Abstract
Endothelial cells (EC) possess at least two membrane receptors for von Willebrand factor (vWF ), the vitronectin receptor (VNR, αvβ3 ), which recognizes an Arg-Gly-Asp (RGD) sequence in the C-terminus of vWF, and glycoprotein Ibα (GP Ibα), which interacts with a region in the N-terminal A1 domain of vWF. In the absence of added cytokines, EC attachment to a vWF substratum is mediated largely through the αvβ3 , with a smaller contribution by GP Ibα. In the present study, we have examined the effect of cytokines on the receptor specificity of EC attachment to wild-type vWF (WT-vWF ) and to vWF, which had been mutated in the C-terminal RGDS sequence (RADS-vWF ). Exposure of human umbilical vein EC (HUVEC) to tumor necrosis factor-α (TNF-α) or to TNF-α in combination with interferon-γ (IFN-γ), but not to interleukin-1β (IL-1), increased attachment to RADS-vWF by about twofold. The TNF-α–induced increase in EC attachment was accompanied by an increase in cell surface GP Ibα expression; GP Ibα surface expression was not increased by IL-1. Attachment of untreated HUVEC to WT-vWF could be inhibited 60% to 70% by a monoclonal antibody (MoAb) (LM609) to the VNR and 30% to 40% by the A1 fragment of vWF (containing the GP Ibα binding domain). The pattern of inhibition of attachment to WT-vWF was largely unchanged after TNF-α treatment of HUVEC. In contrast, the attachment to WT-vWF of HUVEC, treated with TNF-α +IFN-γ was completely inhibited by vWF-A1 and inhibited only 35% by the anti-VNR antibody LM609. Two MoAbs to GP Ibα produced similar, but incomplete, inhibition. Pretreatment of HUVEC with the combination of TNF-α +IFN-γ produced a dramatic decrease in VNR expression, confirming previous findings of Defilippi et al. These results suggest that in the presence of the inflammatory cytokines TNF-α +IFN-γ, the endothelial GP Ib complex is a major determinant of HUVEC adhesion to surface-bound vWF.
ENDOTHELIAL CELLS (EC) attach, through cell surface adhesion receptors, to a number of proteins in the underlying matrix, including, but not limited to, fibronectin, vitronectin, the collagens, laminin and vWF.1,2 These interactions may be of particular importance during wound healing, vascular repair, and angiogenesis.3 EC express on their surface, in addition to a number of β1 integrins,4 the integrin αvβ3 , receptor for vitronectin and other RGD-containing proteins, generally referred to as the vitronectin receptor (VNR).5-7 The VNR is capable of mediating EC adhesion, spreading and focal contact formation on a variety of matrix proteins, and appears to be an important component in the processes of metastasis and angiogenesis.8-16
EC also express glycoprotein (GP) Ibα,17,18 the receptor in platelets for von Willebrand Factor (vWF ). Early work from one of these studies18 had shown that GP Ib could mediate ristocetin-induced human umbilical vein endothelial cell (HUVEC) agglutination, some of the initial evidence demonstrating functionality for the EC GP Ib complex. In addition to GP Ibα, EC express the other components of the GP Ib complex, GP Ibβ, GP IX, and GP V.19,20 EC GP Ibα mRNA and protein synthesis are upregulated by pretreatment with tumor necrosis factor-α (TNF-α) alone, or in combination with interferon-γ (IFN-γ); interleukin-1 (IL-1) does not elicit a similar response.21,22 We have previously demonstrated functionality for EC GP Ibα, in that EC adhesion to surface-bound vWF mutated at its C-terminal RGD site could be largely abrogated by inhibitors of the vWF-GP Ibα interaction.23-25 In addition, using specific inhibitors, we have shown that EC adhesion to wild-type vWF is mediated by both the VNR and GP Ibα.24,25
Inflammatory cytokines are known to alter EC migration and proliferation and to stimulate angiogenesis, processes that are dependent on EC adhesion.3,10,11 One mechanism whereby cytokines may exert their multiple effects is through the regulation of receptor expression.26 For example, the expression of the EC VNR in vitro is increased by various growth factors and mediators of signaling pathways, including basic fibroblast growth factor-β (bFGF ), protein kinase C, and platelet-derived growth factor-B,12-14 but is decreased dramatically in the presence of the combination of TNF-α and IFN-γ.15 16 Therefore, we have investigated the effects of TNF-α and IFN-γ on EC adhesion to vWF, with specfic reference to the relative roles of the VNR and GP Ibα.
MATERIALS AND METHODS
Materials.Recombinant IFN-γ was purchased from Sigma Chemical Co, St Louis, MO (specific activity 1 × 106 U/mg). IFN was used at a final concentration of 100 U/mL. IL-1 β (specific activity 5.0 × 107 U/mg) and TNF-α (specific activity 1 × 108 U/mg) were purchased from Boehringer Mannheim Corp, Indianapolis, IN. IL-1 and TNF-α were used at a final concentration of 50 U/mL. The following murine monoclonal antibodies (MoAbs) were used in this study: the anti-GP Ibα MoAb AS-7, subclass IgG127 (kindly provided by Dr Jonathan Miller, State University of New York at Syracuse), Ib128 (a gift of Dr Zaverio Ruggeri, Scripps Institute, La Jolla, CA) and 6D129 (generously supplied by Dr Barry Coller, Department of Medicine, Mount Sinai Medical Center, New York, NY); and the anti-VNR MoAb LM609 (a generous gift of Dr David Cheresh, Scripps Institute). AS-7 was used for all attachment experiments; Ib1 and 6D1 were used in binding experiments. The anti-β3 integrin MoAb 10E2 was prepared and supplied by Dr Guoxin Wu of the Cardeza Foundation (Philadelphia, PA). A control IgG1 MoAb to CD16, the generous gift of Dr Bice Perussia (Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA), was used in our previous study, as well as this study.25 The concentration of IgG1 in this control CD16 antibody, about 1 mg/mL, was similar to the IgG1 content of AS-7 ascites. The composition of M9 medium, used by Cruz et al24 to label vWF-A1 for binding experiments is: 6 g/L Na2HPO4 , 3 g/L KH2PO4 , 0.5 g/L NaCl, 1 g/L NH4Cl, 0.1 mmol/L MgCl2 , 0.1 mmol/L CaCl2 , and 0.2% glucose. The purified vWF fragments vWF-A3, the collagen-binding fragment of vWF, and vWF-A1, the GP Ibα binding fragment, were generously supplied by Dr Miguel Cruz (Brigham and Women's Hospital, Boston, MA).24 30
Recombinant wild-type vWF and vWF mutated at the RGDS sequence (RADS-vWF: gly → ala at position 1745) were prepared and purified as previously described.23 Plasma vWF was purified from cryoprecipitate by gel filtration on Sepharose CL-4B, as described.23 vWF-A1 migrated as a single band of Mr 36 kD on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and inhibited vWF binding to platelet GP Ibα.24 Recombinant vWF-A3,30 Mr 27 kD, the collagen-binding fragment of vWF, was used as a control.
Cell culture, metabolic labeling, and surface labeling.HUVEC were derived from primary cultures of human umbilical veins as described17,25 and used at second passage. To measure HUVEC adhesion, confluent cultures of HUVEC in T-150 flasks were metabolically labeled with 35S by incubation for 4 hours in 10 mL serum-free minimal essential medium (MEM) lacking methionine and cysteine to which 250 μCi each of [35S]methionine and [35S]cysteine (Trans-35S Label; ICN Pharm Inc, Costa Mesa, CA) were added, as described.23 25 Metabolically labeled HUVEC were also used for immunoprecipitation of the VNR (see below).
For studies of cell surface GP Ibα, 10 confluent T-150 flasks for each condition, untreated or cytokine-treated, comprising about 1 × 108 cells, were dissociated by a brief EDTA treatment in lifting buffer, pH 7.5 (2 g/L NaHCO3 , 0.2 g bovine serum albumin [BSA], 20 mmol/L HEPES, 20 mmol/L EDTA, 10 mmol/L EGTA, 2 mmol/L L-phenylmethylsulfonyl fluoride [PMSF] in phosphate-buffered saline [PBS]). After washing in serum-free MEM,25 HUVEC were resuspended in PBS-EDTA, pH 6.8. Surface sialic acid residues were labeled with 3H-NaBH4 , as described for platelets.27 31 After the final PBS-EDTA wash, the surface-labeled HUVEC were lysed and used for immunoprecipitation (see below).
Immunoprecipitation.35S-labeled or 3H-surface–labeled HUVEC were washed three times with PBS. Labeled HUVEC were extracted in 2 mL of immunoprecipitation (IP) buffer: 10 mmol/L Tris-HCl, 0.5% deoxycholate, 0.5% Triton X-100, 1 mmol/L Na2EDTA, 150 mmol/L NaCl, pH 8.5, to which were added 10 μg/mL aprotinin, 2 mmol/L PMSF, 2 mmol/L EDTA, 0.1 mg/mL benzamidine, and 10 μg/mL pepstatin A.25 As a control, platelets were prepared and labeled in parallel. HUVEC and platelet extracts, which had been externally labeled with 3H-NaBH4 were immunoprecipitated as described,25 with the following modifications: for immunoprecipitation of GP Ibα, 1 mg/mL DNase I, grade II (Boehringer Mannheim, Inc) was included to solubilize the actin cytoskeleton.32 To minimize proteolysis in the presence of DNase I, the final concentration of benzamidine was increased to 8 mg/mL.32 Insoluble cell debris was removed by centrifugation at 200g for 10 minutes and aliquots of the supernatant were incubated overnight at 4°C with either AS-7 ascites fluid (final dilution 1:100) or purified nonimmune mouse IgG (final concentration 10 μg/mL). The mixtures were then incubated for 1 hour at 4°C with 20 μL/mL protein A-Sepharose CL-4B beads. The immune complexes bound to protein A-Sepharose were pelleted by centrifugation for 1 minute at 200g, washed four times with IP buffer, and finally washed with TBS (Tris buffered saline: 50 mmol/L Tris, 150 mmol/L NaCl, pH 7.4). The washed immune complexes were released from protein A-Sepharose by resuspension and incubation at 70°C for 5 minutes in reducing sample buffer. Samples were run on 4% to 15% SDS-PAGE gradient gel and labeled proteins were revealed by fluorography of the gel (ENHANCE; New England Nuclear, Inc, Boston, MA) on Kodak X-Omat AR film (Eastman Kodak, Inc, Rochester, NY), as previously described.25 The VNR was immunoprecipitated by the same technique, using LM609 ascites fluid at a final dilution of 1:500.33
Densitometric analysis.Quantitation of the 36-kD vWF-A1 band present in 35S-methionine labeled extracts (described in Radiolabeling vWF-A1 below), and the 140-kD band in the AS-7 immunoprecipitation (see Fig 5), was determined with a Macintosh NIH Image 1.55 software program (written by Wayne Rasbond, NIH, Bethesda, MD) and image analysis system.
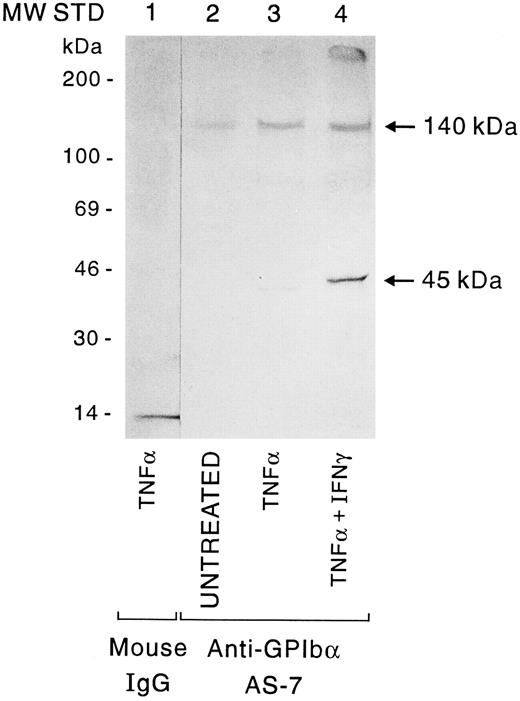
Immunoprecipitation of cytokine-treated HUVEC by the anti-GP Ibα MoAb AS-7. HUVEC were untreated or pretreated with TNF-α or the combination of TNF-α +IFN-γ, and sialic acid residues were labeled with Na3HBH4 as described in Materials and Methods. Whole cell lysates were incubated with nonimmune mouse IgG1 (lane 1) or AS-7 (lanes 2 to 4), followed by protein A-Sepharose. Fluorography of a 4% to 15% gradient SDS-PAGE gel is shown.
Immunoprecipitation of cytokine-treated HUVEC by the anti-GP Ibα MoAb AS-7. HUVEC were untreated or pretreated with TNF-α or the combination of TNF-α +IFN-γ, and sialic acid residues were labeled with Na3HBH4 as described in Materials and Methods. Whole cell lysates were incubated with nonimmune mouse IgG1 (lane 1) or AS-7 (lanes 2 to 4), followed by protein A-Sepharose. Fluorography of a 4% to 15% gradient SDS-PAGE gel is shown.
HUVEC attachment.Adhesion of HUVEC to vWF-coated microtiter wells, (3 μg/well vWF ) was measured as previously described.23,25 HUVEC attachment to WT-vWF and purified plasma vWF were indistinguishable, and experiments with either are labeled ‘WT-vWF’ throughout. HUVEC, which had attained 85% confluence, were treated with 50 U/mL TNF-α for 17 hours before the attachment assay, as described previously.22 Treatment with TNF-α in combination with IFN-γ was performed by preincubating HUVEC with 100 U/mL IFN-γ for 72 hours and 50 U/mL TNF-α for the last 17 hours before use in the attachment assay, as described.21,22 HUVEC were dissociated with trypsin/EDTA for 30 seconds and resuspended in complete medium (Medium 199, containing 10% to 15% fetal bovine serum, 100 μg/mL sodium heparin and 1% EC growth factor). The HUVEC were centrifuged at 300g for 5 minutes, washed twice in serum-free MEM, and allowed to recover at 37°C for 30 minutes before use in the attachment assay. Stock solutions of antibody or vWF fragments were prepared in serum-free MEM and added to achieve a final assay volume of 100 μL. The total number of endothelial cells attached to microtiter wells after 60 minutes of incubation was determined based on the [35S]-methionine/cysteine cpm incorporated/cell, and results of experiments were normalized based on attached cell numbers. Attachment data for cytokine-treated EC, in the presence and absence of inhibitors, was expressed as a percentage of untreated EC attachment in the absence of inhibitors, which was equal to 100% residual adhesion. Adhesion to BSA was determined for untreated, TNF-α–treated and TNF-α +IFN-γ–treated HUVEC and subtracted from the corresponding vWF adhesion to determine ‘specific’ EC attachment. Adhesion of EC to BSA was typically 10% to 15% of adhesion to vWF. Monensin was added to the attachment assay at a concentration of 0.7 μg/mL to prevent the endogenous secretion of vWF by HUVEC.25 Ascites fluid was dialyzed against PBS and varying dilutions of anti-GP Ibα AS-7, control IgG1 (1:100), or the anti-VNR LM609 (1:500) were used in the attachment assays. In our previous study of HUVEC attachment using AS-7 as inhibitor, purified AS-7 IgG1 was used. When directly compared, no difference in the ability of AS-7 ascites or purified AS-7 IgG1 to inhibit HUVEC attachment to vWF was observed. Moreover, because AS-7 ascites reacted specifically with EC GP Ibα, but not other cell surface proteins in immunoprecipitation experiments shown here and in previous studies,25 AS-7 ascites fluid was used for these experiments of HUVEC attachment to vWF.
Radiolabeling of vWF-A1 protein from Escherichia coli. The vWF-A1 cDNA fragment, inserted into the pQE9 vector (Qiagen, Chatsworth, CA), was expressed in E coli (pQE9-vWF-A1), and the vWF-A1 protein was metabolically labeled as described by Cruz et al.24 Bacteria expressing the vWF-A1 protein were washed twice with 50 mL M9 medium and resuspended in 5 mL M9 medium supplemented with 250 μCi Trans-35S Label (ICN Corp) containing a mixture of 35S-methionine and 35S-cysteine and 0.02% of each of the remaining 18 amino acids.24 A final concentration of 1.5 mmol/L isopropyl β-D-thiogalactopyranoside (IPTG) was added to induce expression of vWF-A1.24 The 35S-vWF–A1 protein was solubilized from bacterial inclusion bodies by extraction with 6 mol/L guanidine HCl, 20 mmol/L Tris-HCl, pH 7.4 and dialyzed against 4 mol/L urea, 40 mmol/L HEPES, 200 mmol/L NaCl. Urea was removed by a stepwise dialysis, as previously described,24 to yield 35S-vWF–A1 at a final concentration of 10 μg/mL. The 35S-vWF–A1 fragment was not further purified from these bacterial extracts. However, it was the major band seen on 4% to 20% SDS-PAGE gradient gels (Millipore Corp, Bedford, MA) and comprised 53% of the total protein by quantitative densitometry of the fluorographed gel (data not shown). It should be emphasized, however, that the unlabeled vWF-A1 used in attachment assays and as a competitive inhibitor of 35S-vWF–A1 protein in EC binding assays was the purified Mr 36-kD protein, giving a single band on SDS-PAGE.
Binding of 35S-vWF–A1 to HUVEC monolayers.HUVEC were seeded onto 96-well microtiter plates in complete medium25 and allowed to reach confluence (about 1 × 104 cells/well). Confluent HUVEC were incubated for 30 minutes at 4°C with 0.5 μg 35S-vWF–A1/well alone or in the presence of (1) a 10-fold excess of purified vWF-A1 (to measure nonspecific binding), (2) the anti-GP Ibα MoAb Ib1, AS-7 or 6D1, or (3) 50 μg/mL heparin. Unbound 35S-vWF–A1 was removed by three washes with M9 medium, and bound 35S-vWF–A1 was quantified by β scintillation counting of cell lysates solubilized with 1% SDS. MoAb Ib1, AS-7, or 6D1 were added as ascites fluid at a final dilution of 1:125, an amount that produced maximal inhibition; antibody dilutions of 1:250 and 1:500 produced less inhibition. Total 35S binding represented approximately 50,000 cpm, about 50% of which was inhibited by excess purified unlabeled vWF-A1. The 50% inhibition of 35S counts by vWF-A1 is consistent with the observation that vWF-A1 comprised about 53% of the 35S-vWF–A1 bacterial extract. Thus, the remaining counts are most likely due to the presence of other bacterial proteins detected by SDS-PAGE analysis. Binding data are expressed as specific counts by subtracting the counts not inhibited by excess vWF-A1 from the total counts. Purified vWF-A3 inhibited binding of 35S-vWF–A1 by less than 12%.
Statistical analysis.Standard deviations for attachment assays and vWF-A1 binding studies were determined from a minimum of quadruplicate samples pooled from three experiments, and statistical significance was determined using the Student's paired t-test.
RESULTS
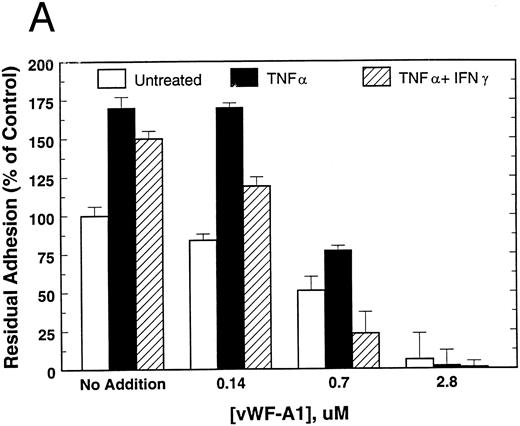
To examine the functionality of EC GP Ibα as a receptor for vWF, attachment was measured to mutant vWF whose C-terminal RGD site was rendered nonfunctional (RGDS → RADS) with respect to integrin binding. As shown in Fig 1A, attachment to RADS-vWF was inhibited in a concentration-dependent manner by vWF fragment A1, containing the GP Ibα binding site. Anti-GP Ibα MoAb AS-7 also produced dose-dependent inhibition, but to a lesser extent (Fig 1B). Treatment with TNF-α, with or without IFN-γ, induced a 50% to 75% increase in HUVEC attachment to RADS-vWF, an attachment that could be totally inhibited by vWF-A1 (Fig 1A) and, to a lesser extent, by AS-7 (Fig 1B). In contrast, vWF-A3, the collagen binding fragment of vWF, had no effect on HUVEC attachment to RADS-vWF (data not shown).
Attachment of untreated, TNF-α–treated, and TNF-α + IFN-γ–treated HUVEC to RADS-vWF in the presence of (A) vWF-A1 or (B) the GP Ibα MoAb AS-7. HUVEC were pretreated with TNF-α for 17 hours (▪) or with IFN-γ for 72 hours and TNF-α for the last 17 hours (▨), or were left untreated (□). HUVEC attachment was measured at 60 minutes as described in Materials and Methods.
Attachment of untreated, TNF-α–treated, and TNF-α + IFN-γ–treated HUVEC to RADS-vWF in the presence of (A) vWF-A1 or (B) the GP Ibα MoAb AS-7. HUVEC were pretreated with TNF-α for 17 hours (▪) or with IFN-γ for 72 hours and TNF-α for the last 17 hours (▨), or were left untreated (□). HUVEC attachment was measured at 60 minutes as described in Materials and Methods.
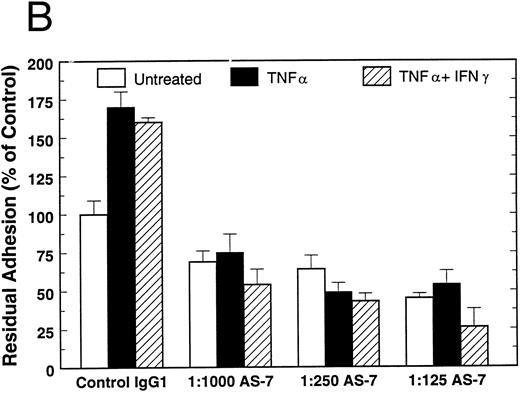
To study the relative contributions of GP Ibα and the VNR in the attachment of EC to WT-vWF, attachment of untreated and cytokine-treated HUVEC was measured in the presence of vWF-A1, the anti-VNR MoAb LM609, or both of these inhibitors. Although the data in Figs 1 and 2 are presented as percent of untreated controls, we found, consistent with our previous studies,23,25 that attachment of untreated HUVEC to WT-vWF was 50% to 75% higher than to RADS-vWF (19,000 cells attached/well v 12,000 cells attached/well). However, in contast to RADS-vWF, cytokine treatment did not increase HUVEC attachment to WT-vWF in the absence of inhibitors (Fig 2, control IgG1). Attachment of untreated HUVEC was inhibited approximately 70% by LM609, 40% by vWF-A1, and 100% by the combination of these agents (Fig 2). Thus, attachment under these conditions appears to be mediated largely by the VNR, with a smaller component dependent on GP Ibα; the two receptors account fully for attachment. vWF-A3 failed to inhibit HUVEC attachment to WT-vWF (data not shown).
Attachment of HUVEC to WT-vWF. Effect of vWF-A1 and the anti-VNR. The anti-VNR MoAb LM609 and 3 μmol/L of the vWF-A1 polypeptide, were added individually or in combination to untreated and cytokine-treated HUVEC and attachment was measured as described above after 60 minutes. Attachment to BSA was measured in parallel and subtracted from all values.
Attachment of HUVEC to WT-vWF. Effect of vWF-A1 and the anti-VNR. The anti-VNR MoAb LM609 and 3 μmol/L of the vWF-A1 polypeptide, were added individually or in combination to untreated and cytokine-treated HUVEC and attachment was measured as described above after 60 minutes. Attachment to BSA was measured in parallel and subtracted from all values.
Attachment of TNF-α–treated HUVEC also is mediated by both receptors (Fig 2), although the sensitivity to inhibition by vWF-A1 or LM609 above suggests a more complex relationship between the two EC adhesion receptors, raising the possibility that cooperativity may exist between these receptors. Consistent with this notion is our observation that MoAb LM609 inhibits untreated HUVEC attachment to RADS-vWF by about 30% (data not shown), although previous data suggests strongly that RADS-vWF has no integrin-binding activity. When HUVEC were treated with the combination of TNF-α and IFN-γ, however, the mechanism of attachment to WT-vWF was significantly shifted; vWF-A1 inhibited attachment by about 85%, while LM609 inhibited attachment by about 30%. Attachment to WT-vWF of cytokine-treated EC, like that of untreated cells, can be completely accounted for by the VNR and GP Ibα. As shown in Fig 3, the anti-GP Ibα MoAb AS-7 also inhibited HUVEC attachment to WT-vWF, but to a lesser extent than seen with vWF-A1.
Attachment of untreated, TNF-α–treated, and TNF-α + IFN-γ–treated HUVEC to WT-vWF in the presence of the GP Ibα MoAb AS-7. Attachment was determined at 60 minutes in the absence or presence of increasing amounts of the anti-GP Ibα AS-7 MoAb and compared with attachment to WT-vWF in the presence of control IgG1 (1:125 dilution).
Attachment of untreated, TNF-α–treated, and TNF-α + IFN-γ–treated HUVEC to WT-vWF in the presence of the GP Ibα MoAb AS-7. Attachment was determined at 60 minutes in the absence or presence of increasing amounts of the anti-GP Ibα AS-7 MoAb and compared with attachment to WT-vWF in the presence of control IgG1 (1:125 dilution).
The ability of MoAb AS-7 to inhibit HUVEC attachment to RADS-vWF was less than that of vWF-A1 vWF under all conditions tested. To determine whether this was due to properties of this specific MoAb or to the interaction of vWF-A1 with sites on the EC surface other than GP Ibα, we compared the ability of AS-7 and two other anti-GP Ibα MoAb, Ib1 and 6D1, to inhibit binding of 35S-vWF–A1 to HUVEC. These MoAb, like AS-7, abolish the binding of vWF to platelet GP Ibα.28,29 As shown in Fig 4, a 1:125 dilution of AS-7, the concentration producing maximum inhibition of HUVEC attachment, only partially inhibited specific 35S-vWF–A1 binding to HUVEC, consistent with partial inhibition by AS-7 of EC adhesion to vWF (P < .001, compared with no MoAb). MoAb Ib1 at a 1:125 dilution completely inhibited specific binding of 35S-vWF–A1 to HUVEC, (P < .001), compared with AS-7. In contrast to AS-7, a 1:125 dilution of Ib1 reduced the attachment of TNF-α or TNF-α + IFN-γ–treated HUVEC on a vWF surface to levels observed on BSA (data not shown). MoAb 6D1 was the least effective inhibitor of specific 35S-vWF–A1 binding, consistent with its failure to inhibit HUVEC attachment to either WT-vWF or RGD mutant vWF, previously observed by us25 and by others.34 Because vWF-A1 also contains a heparin-binding domain, the ability of 50 μg/mL heparin to inhibit specific 35S-vWF–A1 binding was investigated. As also shown in Fig 4, heparin inhibited specific 35S-vWF–A1 binding to HUVEC by only 29%. These results suggest that vWF-A1 binding to the HUVEC surface is primarily through interaction with GP Ibα, and that the ability of anti-GP Ibα MoAb to inhibit vWF-A1 binding, and perhaps WT-vWF binding, to HUVEC varies markedly.
Effect of GP Ibα MoAb and heparin on 35S-methionine labeled vWF-A1 binding to HUVEC. 35S-vWF–A1 was added to confluent HUVEC monolayers in microtiter wells and incubated in the presence or absence of a 1:125 dilution of the anti-GP Ibα MoAbs AS-7, Ib1, or 6D1, or 50 μg/mL of heparin. After incubation at 4°C for 30 minutes and repeated washes, bound 35S-vWF–A1 was detected by counting HUVEC lysates. Data are expressed as vWF-A1–specific counts.
Effect of GP Ibα MoAb and heparin on 35S-methionine labeled vWF-A1 binding to HUVEC. 35S-vWF–A1 was added to confluent HUVEC monolayers in microtiter wells and incubated in the presence or absence of a 1:125 dilution of the anti-GP Ibα MoAbs AS-7, Ib1, or 6D1, or 50 μg/mL of heparin. After incubation at 4°C for 30 minutes and repeated washes, bound 35S-vWF–A1 was detected by counting HUVEC lysates. Data are expressed as vWF-A1–specific counts.
To determine directly the effect of cytokine pretreatment on surface GP Ibα expression, untreated, TNF-α–treated, and TNF-α +IFN-γ–treated HUVEC were cell surface-labeled with 3H-NaBH4 . GP Ibα was immunoprecipitated from equivalent numbers of endothelial cells with MoAb AS-7. As shown in Fig 5, small amounts of radiolabeled bands of 140 and 45 kD were immunoprecipitated from untreated EC; treatment with TNF-α increased the 140-kD band approximately twofold and treatment with TNF-α +IFN-γ increased the band approximately fourfold over untreated cells (compare lanes 2, 3, and 4). Treatment of HUVEC with IL-1 did not increase the amount of immunoprecipitated material above that seen in the untreated cells (data not shown). No bands were detected using preimmune mouse IgG1 for immunoprecipitation (Fig 5, lane 1). Sprandio et al17 and previous studies from this laboratory25 had also observed in addition to a 140-kD band, a band of 80 to 90 kD and several lower molecular weight species after immunoprecipitation of total HUVEC extracts for GP Ibα, even in the presence of several protease inhibitors. These results suggest that endothelial GP Ibα is extremely susceptible to proteolytic breakdown,17 25 and that the 45-kD band may represent a breakdown product of the 140-kD species.
Defilippi et al15,16 previously showed that VNR expression is dramatically reduced when HUVEC are exposed to the combination of TNF-α and IFN-γ for 72 hours in the presence of βFGF. We evaluated the expression of the VNR in HUVEC under our experimental conditions. Confluent endothelial cells were metabolically labeled and immunoprecipitated using the anti-β3 MoAb 10E2 and the VNR complex-specific MoAb LM609. As shown in Fig 6, both the radiolabeled β3 subunit and small amounts of the αv subunit were immunoprecipitated from untreated HUVEC with either LM609 or 10E2 (Fig 6, lanes 1 and 2, respectively). The amounts of radiolabeled αv and β3 immunoprecipitated by 10E2 after TNF-α treatment (Fig 6, lane 3) were slightly greater than those from untreated cells, consistent with previous results of Defilippi et al.15,16 An increase in VNR expression on TNF-α–treated HUVEC could explain, in part, the ability of the anti-VNR MoAb LM609 to inhibit the attachment of TNF-α–treated HUVEC to a greater extent than untreated HUVEC (Fig 2). In contrast, pretreatment of HUVEC with TNF-α and IFN-γ markedly reduced 10E2-immunoprecipitable radiolabeled β3 and αv subunits (Fig 6, lane 4). Immunoprecipitation of IFN-γ–treated HUVEC with 10E2 yielded amounts of αv and β3 indistinguishable from those seen in untreated HUVEC (data not shown). Thus, in agreement with Defilippi et al,15 16 our data indicate that the combination of TNF-α and IFN-γ is required to achieve decreased VNR expression.
Immunoprecipitation of cytokine-treated HUVEC by the β3 VNR antibody 10E2 and by the VNR complex antibody LM609. HUVEC were pretreated with IFN-γ and TNF-α and metabolically labeled with [35S] methionine and [35S]cysteine for the last 17 hours, as described. Immunoprecipitation of untreated HUVEC was performed on equivalent amounts of cell lysate with LM609 (lane 1) or the anti-β3 MoAb 10E2 (lane 2). TNF-α–treated (lane 3) and TNF-α +IFN-γ–treated (lane 4) HUVEC were immunoprecipitated with 10E2. The αv and β3 bands, which migrated at Mr = 140,000 and 110,000, respectively, are indicated by the arrows.
Immunoprecipitation of cytokine-treated HUVEC by the β3 VNR antibody 10E2 and by the VNR complex antibody LM609. HUVEC were pretreated with IFN-γ and TNF-α and metabolically labeled with [35S] methionine and [35S]cysteine for the last 17 hours, as described. Immunoprecipitation of untreated HUVEC was performed on equivalent amounts of cell lysate with LM609 (lane 1) or the anti-β3 MoAb 10E2 (lane 2). TNF-α–treated (lane 3) and TNF-α +IFN-γ–treated (lane 4) HUVEC were immunoprecipitated with 10E2. The αv and β3 bands, which migrated at Mr = 140,000 and 110,000, respectively, are indicated by the arrows.
DISCUSSION
Inflammation of the vessel wall is a part of the response to tissue injury, setting the stage for the process of wound repair. One characteristic of the inflammatory process is the production and release of a variety of cytokines, amongst them TNF-α.35-37 TNF-α has a multiplicity of effects on endothelial cells, including upregulation of endothelial leukocyte adhesion molecule 1 (ELAM-1), intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and class I major histocompatability complex (MHC) molecules,35-37 thereby inducing a proadhesive phenotype.35 The combination of TNF-α and IFN-γ produces additional effects on endothelial cells, such as synergism in the upregulation of class I MHC antigens and downregulation of the VNR.37
Angiogenesis also depends on cytokines and is characterized by the invasion, migration, and proliferation of smooth muscle and endothelial cells, implicating vascular cell adhesion molecules in its regulation.10 As Friedlander et al3 have demonstrated recently, angiogenesis induced in vivo by TNF-α or basic fibroblast growth factor (bFGF ) is mediated by the αvβ3 VNR in the chick chorioallantoic membrane system. In these studies, HUVEC were cultured in the presence of bFGF and/or TNF-α, consistent with the presence of the αvβ3 vitronectin receptor on the HUVEC surface. Thus, specific cytokines or growth factors can differentially regulate the activity of distinct adhesion receptors, suggesting that adhesion processes mediating angiogenesis depend on redundant molecular events modulated by different agents.3
We and others have demonstrated previously that EC synthesize GP Ibα and express this vWF receptor in their membranes,17-22 that TNF-α upregulates EC GP Ibα,22 and that EC GP Ibα can support HUVEC attachment to vWF.25 We initiated this study to investigate whether the interaction of EC with a matrix protein, vWF, capable of binding to both the VNR and GP Ibα, depends on one or both receptors, and whether cytokines could alter these interactions. Our current studies demonstrate that unstimulated endothelial cells depend on both the VNR and the GP Ib complex for binding to a vWF surface, with the VNR effect being the predominant one. TNF-α stimulation does not significantly alter quantitatively or qualitatively the nature of the interaction of HUVEC with WT-vWF. Although TNF-α increases the amount of GP Ibα on the cell surface, our data (Fig 6) suggest that the VNR may also be increased on the HUVEC surface. Thus, it appears that when substantial amounts of the VNR are present on the HUVEC surface, endothelial cell adhesion is driven predominantly by an αvβ3 -dependent mechanism.
When HUVEC are exposed to the combination of TNF-α and IFN-γ HUVEC, however, adhesion to WT-vWF, although quantitatively unchanged, is dependent almost entirely on the GP Ib complex. This change in adhesive mechanism is unlikely to be related to the shape change induced by TNF-α, as cells treated with TNF-α alone or the combination of TNF-α and IFN-γ had a similar morphology. Rather, the shift in adhesion mechanism appears to require two, opposing effects: upregulation of surface GP Ibα and downregulation of the VNR.
Thus, our results indicate that a continuum may exist in dependence of HUVEC adheison to vWF on αvβ3 and the GP Ib complex: untreated cells adhere largely via αvβ3 , while at least some combinations of cytokines alter the balance toward the GP Ib complex.
Using surface labeling followed by immunoprecipitation, we have demonstrated that TNF-α induces an increase in surface expression of GP Ibα. Our previous study showed a major band of 140 kD with minor components of 90 kD, 70 kD, and 55 kD using metabolically labeled HUVEC lysates immunoprecipitated with the AS-7 anti-GP Ibα MoAb.25 Likewise, Sprandio et al17 using the anti-GP Ibα MoAb AP-1, showed protein bands of similar size by immunoprecipitation analysis. These observations suggest that EC GP Ibα may be particularly susceptible to proteolytic cleavage, even in the presence of several protease inhibitors used to inhibit proteolysis of immunoprecipitated platelet GP Ibα.32 Our results are consistent with those of Wu, Shapiro et al (manuscript submitted for publication), who have observed by flow cytometry a 2.5-fold to threefold increase in EC surface expresssion of all four components of the GP Ib complex induced by TNF-α.
Our data suggest that the EC GP Ib complex may play a significant role in EC-matrix interactions, particularly in conditions characterized by increased production of cytokines, such as inflammation and wound healing. Nevertheless, there is little evidence that patients with the Bernard-Soulier syndrome,38-40 who presumably also lack a functional EC GP Ib complex, have major clinical problems in these two areas. Whether this is because of the limited clinical data on the few patients with this disorder or because there are redundant mechanisms for EC-matrix interactions remains to be seen. A similar potential problem arises in assessing the significance of the αvβ3 , as patients with the β3 mutant form of thrombasthenia,41 who presumably lack the EC VNR, have not been reported to have problems with wound healing or angiogenesis. Clearly, more work is needed to decipher the pathophysiological interactions of endothelial cells with their underlying matrix. We are currently extending our studies to examine EC interactions with more complex matrix preparations.
ACKNOWLEDGMENT
The authors thank Dr Miguel Cruz for his gift of purified vWF-A1 and vWF-A3 proteins and for discussions pertaining to their effective use in these experiments. We would also like to thank Andrew Likens for photographic technical expertise, Dr Derrick Grant for his assistance with the NIH Image Analysis 1.55 software system, and Coleen Calviello for technical assistance.
Supported in part by Grant No. 080-01084 (to D.A.B.) from the Southeastern Pennsylvania Affiliate of the American Heart Association (Conshohocken, PA), and by Grant No. HL09163 from the National Institutes of Health, Bethesda, MD (to S.S.S.).
Address reprint requests to Dorothy A. Beacham, PhD, Cardeza Foundation for Hematologic Research, Department of Medicine, Jefferson Medical College of Thomas Jefferson University, Philadelphia, PA 19107-5099.




![Fig. 6. Immunoprecipitation of cytokine-treated HUVEC by the β3 VNR antibody 10E2 and by the VNR complex antibody LM609. HUVEC were pretreated with IFN-γ and TNF-α and metabolically labeled with [35S] methionine and [35S]cysteine for the last 17 hours, as described. Immunoprecipitation of untreated HUVEC was performed on equivalent amounts of cell lysate with LM609 (lane 1) or the anti-β3 MoAb 10E2 (lane 2). TNF-α–treated (lane 3) and TNF-α +IFN-γ–treated (lane 4) HUVEC were immunoprecipitated with 10E2. The αv and β3 bands, which migrated at Mr = 140,000 and 110,000, respectively, are indicated by the arrows.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.4071/4/m_bl_0033f6.jpeg?Expires=1769474770&Signature=gcKLMoi4meW1O~lJltaxXWDLhXnQtrJsOoIpZX8FXQ5cRIfM~k6U24FabOWztSEveWRS1RK-uA~hN5Sm-is7Q3PuK7BVYjTSUlXE871ZKbrg3xz2J7SjECMIy2BiUUq61jTup-hyiOxINgkM72q5AjkfEsrjzGFMy-P0r~iyqW9008fgnBP1c3zKaY1tSkvMUNrijpxTmFrVjf717a-BS3l727WLh1oO3XuHiLuLz3sFKjamEHXlXYleITBFm4nn-P9mbT~7-lb2LkzQZxuySgXXFfdPV~esknQrVGa7A5E8B-iZ~kONZ-kecIaSTWwVHRpwbbUUklm1V8W2CO1oRA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal