were treated with 10 ng/50 μL MCP-1 there was an appreciable effect in HDC mRNA generation (lane 5), but it was less effective than RANTES (lane 4).
Effect of RANTES 10 ng/50 μL (lane 4), MCP-1 (lane 5), LPS 20 (lane 2), FMLP 10−6 mol/L (lane 3) and PBS 50 μL (lane 1) on histidine decarboxylase mRNA expression after 4 hours of incubation. This is a representative experiment of four.
Effect of RANTES 10 ng/50 μL (lane 4), MCP-1 (lane 5), LPS 20 (lane 2), FMLP 10−6 mol/L (lane 3) and PBS 50 μL (lane 1) on histidine decarboxylase mRNA expression after 4 hours of incubation. This is a representative experiment of four.
Percent histamine release in the injection sites of the skin.RANTES and MCP-1 have been presented as potent chemotactic factors for inflammatory cells.12,23 27 To evaluate this effect more fully we studied the percent release of histamine in the injected sites of the skin exposed to PBS (control), FMLP, LPS, MCP-1, and RANTES (Table 3).
Percentage of Histamine Release After Intradermal Injection of MCP-1 and RANTES
| Treatment . | . | P< . |
|---|---|---|
| PBS (control) | ND | — |
| FMLP 10−6 mol/L | 30 ± 5 | — |
| LPS 10 ng | 42 ± 8 | — |
| MCP-1 10 ng | 20 ± 3 | (*) |
| MCP-1 20 ng | 25 ± 6 | (**) |
| RANTES 10 ng | 34 ± 5 | .05 |
| RANTES 20 ng | 40 ± 7 | .05 |
| Treatment . | . | P< . |
|---|---|---|
| PBS (control) | ND | — |
| FMLP 10−6 mol/L | 30 ± 5 | — |
| LPS 10 ng | 42 ± 8 | — |
| MCP-1 10 ng | 20 ± 3 | (*) |
| MCP-1 20 ng | 25 ± 6 | (**) |
| RANTES 10 ng | 34 ± 5 | .05 |
| RANTES 20 ng | 40 ± 7 | .05 |
Percent release of total histamine by a 50 μL intradermal injection in skin tissue treated with PBS, FMLP, LPS, MCP-1, and RANTES. P values were calculated comparing MCP-1 10 ng (*) with RANTES 10 ng and MCP-1 20 ng (**) with RANTES 20 ng.
The injection of RANTES and LPS produced higher effects in terms of histamine release from 4 hours of exposure (40 ± 7 and 42 ± 7, respectively). The effect of RANTES and FMLP were substantially different (34 ± 5 and 30 ± 5, respectively), while MCP-1 (20 and 10 ng/50 μL) had a moderate effect compared with other activator compounds (25 ± 6 and 20 ± 3, respectively).
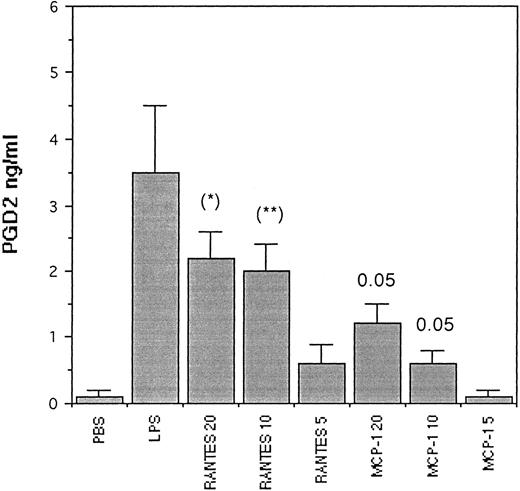
Generation of PGD2 from skin injection sites.Since rat basophilic leukemia (RBL) cells (which have been shown to be homologous to mast cell/and rat peritoneal mast cell) are capable of generating large amounts of PGD2 ,28 in these studies we evaluated the generation of PGD2 from tissue skin injection sites (Fig 7). Here we show the amount of PGD2 pg/mL released after injection of PBS 50 μL (control), LPS 10 ng/50 μL, RANTES (20, 10, and 5 ng/50 μL), and MCP-1 (20, 10, and 5 ng/50 μL). Here the detected PGD2 was enhanced by LPS (positive control) but not by PBS (control). When RANTES was injected, the production of PGD2 was strongly stimulated in a dose-dependent concentration, while MCP-1 was less effective (P < .05). However, MCP-1 5 ng failed to stimulate PGD2 . PGD2 was produced by inflamed tissues in the injection site of the skin.
Generation of PGD2 in rat skin tissue injection sites treated for 4 hours with scalar concentration of RANTES and MCP-1 in 50 μL bolus. The values represent the mean ± SD of three experiments in triplicate. The P values (Student's t-test) are calculated by comparing tissue treated with RANTES (20-10 ng) (* and **), with tissue treated with MCP-1 (20-10 ng), respectively.
Generation of PGD2 in rat skin tissue injection sites treated for 4 hours with scalar concentration of RANTES and MCP-1 in 50 μL bolus. The values represent the mean ± SD of three experiments in triplicate. The P values (Student's t-test) are calculated by comparing tissue treated with RANTES (20-10 ng) (* and **), with tissue treated with MCP-1 (20-10 ng), respectively.
DISCUSSION
Mast cells reside near the mucosal surface in the submucosa around venules and in skin in the dermis where they can act as key mediators in allergen-IgE-dependent complex inducible hypersensitivity reaction.29 It has been reported that RANTES, like MCP-1, -2, -3, MIP-1α, and MIP-1β, is known to be a potent monocyte chemoattractant2-4,31; however, little is known about RANTES and MCP-1 and mast cell migration in inflammatory states.
In the study presented here, after injection of RANTES and MCP-1 chemokines in the rat, an inflammatory reaction ensued as evidenced by way of histologic characterization of cell extravasation and Evan's blue extravasation. RANTES (10 ng/50 μL) generated a greater response than MCP-1 (10 ng/50 μL). RANTES and MCP-1 activity is evident not only for basophilic cells but also for monocytes and eosinophils (Fig 4). Results show that RANTES and MCP-1 are potent pro-inflammatory proteins and can chemoattract mast cells,30,31 in addition to other inflammatory cells. Data obtained in our in vivo model indicate that RANTES provokes a dose-dependent increase in HDC mRNA synthesis in tissue homogenates biopsed at the injection site. The induction of HDC transcripts occurred within 4 hours. The stimulatory effect of RANTES on HDC mRNA expression was compared with that of LPS (positive control). This effect was abolished in the presence of actinomycin D,17 which inhibits mRNA transcripts. Again the effect of MCP-1 on HDC mRNA generation was less effective than RANTES. To better evaluate the role of RANTES and MCP-1 in basophilic cell activation, we also studied the generation of PGD2 in the skin injection sites and found that RANTES strongly stimulates the release of PGD2 , while MCP-1 is less effective in this bioassay.
These results support previous observations that suggest a role for RANTES stimulation of eosinophils and monocytes in inflamed intradermal sites.27 Moreover, Meurer et al reported that a single intradermal injection of RANTES in the dog resulted in an eosinophil and macrophage-rich inflammatory site within 4 hours, providing the first direct evidence in vivo that RANTES has significant proinflammatory activity.27 In accordance with this finding and the temporal profile of chemoattraction, here we describe, for the first time, that RANTES is a potent proinflammatory agonist protein and that it is more active than MCP-1 in the chemoattraction of basophilic cells and the formation of lipid mediator of inflammation. These data provide new evidence that suggests that RANTES and MCP-1 may play an important role in the inflammatory process via recruitment and activation of basophilic cells among other cell types.
In our experiments we found that LPS has the highest activity in our in vivo model. Possibly, since it has been found that RANTES is expressed in response to endotoxemia, this protein could play an important role in the LPS-induced recruitment of macrophages and polymorphonuclear leukocytes.32-34 In our results we show that RANTES is more potent as a chemotactic protein on basophilic cells than MCP-1 in intradermal injections sites.
These properties make RANTES and MCP-1 candidate for a role in important inflammatory processes.
ACKNOWLEDGMENT
We thank Dr Alfredo Grilli, Biology Department, University of Chieti, for the realization of the photos provided for this manuscript.
Supported by a grant from the Ministry of University, Scientific and Technological Research, Rome, Italy.
Address reprint requests to Prof Pio Conti, Immunology Division, University of Chieti School of Medicine, Via dei Vestini, 66013 Chieti, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal