Abstract
Previous in vivo studies have established that plasma thrombopoietin (TPO) levels are regulated by binding to c-Mpl on platelets and that, in vitro, platelets bind and degrade TPO. To determine if the in vivo metabolism of TPO was specific and saturable, we injected normal CD-1 mice IV with trace amounts of 125I-rmTPO with or without a saturating concentration of rmTPO. The amount of radioactivity present in the spleen, blood cell fraction, platelet fraction, tibia/fibula, and femur was significantly greater in the mice receiving 125I-rmTPO alone. Conversely, the amount of radioactivity present in the plasma was significantly greater in the mice receiving both 125I-rmTPO and rmTPO, thus suggesting the uptake of rmTPO by the spleen, platelets, and bone marrow in vivo was saturable. Platelet and spleen homogenates from animals receiving 125I-rmTPO alone showed a degradation pattern of 125I-rmTPO similar to that observed in vitro using mouse platelet rich plasma. To determine the in vivo binding dynamics for rmTPO, mice were injected with 125I-rmTPO alone or with increasing concentrations of rmTPO; spleen and blood cell-associated radioactivity was determined at 2 hours postinjection. A 4-parameter curve fit of the data indicated that the “in vivo binding affinity” for rmTPO was approximately 6.4 μg/kg. These data indicate that after a dose of approximately 6.4 μg/kg, 50% of all c-Mpl receptors will be saturated with rmTPO. Electron microscopy indicated that radioactivity was present bound to and within megakaryocytes and platelets in both sternum and spleen and platelets in circulation. Together these data demonstrate that in vivo, 125I-rmTPO is mainly metabolized by platelets and to a small extent by cells of the megakaryocyte lineage, via a specific and saturable mechanism.
RECENTLY it has been established that the newly identified cytokine thrombopoietin (TPO) signaling through the c-Mpl receptor is responsible for regulating platelet mass by stimulating megakaryocyte growth and differentiation.1-7 In turn, plasma TPO levels appear to be inversely related to platelet mass and it has been suggested that platelets themselves may regulate plasma TPO.8-13 We have recently shown direct regulation of plasma TPO levels by exogenously administered platelets.11 We have also shown that platelets from normal mice can bind TPO in vivo and in vitro, whereas platelets from genetically engineered mice lacking the c-Mpl receptor (c-Mpl−/−) could not. The lack of in vivo platelet binding of TPO in c-Mpl−/− mice led to a fivefold decrease in the clearance of 125I-rmTPO, when compared to controls. These data indicate that both endogenous plasma levels and clearance of exogenous TPO are regulated by binding to platelets via the c-Mpl receptor.11,14 15 Since platelets themselves can regulate the clearance of TPO, it is then logical to theorize that the capacity to clear an exogenously administered dose of TPO will be dependent on both number of platelets and c-Mpl binding sites per platelet. If true, then a thrombocytopenic patient may require a lower dose of TPO to achieve the same plasma TPO levels as a patient with normal platelet levels.
Recent in vitro studies have shown that on binding TPO, platelets metabolize it via specific proteolysis.11,15 In vitro data also indicate that both mouse and human platelets bind TPO with high affinity and that both the binding and degradation of TPO by platelets are saturable at low protein concentrations.11,15 Because the binding and degradation of TPO by platelets in vitro are saturable, it is likely that a similar mechanism exists in vivo. In vivo, specific uptake of radiolabeled TPO was found in blood cells, spleen, and bone marrow, all of which contain either platelets or megakaryocytes.11 15-17 Thus, if exogenous TPO is given at a high enough dose in vivo, all TPO binding sites on platelets and megakaryocytes would then be occupied/saturated. Determination of this in vivo “binding affinity” may help to determine optimal dosing strategies based on platelet and megakaryocyte mass.
MATERIALS AND METHODS
Test materials.Full-length recombinant murine thrombopoietin (rmTPO) was supplied as a clear solution of 0.44 mg/mL.18125I-rmTPO was prepared by the Indirect Iodogen Method,19 purified by size-exclusion chromatography, and formulated in 10 mmol/L Tris, 0.15 mol/L NaCl, 0.01% Tween 20, pH 7.4 (TNT Buffer). The specific activity of the tracer preparations ranged from 50 to 80 μCi/μg and tracers were generally prepared the day before each in vivo study. In preliminary experiments, iodinated rmTPO retained approximately full biological activity as determined by its ability to bind to the c-Mpl receptor on mouse platelets (data not shown).
In vitro metabolism of 125I-rmTPO.Murine platelet-rich plasma (PRP) was prepared from the whole citrated blood by centrifugation for 3 seconds at 14,000 rpm in a microcentrifuge. The samples were then allowed to settle for 5 minutes before the PRP was obtained from the layer above the blood cell pellet. Prostaglandin I2(PGI2 ) was added to the PRP to a final concentration of 300 ng/mL.20 PRP prepared this way typically has approximately 800,000 to 1,000,000 platelets per microliter PRP (0.17 mL) was incubated for 1 hour at 37°C with 125I-rmTPO or 125I-rmTPO and 10 μg rmTPO, or at 4°C with 125I-rmTPO. The PRP was then washed with 0.75 mL of ice-cold TNT buffer, centrifuged at 14,000 rpm for 5 minutes and the supernatant was removed. The resulting pellets were counted in a gamma counter (Wallac 1470; Wallac Inc, Gaithersburg, MD); 0.2 mL of sodium dodecyl sulfate (SDS)-sample buffer containing 10 mmol/L dithiothreitol (DTT) was added, and the sample was vortexed and boiled at 100°C for 5 minutes. 0.15 mL was analyzed by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE). The gels were dried and exposed to X-Omat film (Eastman Kodak, Rochester, NY) for 3 to 7 days at −70°C.
In vivo studies.Female CD-1 mice (8 to 10 weeks old from Charles River, Wilmington, MA) were housed with environmental controls set to maintain a temperature of 66°F to 77°F, a relative humidity of 50% ± 20%, and a 12-hour light-dark cycle. To prevent the accumulation of 125I in the thyroid of the animals, mice were injected subcutaneously with 0.1 mL of a 50 mg/mL NaI solution at 48, 24, and 1 hour before administration of test material. All studies were approved by the Genentech Animal Use and Care Committee.
In vivo metabolism of 125I-rm TPO.Two groups of CD-1 mice (n = 6) received single intravenous (IV) bolus injections (0.1 mL) of either 125I-rmTPO (∼4 μCi) or 125I-rmTPO (∼4 μCi) + 10 μg rmTPO (to displace any specifically bound 125I-rmTPO) via a lateral tail vein. All animals were killed at 3-hours postdose, and citrated blood (0.38%) was collected via cardiac puncture and stored on ice until radioanalysis and processing for plasma. Spleens were obtained sectioned, rinsed in ice-cold phosphate-buffered saline (PBS), blotted dry, weighed, and stored on dry ice until radioanalysis.
To determine the amount of 125I-rmTPO in the blood cell versus plasma fractions, whole blood from each animal (0.1 mL) was first counted for 1 minute in a gamma counter (counts per minute [cpm 1]). Next, 1 mL of PBS was added to each sample, vortexed, and centrifuged at 2,950g for 10 minutes. The resulting supernatant was aspirated and the remaining blood cell radioactivity counted (cpm 2). The data was then expressed as percent blood cell-associated for the 0.1 mL sample (cpm 2/cpm 1 × 100). The remainder of the citrated blood not used was centrifuged at 2,950g for 10 minutes and the plasma procured. Citrate plasma (0.05 mL) was first counted for 1 minute in a gamma counter (cpm 3) and then 0.5 mL PBS + 1% bovine serum albumin was added to each sample. The samples were then vortexed, 0.5 mL of 20% trichloroacetic acid added, and the samples incubated at 4°C for 15 minutes. The samples were then centrifuged at 2,950g for 10 minutes, the liquid phase aspirated, and the pellet associated radioactivity determined (cpm 4). Data were (both cpm 3 and cpm 4) then expressed as cpm/mL of plasma.
To determine if platelets bound and degraded 125I-rmTPO in vivo, PRP was prepared from 0.4 mL of whole blood from each animal. The PRP (0.2 mL) was washed with PBS (0.80 mL) and then centrifuged at 14,000 rpm and the supernatant removed by aspiration. The resulting pellets were counted in a gamma counter for 1 minute; 0.2 mL of SDS-sample buffer containing 10 mmol/L DTT was added, and the samples were vortexed and boiled at 100°C for 5 minutes. 0.15 mL was analyzed by SDS-PAGE as above. Plasma (0.005 mL) was also subjected to SDS-PAGE to determine if rmTPO was degraded in the plasma.
To determine the amount and composition of the 125I-rmTPO present in the spleen, spleens were first counted for 1 minute in a gamma counter and the values were corrected for tissue weight and expressed as cpm/g. Next the spleens were homogenized in buffer containing: 10 mmol/L HEPES, 150 mmol/L NaCl, 5 mmol/L EDTA, 1% Triton X-100 (Sigma, St Louis, MO), 0.1% SDS, 1 μg/mL Aprotinin, 1 μg/mL Leupeptin, and 100 μg/mL Pefabloc (Boehringer Mannheim, Mannheim, Germany), pH 7.4 at 4°C and 0.06 mL of the homogenate was diluted in SDS-sample buffer containing 10 mmol/L DTT then analyzed by SDS-PAGE as above.
In vivo binding affinity.Mice were randomized into 9 groups (n = 3 per group), which received a single intravenous bolus injection (0.1 mL) of either 125I-rmTPO (∼3 μCi) or 125I-rmTPo (∼3 μCi) combined with 0.01 μg, 0.1 μg, 0.5 μg, 1.0 μg, 5.0 μg, 10.0 μg, or 20.0 μg of rmTPO. All animals were killed 2 hours postdose and citrated blood (0.38%) and spleens were procured and analyzed for total and blood cell-associated radioactivity as above. Blood for platelet number determination was collected via orbital puncture from a separate group of 3 mice and stored with EDTA on ice until hematological analysis on a Baker System 9000 Diff Model cell counter (Serono Baker, Allentown, PA). The in vivo binding affinity21 was determined for both tissues by plotting cpm/g (spleen) and % cell-associated (blood) versus dose of rmTPO in μg/kg.
Contribution of bone marrow to the metabolism of 125I-rmTPO.Two groups of CD-1 mice (n = 4) received single IV bolus injections (0.1 mL) of either 125I-rmTPO (∼3 μCi) or 125I-rmTPO (∼3 μCi) + 8.8 μg rmTPO (to displace any specifically bound 125I-rmTPO) via a lateral tail vein. All animals were killed at 3 hours postdose, and citrated blood (0.38%) was collected via cardiac puncture and stored on ice until radioanalysis. Spleens, lungs, tibiae, fibulas, femora, humeri, radii, ulnae, stemums, ribs, pelves, and lower vertebrae were obtained, sectioned, rinsed in ice-cold PBS, blotted dry, weighed, and stored on dry ice until radioanalysis.
The amount of 125I-rmTPO present in the blood cell fraction was determined as above. To correct for the total body radioactivity associated with blood cells, a blood volume of 1.95 mL was assumed for the mice22 and the values corrected for percentage of dose received. To determine the amount of 125I-rmTPO present in the tissues, whole tissues were first counted for 1 minute in a gamma counter and then corrected for percentage of the dose received.
Electron microscopy.Mice received a single intravenous bolus injection of 125I-rmTPO (∼3 μCi) and were killed 3 hours later. The spleen and sternums were removed, washed in PBS, cut into ≈2 mm cubes, and fixed in Karnovsky's fixative (2% paraformaldehyde, 2.5% glutaraldehyde in cacodylate buffer), postfixed in osmium tetroxide, dehydrated, and embedded in Eponate 12 media (Ted Pella Inc, Redding, CA). PRP was prepared from blood as previously and prepared as above. Thin sections were coated with Ilford L4 EM autoradiography emulsion (Ilford, Warrington, PA) and exposed for 3 to 8 weeks.23 Developed sections were stained with lead citrate and uranyl acetate before observation in a CM12 Philips electron microscope (Philips Corp, Eindhoven, The Netherlands).
Data analysis.The in vivo binding affinity data was analyzed by a 4-parameter curve fit using KaleidaGraph (KaleidaGraph; Synergy Software, Reading, PA) with the binding capacity equal to the third parameter as described in the manual. All statistical analyses were performed on mean data using analysis of variance (ANOVA) followed by Turkey's test. Data were considered significantly different if the P value was less than 0.05. Semiquantitative analysis of the electron microscopy data was performed by analyzing sections of each tissue until at least 100 silver grains were counted and images digitized. The digitized images were then used to determine which cell types were associated with the silver grains.
RESULTS
Degradation of 125I-rmTPO by mouse platelets in vitro.The ability of platelets to bind and degrade 125I-rmTPO in vitro is presented in Fig 1. Analysis of the platelets by SDS-PAGE shows that platelets bound (70 to 105 kD band) and degraded 125I-rmTPO into 3 lower MW forms (TB). Both the binding and degradation of 125I-rmTPO were blocked by the addition of 10 μg rmTPO to the reaction (NSB). The majority of the binding and all of the degradation of 125I-rmTPO was absent when the reaction was performed at 4°C. Little degradation was observed when 125I-rmTPO was incubated in buffer alone at either 4°C or 37°C.
Autoradiograph showing the in vitro binding and degradation of 125I-rmTPO by platelets. Mouse PRP was incubated with 125I-rmTPO (TB) or 125I-rmTPO + 10 μg rmTPO (NSB) at 37°C, or with 125I-rmTPO alone at 4°C for 1 hour. 125I-rmTPO was also incubated with buffer at 4°C or 37°C. Samples were then analyzed by SDS-PAGE. Approximate MW were determined from MW standards run on the same gel.
Autoradiograph showing the in vitro binding and degradation of 125I-rmTPO by platelets. Mouse PRP was incubated with 125I-rmTPO (TB) or 125I-rmTPO + 10 μg rmTPO (NSB) at 37°C, or with 125I-rmTPO alone at 4°C for 1 hour. 125I-rmTPO was also incubated with buffer at 4°C or 37°C. Samples were then analyzed by SDS-PAGE. Approximate MW were determined from MW standards run on the same gel.
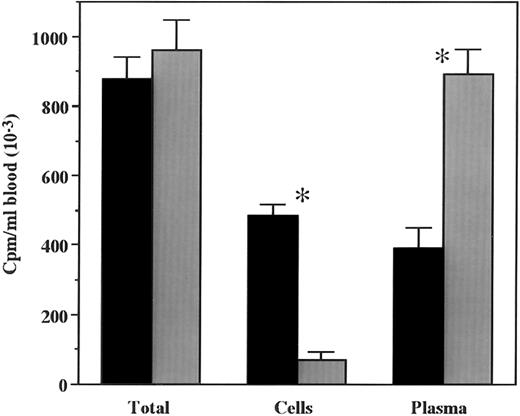
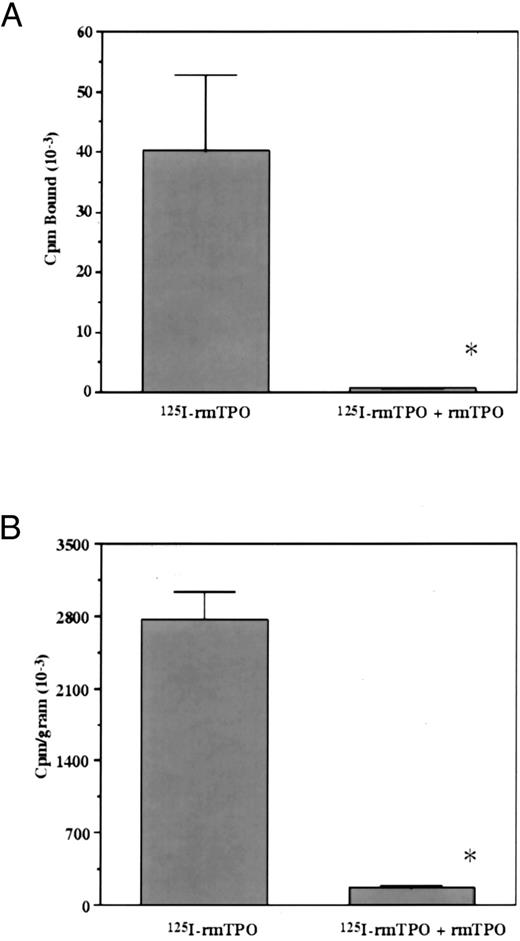
In vivo metabolism of 125I-rmTPO.To determine if the in vivo metabolism of 125I-rmTPO was saturable, mice were injected IV with either 125I-rmTPO alone or in the presence of a saturating amount of rmTPO. A significant difference in the amount of 125I-rmTPO present in the plasma was observed between the groups (Fig 2). The amount of 125I-rmTPO present in the plasma of mice injected with tracer alone was much less than the mice that received tracer + cold rmTPO, indicating that the plasma concentrations were dose-dependent. The amount of 125I-rmTPO present in the plasma appears to be inversely related to the amount present in the blood cell fraction (Fig 2). These data indicate that plasma levels of 125I-rmTPO are dependent on the availability of binding sites (c-Mpl) present on platelets. A similar pattern was observed for the amount of 125I-rmTPO present in platelets isolated from these mice (Fig 3A). Platelets from mice injected with 125I-rmTPO alone contained significantly more radioactivity than platelets from mice receiving 125I-rmTPO + rmTPO, indicating that binding to platelets in vivo is a saturable phenomenon. These data also suggest that the majority of the radioactivity in the blood cell fraction (Fig 2) was due to 125I-rmTPO binding to platelets and not other blood cells. The amount of 125I-rmTPO present in the spleen was consistent with the above data (Fig 3B). Again, the mice receiving 125I-rmTPO alone had significantly more radioactivity present in the spleen than the mice receiving 125I-rmTPO + rmTPO. Together these data demonstrate that the removal of plasma 125I-rmTPO by platelets and the spleen involves a specific and saturable mechanism.
Overall mean concentration of total radioactivity associated with plasma or blood cells following IV administration of 125I-rmTPO. Mice received a single IV bolus injection of 125I-rmTPO or 125I-rmTPO + 10 μg rmTPO and were sacrificed 3 hours later. Citrated blood was collected and the amount of radioactivity per milliliter of blood determined. The blood was washed, centrifuged and the radioactivity associated with the blood cells determined. The remaining radioactivity was associated with plasma. The data were expressed as cpm/mL of blood. Black bars represent 125I-rmTPO and gray bars represent 125I-rmTPO + 10 μg rmTPO. *Indicates values are significantly different (P < .05).
Overall mean concentration of total radioactivity associated with plasma or blood cells following IV administration of 125I-rmTPO. Mice received a single IV bolus injection of 125I-rmTPO or 125I-rmTPO + 10 μg rmTPO and were sacrificed 3 hours later. Citrated blood was collected and the amount of radioactivity per milliliter of blood determined. The blood was washed, centrifuged and the radioactivity associated with the blood cells determined. The remaining radioactivity was associated with plasma. The data were expressed as cpm/mL of blood. Black bars represent 125I-rmTPO and gray bars represent 125I-rmTPO + 10 μg rmTPO. *Indicates values are significantly different (P < .05).
Overall mean concentration of total radioactivity associated with platelets and spleens following IV administration of 125I-rmTPO. Mice received a single IV bolus injection of 125I-rmTPO or 125I-rmTPO + 10 μg rmTPO and were killed 3 hours later. (A) Citrated blood was collected and the amount of radioactivity bound to platelets determined. The data were expressed as cpm bound. (B) Spleens were dissected and the amount of radioactivity associated with the spleens determined and reported as cpm/gram. *Indicates values are significantly different (P < .05).
Overall mean concentration of total radioactivity associated with platelets and spleens following IV administration of 125I-rmTPO. Mice received a single IV bolus injection of 125I-rmTPO or 125I-rmTPO + 10 μg rmTPO and were killed 3 hours later. (A) Citrated blood was collected and the amount of radioactivity bound to platelets determined. The data were expressed as cpm bound. (B) Spleens were dissected and the amount of radioactivity associated with the spleens determined and reported as cpm/gram. *Indicates values are significantly different (P < .05).
The in vivo metabolic fate of the 125I-rmTPO bound by platelets and the spleen is presented in Fig 4A and B. As shown in Fig 4A, platelet samples from mice receiving 125I-rmTPO alone display an intact band (≈70 to 100 kD) as well 3 major proteolyzed fragments (≈30 to 50 kD) of 125I-rmTPO. However, it should be noted that these fragments might appear nondiscrete in the autoradiographs due to differences in glycosylation of the proteolyzed fragments. Little, if any 125I-rmTPO was present in the platelet samples from mice receiving excess rmTPO. These in vivo data are similar to those presented in Fig 1, which show that in vitro, platelets degrade 125I-rmTPO into 3 lower MW fragments. Although there is a clear difference in the amount of 125I-rmTPO present in the plasma between the two groups, there was little if any degraded 125I-rmTPO present in the plasma. A similar degradation pattern of 125I-rmTPO was also observed in samples from the spleens (Fig 4B). These results indicate that 125I-rmTPO is very stable in plasma, but is metabolized into proteolytic fragments in the blood by platelets and by cells in the spleen.
Autoradiographs showing the in vivo binding and degradation of 125I-rmTPO by platelets and spleen tissue following IV administration of 125I-rmTPO. Mice received a single IV bolus injection of 125I-rmTPO or 125I-rmTPO + 10 μg rmTPO and were killed 3 hours later. (A) Citrated blood, (B) spleens, and plasma were obtained and processed. These samples were then analyzed by SDS-PAGE. Approximate MW were determined from MW standards run on the same gel.
Autoradiographs showing the in vivo binding and degradation of 125I-rmTPO by platelets and spleen tissue following IV administration of 125I-rmTPO. Mice received a single IV bolus injection of 125I-rmTPO or 125I-rmTPO + 10 μg rmTPO and were killed 3 hours later. (A) Citrated blood, (B) spleens, and plasma were obtained and processed. These samples were then analyzed by SDS-PAGE. Approximate MW were determined from MW standards run on the same gel.
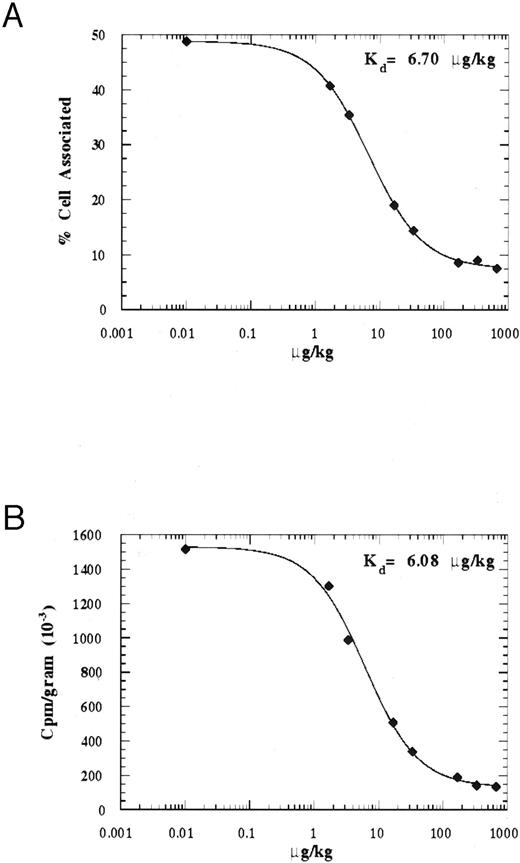
Determination of the in vivo binding affinity of 125I-rmTPO.To determine the in vivo binding affinity of 125I-rmTPO, mice were injected IV with 125I-rmTPO ± increasing concentration of rmTPO and the amount of radioactivity associated with the blood cells and spleen was determined after 2 hours. These data are presented in Figs 5A and B. As shown in Fig 5A, as the concentration of rmTPO increased, the amount of 125I-rmTPO bound to the blood cells decreased. Expressing the data as % blood cell associated versus amount of rmTPO injected (μg/kg) and using a 4-parameter fit to determine the binding constant, we calculated an in vivo binding affinity of ≈6.70 μg/kg. Expressing the amount of radioactivity present in the spleen as cpm per gram, we calculated a very similar in vivo binding affinity of ≈6.08 μg/kg, suggesting that a saturable binding mechanism is present in both tissues. Together these data indicate that at an IV dose of ≈6.4 μg/kg, 50% of all c-Mpl receptors will be occupied with rmTPO.
Graphical representation of the in vivo binding affinities of blood cells and spleen tissue following IV administration of 125I-rmTPO. Mice received a single IV bolus injection of 125I-rmTPO or 125I-rmTPO + increasing concentrations of rmTPO and were killed 2 hours later. Citrated blood and spleens were procured and blood cell and spleen-associated radioactivity were determined. The binding affinities were determined by 4 parameter fits of % blood cell-associated (A) and spleen-associated cpm/gram (B) versus dose of rmTPO expressed in μg/kg.
Graphical representation of the in vivo binding affinities of blood cells and spleen tissue following IV administration of 125I-rmTPO. Mice received a single IV bolus injection of 125I-rmTPO or 125I-rmTPO + increasing concentrations of rmTPO and were killed 2 hours later. Citrated blood and spleens were procured and blood cell and spleen-associated radioactivity were determined. The binding affinities were determined by 4 parameter fits of % blood cell-associated (A) and spleen-associated cpm/gram (B) versus dose of rmTPO expressed in μg/kg.
Contribution of bone marrow to the metabolism of 125I-rmTPO.To compare the specific total body radioactivity associated with blood cells, spleen and bone marrow, mice were injected IV with either 125I-rmTPO alone or in the presence of a saturating amount of rmTPO. A significant difference in the amount of 125I-rmTPO present (expressed as % of added total dose) in the blood cells, spleen, tibia/fibula, and femur was observed between the groups (Fig 6). Tissues from mice injected with 125I-rmTPO alone contained significantly more radioactivity than the tissues from mice receiving 125I-rmTPO + rmTPO, again indicating that binding to platelets and megakaryocytes in vivo is a saturable phenomenon. However, the total amount of 125I-rmTPO in the blood cells and spleen was much greater than that found in the whole bones (Fig 6). Combined, the blood cells and spleen contained 12.45% of the total dose of 125I-rmTPO 3 hours after injection, whereas all the bones combined contained only 3.26%. However, some of the 125I-rmTPO present in the bones is likely also due to the presence of platelets within the vascular compartment of the bones of which we could not control for. Together these data suggest that the platelets and spleen are the major tissues involved in the binding and metabolism of 125I-rmTPO in vivo, although cells of the megakaryocyte lineage also contribute to this process.
Percent of total dose associated with blood cells and tissues following IV administration of 125I-rmTPO. Mice received a single IV bolus injection of 125I-rmTPO or 125I-rmTPO + 8.8 μg rmTPO and were killed 3 hours later. Citrated blood was collected and the amount of radioactivity associated with the blood cells determined per animal based on 1.95 mL total blood volume. Whole tissues were dissected and the amount of radioactivity associated with each tissue determined. The data were expressed as percent of total dose. Black bars represent 125I-rmTPO and striped bars represent 125I-rmTPO + 8.8 μg rmTPO. *Indicates values are significantly different (P < .05).
Percent of total dose associated with blood cells and tissues following IV administration of 125I-rmTPO. Mice received a single IV bolus injection of 125I-rmTPO or 125I-rmTPO + 8.8 μg rmTPO and were killed 3 hours later. Citrated blood was collected and the amount of radioactivity associated with the blood cells determined per animal based on 1.95 mL total blood volume. Whole tissues were dissected and the amount of radioactivity associated with each tissue determined. The data were expressed as percent of total dose. Black bars represent 125I-rmTPO and striped bars represent 125I-rmTPO + 8.8 μg rmTPO. *Indicates values are significantly different (P < .05).
Electron microscopy.Electron microscopy analysis of sections of the sternum and spleen following a single IV bolus injection revealed the presence of silver grains corresponding to 125I-rmTPO over megakaryocytes, preplatelets, and platelets. A representative micrograph of platelets from PRP containing silver grains is presented in Fig 7A. In the PRP, 87% of the silver grains were associated with platelets, with the rest being found over red blood cells or cell-free portions of the sections. The majority of silver grains present in the spleens of the above mice were also found associated with platelets (71%), although silver grains were also associated with megakaryocytes or other cell types including RBCs, macrophages, and lymphocytes. However, the platelets clearly appear to be the major binding site for 125I-rmTPO in the spleen (Fig 7B). Figure 7C shows a megakaryocyte present within the bone marrow of a sternum section, most silver grains were found within the megakaryocytes (70%). Some silver grains were found associated with platelets and preplatelets (12%) or other cell types within the marrow and vasculature of the sternum. Of the silver grains present in both platelets and megakaryocytes (on examining multiple samples), some appeared to be associated with vesicles, whereas others were found in the cytoplasm (data not shown). Because of the complex nature of the cytoplasm structure in megakaryocytes and platelets, as well as the relative insensitivity of autoradiography techniques, care should be taken when interpreting the data. However, they clearly indicate that both megakaryocytes and platelets bind and internalize 125I-rmTPO in vivo. The fact that few silver grains were associated with the other blood cell types indicate that the specific cellular uptake of 125I-rmTPO within the bone marrow and spleen was likely restricted to blood cells of the megakaryocyte lineage.
Electron microscopy autoradiographs showing cellular localization of 125I-rmTPO within platelets, spleen, and sternum. Mice received a single IV bolus injection of 125I-rmTPO and were killed 3 hours later. Sections of platelets, spleen, and sternum were fixed and processed for electron microscopy. Silver grains were found associated with platelets in (A) PRP and (B) spleen, and (C) megakaryocytes in sternum. The bar in the autoradiographs is equal to 1 μm.
Electron microscopy autoradiographs showing cellular localization of 125I-rmTPO within platelets, spleen, and sternum. Mice received a single IV bolus injection of 125I-rmTPO and were killed 3 hours later. Sections of platelets, spleen, and sternum were fixed and processed for electron microscopy. Silver grains were found associated with platelets in (A) PRP and (B) spleen, and (C) megakaryocytes in sternum. The bar in the autoradiographs is equal to 1 μm.
DISCUSSION
Recent studies have implicated TPO as the major cytokine responsible for the regulation of circulating platelet numbers through stimulation of megakaryopoiesis via the c-Mpl receptor.1-7 Conversely, it has been recently shown that plasma TPO levels are directly regulated by the platelets themselves, and to some extent by megakaryocytes, via binding to the c-Mpl receptor.8-13 However, the data we have presented clearly indicates that platelets are the main contributing factor towards regulation of TPO levels in vivo. Because platelets in vitro have the ability to specifically bind and degrade TPO via a saturable mechanism, it is likely that in vivo, the ability of platelets to regulate TPO levels will also be saturable.11,16 Therefore, during thrombocytopenia, plasma TPO levels should increase because of the decrease in platelets and in c-Mpl binding sites. This, in turn, would allow the bone marrow to be exposed to a greater concentration of TPO.24,25 As platelet levels increase to normal or supranormal levels, plasma TPO levels should decrease due to binding to platelets.7,8 11 In vivo, the number of platelet binding sites available relative to the amount of TPO administered will also likely affect the clearance of administered TPO. These studies were designed to clarify the role of platelets in the clearance of TPO in vivo, as well as to determine the in vivo binding affinity for TPO in mice.
First, we compared the metabolism of TPO by platelets both in vivo and in vitro. An earlier study comparing c-Mpl deficient mice to normal mice indicated that normal mice specifically sequestered 125I-rmTPO in both blood cells and the spleen while the c-Mpl−/− mice did not display specific uptake in any tissue.11 In the present studies, 125I-rmTPO was specifically bound in both tissues as well as the tibia/fibula and femur. Conversely, significantly more radioactivity was present in the plasma in mice receiving both 125I-rmTPO and rmTPO. These data strongly suggest that the ability of these tissues to remove 125I-rmTPO from plasma is specific and saturable.
In vitro, TPO is bound with high affinity and proteolyzed by both mouse and human platelets.11 15 In vivo data from both platelet and spleen samples show that these tissues also specifically degrade 125I-rmTPO. Little degradation of 125I-rmTPO was evident in the plasma from either group of mice indicating that metabolism of TPO does not take place in the plasma. In similar studies, we have measured the presence of intact 125I-rmTPO in plasma 24 hours after an IV injection, further showing its stability in plasma (data not shown). Together, these data indicate that in vivo TPO is removed from the plasma by binding mainly to platelets and megakaryocytes cells in the spleen, blood, and bone marrow where it is subsequently metabolized. Data also indicate that when expressed as % of total added dose, the total contribution of the spleen and blood cells to the binding and metabolism of 125I-rmTPO was greater than that of the bone marrow.
Because the in vivo clearance and metabolism of TPO by platelets and spleen cells is specific, saturable, and mediated via one class of receptors, it was possible to determine the in vivo binding affinity of TPO in mice using the principles of ligand-receptor interactions.19,26 Data indicated that following a dose of ∼6.4 μg/kg of rmTPO, 50% of available binding sites in tissue and blood should be occupied at 2-hours postinjection. Consequently, doses greater than the theoretical binding affinity may not be any more efficacious due to lack of binding sites available on megakaryocyte precursor cells. In support of this, significant differences in maximum platelet response were not observed in mice given a single IV bolus injection of either 5 or 25 μg/kg of rmTPO (data not shown). These data also suggest that at a dose of approximately 6.4 μg/kg of rmTPO, the specific clearance mechanism (binding to c-Mpl) could begin to be saturated and any additional TPO may be cleared by a nonspecific mechanism. This assumption is supported by pharmacokinetic studies using c-Mpl deficient mice, in which an injected dose of 125I-rmTPO was cleared at a much slower rate in the c-Mpl deficient mice than in normal mice.11 This dramatic decrease in clearance was because of a lack of TPO binding to c-Mpl on platelets and megakaryocytes.
Human platelets also have the ability to bind and remove iodinated recombinant human thrombopoietin (125I-rhTPO) from plasma via internalization and enzymatic degradation.15,27,28 Similar to the data obtained in mice, the binding and degradation of 125I-rhTPO by human platelets in vitro was specific and saturable at low protein concentrations, with both murine and human ligands binding to their respective receptor with similar affinities (200 to 500 pmol/L).11,15 Therefore, it is likely that a similar mechanism exists in humans which is saturable at relatively low concentrations of rhTPO. By using the in vivo binding affinity of approximately 6.4 μg/kg for mice and the relative platelet numbers and number of binding sites per platelet, we can estimate the theoretical dissociation constant (Kd ) of rhTPO in humans. Mice have approximately fourfold more platelets than humans and approximately 2 to 4 times the number of binding sites for TPO per platelet.11,15 If the in vivo Kd for rmTPO in mice is approximately 6.4 μg/kg, then by correcting the data for differences in platelet and binding site numbers, we calculate an approximate in vivo Kd for humans of 0.8-1.7 μg/kg. If correct, these data suggest that in a human with a normal platelet count, a single IV bolus of the above dose should saturate approximately 50% of available binding sites and produce a near-maximal biological response. Therefore it may be possible to approximate what dose to administer to patients based on number of platelets and number of c-Mpl binding sites per platelet. In support of this assumption, recent clinical data has demonstrated that single IV doses of rhTPO at concentrations of 0.3 to 2.6 μg/kg stimulated a dose-dependent increase in platelet levels.29 These data also suggest that in human doses higher than the in vivo Kd may begin to saturate the specific clearance of rhTPO.
Because there is an inverse relationship between platelet number and plasma TPO levels,7-13 patients with normal or high platelet counts would likely require a greater dose of TPO to achieve the same plasma concentrations of free TPO than thrombocytopenic patients.29 A similar phenomenon has been noted in chemotherapy patients receiving granulocyte colony-stimulating factor (G-CSF )30 who showed an increase in the clearance of G-CSF as absolute neutrophil numbers increased. Again, these data support the presence of end-cell regulation of cytokine levels by some of the hematopoietic lineages.30 31
The electron microscopy results support earlier observations that the platelets and spleen are the major tissues involved in the removal of 125I-rmTPO from plasma.11,16 Although silver grains were present in megakaryocytes within the spleen, most appeared to be associated with platelets. These data were expected since close to one-third of the total circulating platelets are present within the spleen in mice.11 Within the bone marrow, 125I-rmTPO was associated with megakaryocytes, platelets, and preplatelets. The fact that not all silver grains were associated with membranes or organelles and some were present free in the cytoplasm suggests that either more than one internalization pathway may exist in these cells, or that the silver grains found in the cytoplasm represent cellularly metabolized fragments of 125I-rmTPO. However, these data clearly show that both megakaryocytes and platelets bind and internalize 125I-rmTPO in vivo.
In summary, our data show that the in vivo binding and metabolism of 125I-rmTPO in mice is specific and saturable. The major cells involved in the binding and metabolism of 125I-rmTPO appear to be platelets present in the blood and spleen. Electron microscopy studies confirm that 125I-rmTPO was bound and internalized by both platelets and megakaryocytes in vivo. Data also suggest that the in vivo Kd for 125I-rmTPO in mice was approximately 6.4 μg/kg. Together these in vivo data from mice combined with in vitro data from humans may provide a method for determining the approximate dose of TPO to be administered to patients based on platelet numbers and number of c-Mpl on platelets.
ACKNOWLEDGMENT
The authors thank Allison Nixon, Anne Walters, and Elizabeth Tomlinson for their help with the animals and Yvonne Lin for her editorial comments.
Address reprint requests to Paul J. Fielder, PhD, Department of Pharmacokinetics and Metabolism, Mail Stop 70, Genentech Inc, 460 Point San Bruno Blvd, S San Francisco, CA 94080.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal