Abstract
We have used our previously described baboon model of infusion of both a sublethal dose of Escherichia coli and C4b binding protein to assess the impact of inhibiting platelet function with the F(ab′ )2 fragment of the monoclonal antibody 7E3, directed against the platelet glycoprotein (GP)IIb/IIIa receptor, on the characteristic microvascular changes. At a dose of 0.25 to 0.35 mg/kg bolus plus an infusion of 0.25 to 0.35 mg/kg over 6 hours, c7E3 F(ab′ )2 had only a minimal impact on fibrinogen consumption and delayed but did not prevent, the development of thrombocytopenia. Treatment with 7E3 F(ab′ )2 , however, produced significant protection from the development of microangiopathic hemolysis and renal insufficiency. Histologic examination supported these observations, with treated animals having fewer schistocytes on blood smear and less evidence of ischemic renal changes. Treated animals also had more rapid recovery of peripheral white blood counts, suggesting a possible protective effect of treatment on ischemic damage to the bone marrow. These data indicate that potent inhibition of platelet function via GPIIb/IIIa receptor blockade can decrease ischemic organ damage in this animal model that has features similar to those found in diffuse intravascular coagulation, hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura.
WE PREVIOUSLY described a baboon model in which infusion of C4b-binding protein (C4bBP) before infusion of a sublethal dose of Escherichia coli produced a consumptive coagulopathy characterized by marked reductions in platelets and plasma fibrinogen, microangiopathic hemolytic anemia, and microvascular thrombosis-induced renal failure.1 The C4bBP infusion decreased the plasma protein S levels, thus inhibiting the protein C anticoagulant pathway and predisposing the animals to thrombotic complications.2 This most likely accounts for the exaggerated response to the sublethal E coli infusion, which otherwise only produces moderate thrombocytopenia and leukocytosis. The syndrome produced by infusing the combination of C4bBP and sublethal E coli has elements that are similar to those observed in human diffuse intravascular coagulation, thrombotic thrombocytopenic purpura, and hemolytic uremic syndrome.3-6 Since platelets have been implicated in mediating, at least in part, the tissue damage associated with all three of these syndromes, we evaluated the effect of treating animals with a potent antiplatelet agent, the F(ab′ )2 fragment of the murine monoclonal antibody (MoAb) 7E3, which binds to platelet glycoprotein (GP)IIb/IIIa receptors and inhibits platelet aggregation.7-9 Previous studies with 7E3 F(ab′ )2 , 7E3 Fab, and a mouse/human chimeric 7E3 Fab, have shown that all three agents nearly eliminate platelet aggregation, prolong the bleeding time, and protect against arterial thrombosis in both animals and humans.7,10 11
MATERIALS AND METHODS
Materials.C4b binding protein (C4bBP) was prepared from human plasma as described previously.1 Protein S is a cofactor for activated protein C, accelerating the latter's cleavage and inactivation of factors Va and VIIIa. Inhibition of protein S cofactor activity by C4bBP was determined using a factor Xa one-stage coagulation assay to which activated protein C was added as described previously.1 Adding activated protein C to the assay in the presence of protein S increased the clotting time from 35 seconds to 100 seconds, and the addition of approximately 400 μg/mL of C4bBP neutralized sufficient protein S to reduce the clotting time back to 35 seconds.
Intact murine MoAb and the 7E3 F(ab′ )2 fragment, directed against the platelet GPIIb/IIIa receptor, were prepared as previously described12 13 and provided by Centocor (Malvern, PA).
E coli organisms (33 985 Type B7-086a:K1; American Type Culture Collection, Rockville, MD) used in the infusion study were isolated from a stool specimen at Children's Memorial Hospital (Oklahoma City, OK). They were stored in the lyophilized state at 4°C after growth in tryptic soybean agar and reconstituted and characterized as described previously.14
Intact 7E3 was radiolabeled with 125I and used to assess the number of GPIIb/IIIa receptors on the surface of platelets as previously described.13 15 Two animals were studied for GPIIb/IIIa receptor blockade.
An anti-C4bBP MoAb, BP-91, was used in an enzyme-linked immunosorbent assay (ELISA) assay to determine the plasma level of C4bBP: the antibody was prepared and isolated from ascites fluid as described previously.2
Pre-experimentation and experimentation procedures.The study protocol received prior approval by the Institutional Animal Care and Use Committees of both the Oklahoma Medical Research Foundation and the University of Oklahoma Health Sciences Center (OUHSC).
Papiocyanocephalus cynocephalus or Papio cyanocephalus anubis baboons were purchased from either a breeding colony maintained at OUHSC or Biomedical Research Foundation, Inc (Houston, TX). Animals weighed 2 to 14 kg, had leukocyte counts of 5,000 to 10,000/μL, and hematocrits exceeding 36%. They were free of tuberculosis. Animals were held for 30 days at the OUHSC animal facility where the infusion studies were performed. All animals were observed continuously during the first 6 hours after infusion of the test materials.
Infusion procedures.Experiments were performed on 17 baboons. Animals were fasted overnight before each experiment, but were allowed water ad libitum. Each animal was sedated with ketamine hydrochloride (14 mg/kg, intramuscularly) on the morning of the study, and then, using a percutaneous catheter in the cephalic vein, anesthetized with sodium pentobarbital (2 mg/kg initially and with additional amounts approximately every 20 minutes for 6 hours to maintain a light level of surgical anesthesia).1,2 Animals were intubated orally and allowed to breathe spontaneously. The femoral artery and vein were cannulated aseptically and used for measuring arterial pressure and obtaining blood samples, respectively. A percutaneous catheter was placed in the saphenous vein and used to infuse the E coli organisms.1 2 Each anesthetized baboon was positioned on its side in contact with controlled temperature heating pads.
Experimental groups.Table 1 shows the four groups of animals studied. Group 1 was infused with C4bBP alone, Group 2 was infused with sublethal E coli alone, Group 3 was infused with C4bBP plus sublethal E coli (“untreated”), and Group 4 was infused with 7E3 F(ab′ )2 plus C4bBP plus sublethal E coli (“treated”). Groups 1 and 2 are historical controls studied previously,1 2 while Groups 3 and 4 were studied as integral parts of this study.
Experimental Groups
| Exp. No. . | Sex . | Weight (kg) . | E coli Infused (CFU/kg × 1010) . | E coli Colony Count at T = 2 h (CFU/mL × 106) . | C4bBP (mg/kg) . | 7E3 F(ab′)2 (mg/kg) . | Survival (h) . |
|---|---|---|---|---|---|---|---|
| Group 1 — C4bBP alone | |||||||
| 1 | F | 4.6 | — | — | 20 | — | 168 |
| 2 | M | 5.2 | — | — | 20 | — | 168 |
| 3 | F | 8.2 | — | — | 20 | — | 168 |
| Average | — | 6.0 | — | — | 20 | — | 168 |
| SE | — | 0.4 | — | — | 0 | — | 0 |
| Group 2-Sublethal E coli alone | |||||||
| 4 | M | 6.1 | 0.93 | 3.20 | — | — | 168 |
| 5 | M | 7.1 | 0.93 | 1.04 | — | — | 168 |
| 6 | F | 14.6 | 0.84 | 7.60 | — | — | 168 |
| 7 | F | 6.4 | 1.07 | 0.28 | — | — | 168 |
| 8 | M | 5.7 | 1.13 | 0.61 | — | — | 168 |
| Average | — | 7.9 | 0.98 | 2.54 | — | — | 168 |
| SE | — | 1.67 | 0.05 | 0.28 | — | — | 0 |
| Group 3-Sublethal E coli plus C4bBP (“untreated”) | |||||||
| 9 | M | 3.9 | 1.24 | 0.48 | 20 | — | 25(s)* |
| 10 | M | 2.7 | 1.71 | 0.87 | 20 | — | 24(s) |
| 11 | F | 2.3 | 0.76 | 0.03 | 20 | — | 23(s) |
| 12 | M | 5.2 | 0.55 | 0.11 | 20 | — | 14 |
| 13 | M | 7.5 | 1.34 | 0.73 | 20 | — | 30(s) |
| Average | — | 4.32 | 1.12 | 0.44 | 20 | — | — |
| SE | — | 0.94 | 0.20 | 0.17 | 0 | — | — |
| Group 4-7E3 F(ab′)2 plus sublethal E coli plus C4bBP (“treated”) | |||||||
| 14 | F | 7.3 | 1.2 | 0.92 | 20 | 0.25 bolus | 48(s) |
| 0.25 infusion | |||||||
| 15 | F | 7.7 | 1.1 | 0.26 | 20 | 0.35 bolus | 72(s) |
| 0.35 infusion | |||||||
| 16 | M | 6.8 | 1.2 | 0.61 | 20 | 0.35 bolus | 48(s) |
| 0.35 infusion | |||||||
| 17 | F | 8.2 | 1.3 | 0.22 | 20 | 0.35 bolus | 7 days(s) |
| 0.35 infusion | |||||||
| Average | — | 7.5 | 1.20 | 0.50 | 20 | — | — |
| SE | — | 0.30 | 0.04 | 0.16 | 0 | — | — |
| Exp. No. . | Sex . | Weight (kg) . | E coli Infused (CFU/kg × 1010) . | E coli Colony Count at T = 2 h (CFU/mL × 106) . | C4bBP (mg/kg) . | 7E3 F(ab′)2 (mg/kg) . | Survival (h) . |
|---|---|---|---|---|---|---|---|
| Group 1 — C4bBP alone | |||||||
| 1 | F | 4.6 | — | — | 20 | — | 168 |
| 2 | M | 5.2 | — | — | 20 | — | 168 |
| 3 | F | 8.2 | — | — | 20 | — | 168 |
| Average | — | 6.0 | — | — | 20 | — | 168 |
| SE | — | 0.4 | — | — | 0 | — | 0 |
| Group 2-Sublethal E coli alone | |||||||
| 4 | M | 6.1 | 0.93 | 3.20 | — | — | 168 |
| 5 | M | 7.1 | 0.93 | 1.04 | — | — | 168 |
| 6 | F | 14.6 | 0.84 | 7.60 | — | — | 168 |
| 7 | F | 6.4 | 1.07 | 0.28 | — | — | 168 |
| 8 | M | 5.7 | 1.13 | 0.61 | — | — | 168 |
| Average | — | 7.9 | 0.98 | 2.54 | — | — | 168 |
| SE | — | 1.67 | 0.05 | 0.28 | — | — | 0 |
| Group 3-Sublethal E coli plus C4bBP (“untreated”) | |||||||
| 9 | M | 3.9 | 1.24 | 0.48 | 20 | — | 25(s)* |
| 10 | M | 2.7 | 1.71 | 0.87 | 20 | — | 24(s) |
| 11 | F | 2.3 | 0.76 | 0.03 | 20 | — | 23(s) |
| 12 | M | 5.2 | 0.55 | 0.11 | 20 | — | 14 |
| 13 | M | 7.5 | 1.34 | 0.73 | 20 | — | 30(s) |
| Average | — | 4.32 | 1.12 | 0.44 | 20 | — | — |
| SE | — | 0.94 | 0.20 | 0.17 | 0 | — | — |
| Group 4-7E3 F(ab′)2 plus sublethal E coli plus C4bBP (“treated”) | |||||||
| 14 | F | 7.3 | 1.2 | 0.92 | 20 | 0.25 bolus | 48(s) |
| 0.25 infusion | |||||||
| 15 | F | 7.7 | 1.1 | 0.26 | 20 | 0.35 bolus | 72(s) |
| 0.35 infusion | |||||||
| 16 | M | 6.8 | 1.2 | 0.61 | 20 | 0.35 bolus | 48(s) |
| 0.35 infusion | |||||||
| 17 | F | 8.2 | 1.3 | 0.22 | 20 | 0.35 bolus | 7 days(s) |
| 0.35 infusion | |||||||
| Average | — | 7.5 | 1.20 | 0.50 | 20 | — | — |
| SE | — | 0.30 | 0.04 | 0.16 | 0 | — | — |
(s) = Sacrificed.
C4bBP was infused at a concentration of 20 mg/kg as a bolus at T = −0.5 hour in all groups in which it was given. The C4bBP plasma concentration at T = 0 averaged 834 ± 150 μg/mL. The 7E3 F(ab′ )2 was infused first as either a 0.25 or 0.35 mg/kg bolus at T = −10 minutes, and then as a continuous infusion of either 0.25 or 0.35 mg/kg given over 6 hours beginning at T = 0. The percentage of platelet GPIIb/IIIa receptors blocked by the 7E3 F(ab′ )2 averaged 94% and 86% at T = 0 and T = 6 hours, respectively, in the two animals studied. Sublethal E coli doses administered ranged from 0.55 × 1010 to 1.71 × 1010 CFU/kg, with mean values of 0.98, 1.12, and 1.20 × 1010 CFU/kg in the three groups given E coli. The E coli were infused over two hours and then colony counts were performed on blood taken at that time. The blood concentrations (mean ± SE) of E coli at T = 2 hours in the animals in Groups 2, 3, and 4 were 2.54 ± 0.28 × 106, 0.44 ± 0.17 × 106, and 0.50 ± 0.16 × 106 CFU/mL, respectively.
Animals in Groups 1 and 2 were not killed, but were returned to the colony after observation of the hemostatic and hematologic responses during the first 24 hours; previous studies of animals receiving these regimens showed that these agents do not cause significant pathology at the light microscopic levels when given alone.1 2 Animals in Groups 3 and 4 were killed at the times indicated in Table 1 with the exception of animal no. 12, which died at 14 hours.
Sampling and assays.Mean systemic arterial pressure (MSAP) and heart rate were monitored with a Stathem pressure transducer and Hewlett Packard (Avondale, PA) recorder. Rectal temperature was measured with a telethermometer (Yellow Springs Instrument Co, Yellow Springs, OH). The above measurements were made, and blood samples were collected, at T = −0.5, 0, +1, +2, +3, +4, +6, and +24 hour(s). T = 0 designated the point at which the infusion of E coli was started. Less than 10 percent of the animals' calculated blood volumes (70 mL/kg) were withdrawn over the 6-hour monitoring period. The blood collection volumes and times of collection, as well as assays performed, are shown in Table 2. At the time of death, tissue specimens were removed from the animal's lungs, kidney, liver, adrenal glands, heart, and spleen for light microscopic examination.
Blood Sample Collection Schedule
| Collection TIme (h) . | Blood Samples and Anticoagulants . | |||||
|---|---|---|---|---|---|---|
| . | EDTA (1 mL) (CBC) . | Citrate (2 mL) (Fibrinogen16 ) (C4bBP1 ) . | Trayslol-Thrombin (0.5 mL) (FDP*)17 . | Citrate (2 mL) (GPIIb/IIIa receptors)*7,13,15 . | No Anticoagulant (3 mL) (BUN18) (Cr19 ) (SGPT18 ) . | No Anticoagulant (0.5 mL) (colony counts14 ) . |
| 0 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 1 | ✓ | ✓ | — | — | — | |
| 2 | ✓ | ✓ | — | — | ✓ | |
| 3 | ✓ | ✓ | — | — | — | |
| 4 | ✓ | ✓ | — | — | — | |
| 6 | ✓ | ✓ | ✓ | ✓ | ✓ | — |
| 24 | ✓ | ✓ | ✓ | ✓ | — | |
| Collection TIme (h) . | Blood Samples and Anticoagulants . | |||||
|---|---|---|---|---|---|---|
| . | EDTA (1 mL) (CBC) . | Citrate (2 mL) (Fibrinogen16 ) (C4bBP1 ) . | Trayslol-Thrombin (0.5 mL) (FDP*)17 . | Citrate (2 mL) (GPIIb/IIIa receptors)*7,13,15 . | No Anticoagulant (3 mL) (BUN18) (Cr19 ) (SGPT18 ) . | No Anticoagulant (0.5 mL) (colony counts14 ) . |
| 0 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 1 | ✓ | ✓ | — | — | — | |
| 2 | ✓ | ✓ | — | — | ✓ | |
| 3 | ✓ | ✓ | — | — | — | |
| 4 | ✓ | ✓ | — | — | — | |
| 6 | ✓ | ✓ | ✓ | ✓ | ✓ | — |
| 24 | ✓ | ✓ | ✓ | ✓ | — | |
Abbreviations: EDTA, ethylenediamine tetraacetic acid; CBC, complete blood count (including hematocrit, erythrocyte morphology, platelet and leukocyte counts, and leukocyte differential); FDP, fibrin(ogen) degradation products; CR, creatinine.
Obtained in 2 animals.
A schistocyte was defined as any fragmented erythrocyte and the schistocyte percentage was determined from visual examination of Wright's stained blood smears prepared from blood obtained at T = 0 and T = 24 hours. In Groups 1 and 2, previously prepared blood smears were reviewed retrospectively by one observer who was blinded to the nature of the experiment. For the blood smears obtained from animals in Groups 3 (untreated) and 4 (treated), two separate observers each performed either two or three 100 cell assessments of the slides without knowing from which animal the blood was taken. The means of the separate determinations were averaged and the number of fragmented erythrocytes was expressed as a percentage of the total number of erythrocytes analyzed.
Histologic evidence of ischemic damage to the kidneys was assessed by analyzing renal tissue sections stained with hematoxylin-eosin and with phosphotungstic acid-hematoxylin (PTAH). Abnormalities were graded on a scale of 0 to 10, with 0 representing no features typical of ischemic damage and thrombosis and 10 representing features typical of severe ischemic damage and thrombosis, including fibrin-platelet thrombi in glomerular capillaries, glomerular contractions, prominence of tubular cell nucleoli, and either bland or hemorrhagic infarction. Sections taken from Group 3 (untreated) and Group 4 (treated) animals at the time of death were examined. Slides were graded by two independent pathologists who were blinded as to the identity of the specimens.
Statistical analysis.Results in text are expressed as mean values ± SEM. Fisher's exact probability test was used to determine the significance of the difference in mean values between Group 3 (untreated) and Group 4 (treated) with P < .05 taken as significant. Analysis of variance was used to determine differences between group means at different time points throughout the observation period.
RESULTS
Group 1 (C4bBP alone) and group 2 (sublethal E coli alone).As we previously reported in Group 1, the white blood cell (WBC) counts increased soon after C4bBP infusion and remained mildly and variably elevated for 24 hours. The WBC counts decreased at T = 2 hours in Group 2 and rose to above baseline at T = 24 hours. No increase in schistocytes occurred in either group, but platelets decreased to approximately 45% of control values in both groups. There was minimal fibrinogen consumption in both groups over the first 6 hours, and the fibrinogen concentration in both groups rose to greater than baseline values at T = 24 hours, presumably reflecting an acute phase reaction. Hematocrits decreased by 2 and 6 points, respectively, in the two groups at T = 24 hours. There was a minor increase in blood urea nitrogen (BUN) in Group 2 and minor elevations in serum glutamic-pyruvic transaminase (SGPT) in both groups at T = 24 hours. An additional two animals infused with a 0.35 mg/kg bolus of 7E3 F(ab′ )2 , while showing a 1.5-fold increase in white cell count, showed no evidence of changes in other formed elements (including platelets) of the blood over a 24-hour period.
Group 3 (sublethal E coli plus C4bBP) (untreated) and group 4 (7E3 F(ab′ )2 plus sublethal E coli plus C4bBP) (treated).The nadir mean total white cell count in the untreated group (Group 3) (1,500/μL) was similar to that of the treated group (Group 4) (1,200/μL) and both occurred within the first three hours. However, by T = 24 hours, the total white cell count increased to a significantly higher level in treated compared to untreated animals (13,900 v 2,900/μL, P = .01) (Fig 1). Band forms were significantly higher in Group 4 than Group 3 animals at 4, 6, and 24 hours. Lymphocyte counts decreased in both groups within an hour of the infusion, and remained depressed until T = 6 hours. The mean lymphocyte count remained depressed in Group 3 animals at T = 24 hours, whereas that of the Group 4 animals recovered to a level similar to that at T = 0 (Fig 1).
Responses of total WBCs, band forms (Bands), segmented neutrophils (Segs), and lymphocytes (Lymphs) in animals in Group 3 (sublethal E coli plus C4bBP) versus the responses in animals in group 4 (7E3 F[ab′]2 plus sublethal E coli plus C4bBP): Mean values for untreated animals (Group 3) receiving sublethal E coli plus C4bBP (○) versus treated animals (Group 4) receiving 7E3 F(ab′ )2 plus sublethal E coli plus C4bBP (X). The asterisk denotes that the count of the untreated group is significantly lower than that of the treated group at that time point (P = <.01). Error bars represent ± standard error of the mean.
Responses of total WBCs, band forms (Bands), segmented neutrophils (Segs), and lymphocytes (Lymphs) in animals in Group 3 (sublethal E coli plus C4bBP) versus the responses in animals in group 4 (7E3 F[ab′]2 plus sublethal E coli plus C4bBP): Mean values for untreated animals (Group 3) receiving sublethal E coli plus C4bBP (○) versus treated animals (Group 4) receiving 7E3 F(ab′ )2 plus sublethal E coli plus C4bBP (X). The asterisk denotes that the count of the untreated group is significantly lower than that of the treated group at that time point (P = <.01). Error bars represent ± standard error of the mean.
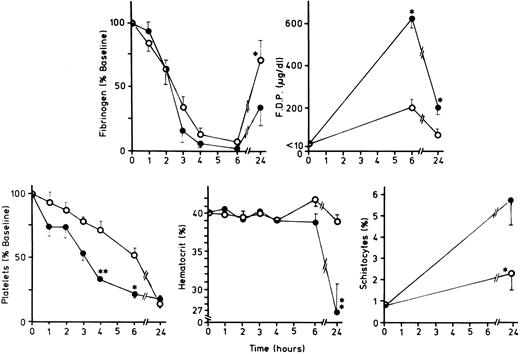
Both groups sustained severe decreases in platelet count over the 24-hour period (Fig 2). However, the platelet counts were significantly higher in the treated animals (Group 4) than in the untreated animals (Group 3) at T = 4 and 6 hours. This difference disappeared at T = 24 hours (Fig 2). There were significant differences in schistocyte and hematocrit measurements between the untreated and treated groups, and these were most pronounced at T = 24 hours (Fig 2). The schistocyte count rose from 1.0 ± 0.3 to 5.9 ± 1.3% by 24 hours in the untreated group, whereas it increased from 1.0 ± 0.3 to only 2.5 ± 0.9% in the treated group. The mean hematocrit values reflected these results, falling from 40.8 to 27.0% at 24 hours in the untreated animals (Group 3), but only falling from 40.0 to 39.0% in the treated animals (Group 4) (Fig 2). Thus, 7E3 F(ab′ )2 treatment had a greater impact on preserving the hematocrit than preventing the increase in schistocytes; this might reflect more rapid clearance of schistocytes in the untreated group, perhaps in partially occluded microvascular sites.
Hemostatic factors, hematocrits, and schistocytes in animals in Group 3 (sublethal E coli plus C4bBP) versus animals in Group 4 (7E3 F(ab′ )2 plus sublethal E coli plus C4bBP): Changes over time in fibrinogen, FDPs, platelets, hematocrit, and schistocyte percentages in untreated animals receiving sublethal E coli plus C4bBP (Group 3) (•) versus treated animals receiving 7E3 F(ab′ )2 plus sublethal E coli plus C4bBP (Group 4) (○). The asterisks denote time points at which there were significant differences between the two groups (*P = <.05, **P = <.01).
Hemostatic factors, hematocrits, and schistocytes in animals in Group 3 (sublethal E coli plus C4bBP) versus animals in Group 4 (7E3 F(ab′ )2 plus sublethal E coli plus C4bBP): Changes over time in fibrinogen, FDPs, platelets, hematocrit, and schistocyte percentages in untreated animals receiving sublethal E coli plus C4bBP (Group 3) (•) versus treated animals receiving 7E3 F(ab′ )2 plus sublethal E coli plus C4bBP (Group 4) (○). The asterisks denote time points at which there were significant differences between the two groups (*P = <.05, **P = <.01).
There was marked fibrinogen consumption during the first 6 hours in both groups, but there was a trend toward higher fibrinogen levels in the treated animals at T = 3 hours. At T = 24 hours the mean fibrinogen level was significantly higher in the treated animals (Group 4) than the untreated animals (Group 3) (P < .05) (Fig 2).
The changes in fibrin(ogen) split product (FDP) concentrations also differed between Groups 3 and 4 increasing to 640 μg/mL by 6 hours in the untreated group, but to only 200 μg/mL in the treated group. Although the FDP levels in both groups decreased by 24 hours, the difference between the two groups remained significant (Fig 2).
Significant differences in renal function and renal pathology were also observed between the untreated and treated groups (Fig 3). The mean BUN rose from 24 mg/dL to 85 mg/dL by 24 hours in Group 3, whereas the comparable values in Group 4 were 15 mg/dL and 40 mg/dL (P < .01). Mean serum creatinine values increased over 24 hours from 0.6 mg/dL to 3.8 mg/dL in Group 3, but from 0.5 mg/dL to only 1.5 mg/dL in Group 4 (P < .001). The histopathologic appearance of the kidneys from the animals in Group 3 at autopsy was characterized by capillary and arteriolar thrombosis, accompanied by evidence of infarction, including focal areas of hemorrhage and tubular degeneration, resulting in a mean renal pathology score of 7.6. In sharp contrast, the mean renal pathology score of the treated group was 1.7, with minimal changes noted (P < .01). It is unlikely that the differences in time from treatment to autopsy between Group 3 and Group 4 animals significantly affected the renal histopathology results because the renal lesions in this model are established by T = 24 hours and are irreversible (100% lethal).1 2 Hence, the lack of renal pathology seen in the treated animals at T = 48 to 72 hours is not due to spontaneous remission.
Renal histopathology in an animal in Group 3 (sublethal E coli plus C4bBP) versus an animal in Group 4 (7E3 F[ab′]2 plus sublethal E coli plus C4bBP): (A) The renal histopathology of the untreated animal in Group 3 was characterized by numerous capillary and arteriolar thrombi, which stained positive for fibrin with the PTAH stain (see arrows). In some cases, the capillary walls were thickened with eosinophilic hyaline or granular material between the basement membrane and endothelium. Kidneys from untreated animals also showed focal areas of hemorrhage, tubular changes, and mononuclear leukocyte infiltration. (B) The renal histopathology of a treated animal in Group 4, which was characterized by mild congestion with no thrombin, hemorrhage, or leukocyte infiltration.
Renal histopathology in an animal in Group 3 (sublethal E coli plus C4bBP) versus an animal in Group 4 (7E3 F[ab′]2 plus sublethal E coli plus C4bBP): (A) The renal histopathology of the untreated animal in Group 3 was characterized by numerous capillary and arteriolar thrombi, which stained positive for fibrin with the PTAH stain (see arrows). In some cases, the capillary walls were thickened with eosinophilic hyaline or granular material between the basement membrane and endothelium. Kidneys from untreated animals also showed focal areas of hemorrhage, tubular changes, and mononuclear leukocyte infiltration. (B) The renal histopathology of a treated animal in Group 4, which was characterized by mild congestion with no thrombin, hemorrhage, or leukocyte infiltration.
DISCUSSION
The major findings from these studies is that treatment of baboons infused with C4bBP and a sublethal dose of E coli with 7E3 F(ab′ )2: (1) had only a modest effect on the decrease in plasma fibrinogen, (2) decreased the level of FDPs, (3) significantly delayed, but did not prevent, the development of thrombocytopenia, (4) attenuated the granulocytopenia and facilitated recovery of the WBC count, (5) decreased the microangiopathic hemolysis, as shown by both a reduction in schistocytes and better preservation of the hematocrit, and (6) dramatically decreased both biochemical and histopathological evidence of renal impairment. Similar results were obtained in a small group of animals using the Fab′ fragment of the mouse-human chimeric 7E3 antibody (c7E3 Fab; abciximab; ReoPro) antibody instead of the murine 7E3 F(ab′ )2. These data support the view that platelets contribute significantly to both the microangiopathic and renal pathology produced by this model.
Although 7E3 F(ab′ )2 treatment significantly decreased the rate of developing thrombocytopenia, it did not prevent the eventual development of severe thrombocytopenia. Since 7E3 F(ab′ )2 treatment did prevent the development of significant microangiopathic hemolytic anemia and renal failure, and since these phenomena presumably reflect microvascular damage due to platelet deposition, the failure of 7E3 F(ab′ )2 treatment to prevent thrombocytopenia is surprising. One possible explanation for these observations is that 7E3 F(ab′ )2 prevented platelet deposition in the microvasculature due to platelet aggregation, but did not prevent clearance through other mechanisms. In vitro, strong platelet agonists such as thrombin can induce the platelet release reaction even when there is high-grade GPIIb/IIIa receptor blockade,20 and this may result in surface expression of α-granule contents such as thrombospondin and perhaps fibrinogen, PF4, and von Willebrand factor. It is possible that activated platelets coated with these proteins may be cleared from the circulation by mechanisms independent of intravascular deposition.
The inability of 7E3 F(ab′ )2 treatment to prevent the marked reduction in plasma fibrinogen suggests that thrombin generation was not dramatically inhibited, but the trend toward higher fibrinogen concentrations in the treated animals, with the T = 24-hour values almost twice as high, raises the possibility of 7E3 having some impact on thrombin generation. Activated platelets are thought to facilitate thrombin generation by releasing factor V(a) and by providing an efficient catalytic surface for several coagulation reactions.21 Evidence from our laboratory indicates that 7E3 can inhibit thrombin generation in a reconstituted system of gel-filtered platelets and defibrinated plasma when low concentrations of tissue factor are used to initiate thrombin generation.22 Similarly, there is evidence that 7E3 can prolong the activated clotting time of heparinized blood either when added in vitro23 or administered to patients in vivo.24 However, in vitro addition of 7E3 did not prolong the activated clotting time of unheparinized blood, perhaps because very large amounts of thrombin were generated.23 Thus, it is likely that 7E3 was unable to dramatically decrease thrombin generation induced by C4bBP infusion and septicemia because this also constitutes a very powerful stimulus, and because the depressed level of protein S prevented normal feedback inhibition of thrombin formation. Animals treated with 7E3 F(ab′ )2 had lower levels of fibrin(ogen) degradation products than untreated animals. This probably is due in part to the attenuated decrease in plasma fibrinogen produced by 7E3 F(ab′ )2 treatment, but it may also be due to the more extensive renal clearance of fibrin(ogen) degradation products in the treated group because renal function was better preserved.
This model has features typical of diffuse intravascular coagulation, as well as thrombotic thrombocytopenia purpura, and hemolytic uremic syndrome. Thus, the initiating insult includes systemic gram negative septicemia, a common trigger for diffuse intravascular coagulation. The profound fibrinogen consumption observed in this model is one of the most prominent features of diffuse intravascular coagulation, but uncommon in thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. Microangiopathic hemolysis is very prominent in this model as it is in thrombotic thrombocytopenic purpura and hemolytic uremic syndrome, but it also may be associated with diffuse intravascular coagulation. There was no gross evidence that the animals suffered any neurological abnormalities typical of thrombotic thrombocytopenic purpura, but this is not helpful in differentiating between the disorders because even in the human disorder, the symptoms can be minor and transient. Renal insufficiency can also accompany diffuse intravascular coagulation, but the damage is usually less apparent than in this model or in hemolytic uremic syndrome or thrombocytopenic purpura. Moreover, the renal histology seen in the baboon model of diffuse intravascular coagulation induced by a lethal dose of E coli typically consists of moderate to severe corticomedullary congestion, and cortical proximal tubular necrosis with granular cast formation25,26 rather than the platelet-fibrin thrombi and infarction found in the present study. In the diffuse intravascular coagulation model, only occasional glomerular fibrin thrombi are seen even though immunostaining reveals linear deposition of fibrin.27 This is not surprising since the very early fibrin thrombi that normally appear in the renal glomerulae of baboons with massive diffuse intravascular coagulation disappear within the first 10 hours.28 This is in contrast to the extensive microvascular thrombi seen after 24 hours or more in this baboon model following C4bBP coinfused with sublethal E coli. Since the renal microvascular thrombosis is established irreversibly by 24 hours in this C4bBP plus sublethal E coli model, there is no possibility of spontaneous resolution in the interval between 24 to 72 hours. This permits comparison of microscopic sections of tissues taken from untreated animals taken at approximately T = 24 hours with tissues taken from treated animals at T = 48 to 72 hours. The harvest of tissues from the treated animals was deliberately delayed to give these animals every opportunity to develop the lesions observed at T = 24 hours in the untreated animals.
Though this was not a survival study, we did allow one animal in the treated group to continue past the 48- to 72-hour sacrifice point to T = 7 days at which time the animal was killed. Seven-day survivors are considered permanent survivors.
An unexpected effect of treatment with 7E3 F(ab′ )2 was its beneficial effect on the recovery of the WBC count. The mean total white cell counts of untreated and treated animals reached similar nadir values within the first three hours. The mean white cell count of the treated animals, however, was more than fourfold higher than that of the untreated animals at T = 24 hours, and at that time point, 24% of the WBCs in the treated animals were immature neutrophils versus only 7% for the untreated animals. The recovery observed in the treated group matches that normally seen in animals receiving sublethal E coli alone. The beneficial effect of treatment with 7E3 F(ab′ )2 raises some interesting questions regarding a potential role for platelet-mediated ischemic damage to the bone marrow in this model, especially since previous studies in humans have documented microthrombi in the bone marrow vasculature.29
The beneficial effect of 7E3 F(ab′ )2 in this animal model raises the possibility that 7E3 treatment may be of benefit to humans at risk of suffering tissue damage from diffuse intravascular coagulation, thrombotic thrombocytopenic purpura, and/or hemolytic uremic syndrome. This is of note because other inhibitors of platelet function have limited efficacy in these disorders, perhaps because they are less potent inhibitors of platelet aggregation. Unfortunately, the differences in pathophysiology between this model and each of the human disorders makes it very difficult to extrapolate from this model to human disease. The most serious potential side effect of such therapy would be exacerbation of hemorrhage. Therefore, it is somewhat reassuring that despite the profound derangements in coagulation and platelet numbers and functions, and despite the extensive instrumentation of these animals, serious hemorrhage was not encountered. In fact, in the treated animals, there was essentially no decrease in hematocrit over the 24-hour period.
Supported in part by National Institutes of Health (Bethesda, MD) Grants No. HL30340 (C.T.E.), HL19278 and HL54469 (B.S.C.), and GM37704 (F.B.T.). C.T.E is an investigator of the Howard Hughes Medical Institute.
Address reprint requests to F.B. Taylor, MD, Cardiovascular Biology Research, Oklahoma Medical Research Foundation, 825 NE 13th St, Oklahoma City, OK 73104.

![Fig. 1. Responses of total WBCs, band forms (Bands), segmented neutrophils (Segs), and lymphocytes (Lymphs) in animals in Group 3 (sublethal E coli plus C4bBP) versus the responses in animals in group 4 (7E3 F[ab′]2 plus sublethal E coli plus C4bBP): Mean values for untreated animals (Group 3) receiving sublethal E coli plus C4bBP (○) versus treated animals (Group 4) receiving 7E3 F(ab′ )2 plus sublethal E coli plus C4bBP (X). The asterisk denotes that the count of the untreated group is significantly lower than that of the treated group at that time point (P = <.01). Error bars represent ± standard error of the mean.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.4078/4/m_bl_0041f1.jpeg?Expires=1769148470&Signature=ydtHUcoDPN0t5SCrtz6e9RKeP~-WxQHiKR83KeMhMpUrMV~rTlIhhQN5betKAk~y3CqjxEmUmiyqC08kTScLrvWTmhIx4nh4qzX0r1wcniQhlme4qMHIXVUP2fskBNpHozXpa-Y2uzdqBhfV1Cdt-jIvs5qKI~Ova6SNWS4wtKwxcQH~voA0zjoRuTvGpkWQxj52Saqckbm6AxkNn5M03Im9QO7sKCYaOF7pwndIbjXnWYA1tjqmVLD0Deo~Ae31ZxmBhRIeCmQGsIg2Y3d9MMHxG56jjrZqfOhJJBpC337lEpSerAndosoFEQSAvrMbVjPObLalrx~DuorFYra6BQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Renal histopathology in an animal in Group 3 (sublethal E coli plus C4bBP) versus an animal in Group 4 (7E3 F[ab′]2 plus sublethal E coli plus C4bBP): (A) The renal histopathology of the untreated animal in Group 3 was characterized by numerous capillary and arteriolar thrombi, which stained positive for fibrin with the PTAH stain (see arrows). In some cases, the capillary walls were thickened with eosinophilic hyaline or granular material between the basement membrane and endothelium. Kidneys from untreated animals also showed focal areas of hemorrhage, tubular changes, and mononuclear leukocyte infiltration. (B) The renal histopathology of a treated animal in Group 4, which was characterized by mild congestion with no thrombin, hemorrhage, or leukocyte infiltration.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.4078/4/m_bl_0041f3.jpeg?Expires=1769148470&Signature=wsTItn2l1DzBaqMhYV8q4F9XjovcamA2PPsdlVjLY3OzFm4tf9wCQS5tballp2YTdtNiO0Uf470-e5rEkPik0qZbxCEr49pNByZ6D3bCNmTOtQiNHJUOM~eIZGVPfnfG-uQ9ajcqzmnF9JQLBF7MirrmUj50zKGVLOgsSlkHGXBmOVxHqqbCckheJQFcryo72uy0z02C3qB4zjIQaNxVXrmmaxrGwi3rcwfehDdL6JGUV7k0IoyZixqGdu-ft8jDiAH2V86LCjQ7zS-XaMOk2VxMqvDNpjEeZt6Xxs1MvrvtEhSJvy5q2glqjRR33AJxnlZeebq3h0kzslB185YAHw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal