Abstract
The U-A10 cell line, a doxorubicin-selected variant of human U-937 myeloid leukemia cells, exhibits a redistribution of anthracyclines into a expanded vesicular compartment. The acidic nature of this compartment was confirmed by vital staining with a pH sensitive dye, LysoSensor yellow/blue DND-160. Identification of the vesicular compartment was performed by immunofluorescence analysis. Staining for the LAMP-1 and LAMP-2 antigens showed that the vesicles are enlarged lysosomes that are eccentrically placed near the nucleus of U-A10 cells. By contrast, the expression of the multidrug resistance-associated protein and the P-glycoprotein was observed predominately on the plasma membrane of the drug-resistant cells. The accumulation of daunorubicin into cellular compartments was quantified using radiolabeled drug. Exposing cells to 3[H]-daunorubicin and then isolating intact nuclei showed that nuclei from U-A10 cells accumulated twofold to threefold less anthracycline than nuclei from U-937 cells. However, when nuclei were isolated first and then exposed to 3[H]-daunorubicin, little difference in net nuclear drug accumulation was detected. Cytoplasts prepared from U-A10 and U-937 cells were exposed to 3[H]-daunorubicin to measure cytoplasmic drug accumulation. At external daunorubicin concentrations of 100 ng/mL or higher, cytoplasts from U-A10 cells accumulated significantly more daunorubicin than cytoplasts from U-937 cells. Moreover, studies with the lysosomotropic agent chloroquine showed that U-A10 cells accumulated twofold more chloroquine and showed twofold enhanced sensitivity to this agent as compared with parental U-937 cells. Fluorescence microscopy showed that chloroquine affects vesicular anthracycline sequestration in U-A10 cells with an associated increase in daunorubicin nuclear fluorescence. Although chloroquine did not alter anthracycline cytotoxicity in parental cells, it restored daunorubicin and doxorubicin sensitivity to U-A10 cells. Taken together, these studies demonstrate that U-A10 cells exhibit a redistribution of the lysosomal compartment. The trapping of drug into an expanded acidic vesicular compartment results in decreased nuclear drug accumulation and decreased cytotoxicity. Lysosomotropic agents, such as chloroquine, warrant further study as modulators of this acquired drug-resistance phenotype.
HUMAN MYELOID leukemia cells selected for resistance to the anthracyclines doxorubicin or daunorubicin generally display a multidrug-resistance phenotype associated with overexpression of MDR1 and/or MRP. Both MDR1 and MRP are members of the ATP-binding cassette superfamily of membrane transporters.1-3 Transfection of the cDNA for either MDR1 or MRP results in cell lines that display diminished sensitivity to the anthracyclines, vinca alkaloids, and epipodophyllotoxins and demonstrate energy-dependent efflux of cytotoxic agents.4-6
In some anthracycline-selected cell lines, the subcellular drug distribution is strikingly different between parental and drug-resistant cell lines.7-13 Whereas most parental cell lines accumulate anthracyclines predominately in the nucleus, certain drug-resistant cells appear to sequester anthracyclines into a vesicular, cytoplasmic compartment. Because the cytotoxic target of the anthracyclines is the nuclear enzyme topoisomerase II,14 non-nuclear drug redistribution may contribute to diminished sensitivity to these agents. Despite these observations, the contribution of vesicular sequestration to the resistance phenotype has been incompletely characterized.
We described a series of U-937 cell lines selected for doxorubicin resistance.12 Cells that were exposed to low levels of drug overexpressed the multidrug resistance-associated protein and showed a vesicular redistribution of anthracyclines into a non-nuclear compartment. We now present data that further define this process. The results indicate that vesicular anthracycline sequestration directly contributes to diminished sensitivity to the anthracyclines. These cells possess a distinct constitutive redistribution of the lysosomal compartment associated with the expression of the lysosomal-associated membrane protein, LAMP-1. Staining for the P-glycoprotein and for MRP suggests that these transporters are expressed independently of the vesicular compartment and are primarily on the plasma membrane in U-A10 cells. Furthermore, this compartment may be a target for modulating agents to partially reverse this resistance phenotype.
MATERIALS AND METHODS
Cell culture.The U-937 human myeloid leukemia cell line was obtained from the American Type Culture Collection (Rockville, MD). U-A10 cells were derived from the parental U-937 line by chronic exposure to a final concentration of 10 ng/mL doxorubicin.12 Both cell lines were routinely grown at 37°C and 5% CO2 in RPMI-1640 medium supplemented with 10% fetal bovine serum, L-glutamine, and penicillin/streptomycin.
Drugs and chemicals.Daunorubicin was purchased from Wyeth-Ayerst Laboratories (Philadelphia, PA). 3[H]-daunorubicin (specific activity, 1.4 Ci/mmol) and 3[H]-water (specific activity, 6.33 mCi/mL) were from DuPont-New England Nuclear (Boston, MA). Silicone oil (specific gravity, 1.035 to 1.045) was from William F. Nye Co (New Bedford, MA). Lysosensor yellow/blue DND-160 (sulfinylbis methane) was from Molecular Probes (Eugene, OR). All other chemicals were purchased from Sigma Chemical Co (St Louis, MO).
Antibodies.The antisera MRP-6KQ was generously provided by M. Center (Kansas State University, Manhattan, KS) and was used at a 1:1,000 dilution.15 The monoclonal antibodies (MoAbs) QCRL-1 and QCRL-3 were kindly provided by S. Cole (Queen's University, Kingston, Ontario, Canada) and were used as described.16 The MoAb C219 was purchased from Signet (Dedham, MA) and was used at 1 μg/mL.17 Antibodies to human LAMP-1 and LAMP-2 were purchased from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA) and were used at the supplier's recommended dilution.18
Fluorescence photomicrographs.Cells or cytoplasts were suspended in RPMI medium and incubated with daunorubicin (500 ng/mL final concentration) for 1 hour. They were then washed twice in phosphate-buffered saline (PBS; 0°C) and kept on ice until examination by fluorescence microscopy. In some experiments, chloroquine (0 to 100 μmol/L) was added to cell suspensions for 2 hours before daunorubicin exposure to examine its effect on vesicular accumulation. Cells or cytoplasts were photographed rapidly to minimize the effect of photo bleaching. Photographs were taken through an Olympus BH-2 fluorescence microscope (Olympus Optical Co, Tokyo, Japan). Exposures were for 4 seconds with Kodak TMAX ASA 3200 film or for 8 seconds with Kodak ASA 1600 colorprint film (Eastman Kodak, Rochester, NY).
Vital staining with pH indicator.Cells (1 × 106/mL) were exposed to the pH sensitive fluorescence dye, Lysosensor yellow/blue DND-160, at a final concentration of 10 μmol/L for 2 hours in PBS-glucose. Cells were washed twice with cold PBS (0°C) and then kept on ice until fluorescence microscopy. After an excitation period of 5 seconds, exposures of 20 seconds were performed with Kodak ASA 400 colorprint film.
Immunofluorescence studies.Cells (1 × 107) were washed with PBS, fixed in 70% ethanol at 0°C for 1 hour, and then incubated in 5% goat serum for 30 minutes while on ice. Primary antibody incubation occurred for 1 hour at 0°C before washing in PBS and incubating with either a goat antimouse or goat antirabbit fluorescein isothiocyanate (FITC)-conjugated second-step antibody (Boehringer Mannheim, Indianapolis, IN) for 45 minutes (0°C). The cells were then washed and kept on ice in the dark until examination by fluorescence microscopy using an Olympus BH-2 fluorescence microscope (Olympus Optical Co, Tokyo, Japan). Cells were photographed using Kodak Tri-PanX ASA 400 film.
Measurement of daunorubicin accumulation.All drug incubations were performed at 37°C. Cells or cytoplasts were resuspended at 106/mL and incubated for 1 hour in RPMI medium containing 3[H]-daunorubicin at final concentrations of 0 to 750 ng/mL as indicated. Drug accumulation was terminated by sedimenting cells or cytoplasts through silicone oil using a previously described technique.19 20 Briefly, after drug incubations, 200 μL of cell suspensions were pelleted through a layer of silicone oil (200 μL; specific gravity, 1.035 to 1.040) into concentrated formic acid (20 μL). The contents of the tube were frozen on dry ice before cutting and transferring the tip into a vial with scintillation fluid and determining the radioactivity. Counts per minute were converted to the amount (in nanograms) of drug using a regression analysis of scintillation counts against a known amount of 3[H]-daunorubicin.
Assessment of nuclear daunorubicin accumulation in intact cells.The nuclear accumulation of daunorubicin was assayed after exposing cells to 3[H]-daunorubicin for 1 hour. Subsequent steps of cell washing and nuclei isolation were performed on ice or in a cold room to minimize drug loss. Cells (106/mL) were pelleted, washed twice, and transferred to 1 mL of buffer containing 0.25 mol/L sucrose in TKMC (50 mmol/L Tris, pH 7.0, 25 mmol/L KCl, 5 mmol/L MgCl2 ).21 The cells were homogenized with 20 strokes of a Dounce homogenizer. The homogenate was mixed with 2.3 mol/L sucrose containing 0.75% Triton-X 100 in TKMC buffer. The mixture was layered over 2.3 mol/L sucrose in TKMC buffer and spun at 30,000 rpm for 70 minutes at 4°C. The pelleted nuclei were recovered and resuspended in 50 μL of 60 mmol/L KCl in TKMC buffer. The number and the purity of the nuclei were assessed using a Coulter Counter (Coulter, Hialeah, FL) and by light microscopy after trypan blue staining. Preparations were observed to be greater than 95% isolated nuclei with no evidence of other attached cellular material. Nuclear drug accumulation was determined by removing aliquots of isolated nuclei, dissolving with 1 N NaOH, neutralizing with 1 N HCl, and subsequent scintillation counting. Counts per minute were converted to amount (in nanograms) of daunorubicin per 106 nuclei.
Assessment of nuclear daunorubicin accumulation in isolated nuclei.The nuclear accumulation of daunorubicin was assessed by incubating isolated nuclei (separated first from cells before drug exposure) in 3[H]-daunorubicin for 15 minutes at 37°C. Cells (107) were washed twice in PBS and resuspended in 5 mL of lysis buffer (10 mmol/L Tris, pH 7.4, 10 mmol/L NaCl, 3 mmol/L MgCl2 , and 0.5% NP-40), and the mixture was sharply tapped. After incubation on ice for 5 minutes, the nuclei were pelleted (1,000g ), resuspended in lysis buffer, tapped, iced, and repelleted. The nuclei were resuspended in TKMC buffer containing 0.25 mol/L sucrose. Nuclei were counted and preparations were observed to be greater than 95% isolated nuclei. After exposure to 3[H]-daunorubicin, nuclei were pelleted through silicone oil and radioactivity determined as described above.
Preparation of cytoplasts.Cytoplasts were prepared as described.22 23 Briefly, cells (∼5 × 107) were pelleted, washed in Dulbecco's PBS, and resuspended in 1 mL of 10% Ficoll-400 previously prepared in RPMI medium containing 10 μg/mL of cytochalasin B. This cell suspension was spun through a discontinuous Ficoll gradient (10% to 25%) at 25,000 rpm for 1 hour at 30°C. Cytoplasts were recovered at the 15% to 16% Ficoll interface, washed, counted, and resuspended in RPMI medium (106 cytoplasts/mL) and incubated at 37°C for 1 hour before exposure to drugs. Aqueous volumes of cytoplasts prepared from U-937 and U-A10 cells were determined by measuring the 2-hour accumulation of tritiated water at 37°C in RPMI medium.
Measurement of cellular accumulation of chloroquine.After 2 hours of exposure to chloroquine, cells (107) were pelleted and washed twice in ice-cold PBS and resuspended in 50 μL of PBS containing 8-hydroxyquinoline as an internal standard. Cells were lysed by adding 800 μL of a 50% solution of acetonitrile in methanol, and the lysate was left on ice for 1 hour before centrifuging on a desk top centrifuge at high speed for 10 minutes. Samples (10 μL) were injected onto a high-performance liquid chromatography (HPLC) column. The HPLC system consisted of a system controller (C-R3A; Shimadzu, Kyoto, Japan), two pumps (LC-6A; Shimadzu), a UV/Vis detector (SPD-6AV; Shimadzu) set at 330 nm, and an integrating recorder (SPD-6AV; Shimadzu). A MOS hypersil C8, 5m, 100 × 2.1 mm column was used (Hewlett-Packard, Palo Alto, CA). The mobile phase contained 80% methanol, 20% water, 50 mmol/L sodium acetate, and 30 mmol/L octadecyl sulfonate adjusted to pH 5.0. Retention times were 2.3 and 4.3 minutes for 8-hydroxyquinoline and chloroquine, respectively. This assay was linear for chloroquine in the range used (r2 > .999; 5 to 500 μmol/L).
Drug sensitivity assays.Cell sensitivity to daunorubicin, doxorubicin, chloroquine, and anthracycline/chloroquine combinations was determined after 96 hours of exposure to drugs using an MTT assay performed in quadruplicate at least three times.24 The IC50 values were determined by fitting the data to a sigmoidal inhibitory effect model with a baseline effect parameter using a nonlinear least squares regression program (Model 108, PCNONLIN ver 4; SCI Software, Stateline, PA). The fold resistance was calculated from the ratio of IC50 value for U-A10 cells to the IC50 value for the parental U-937 cells. The effect of chloroquine on anthracycline cytotoxicity was determined by adding the modulating agent just before the anthracycline. A chloroquine concentration of 10 μmol/L is minimally toxic to cells and is clinically achievable.25
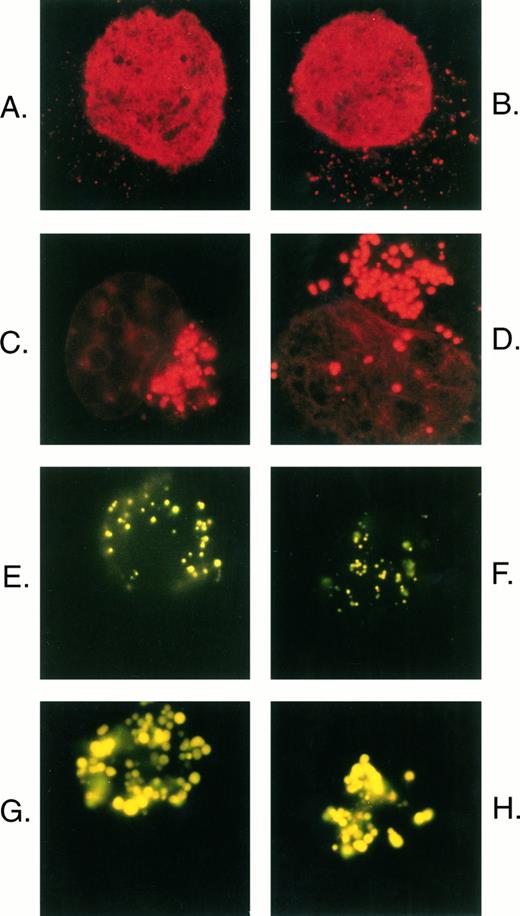
Comparison of daunorubicin and Lysosensor yellow/blue DND-160 distribution in U-937 cells. Parental U-937 cells (A and B) and U-A10 cells (C and D) were exposed to 500 ng/mL of daunorubicin for 1 hour, and the cells were washed, visualized by fluorescence microscopy, and photographed. Parental U-937 cells (E and F ) and U-A10 cells (G and H) were exposed to Lysosensor yellow/blue DND-160 (10 μmol/L) for 2 hours, and the cells were washed, visualized by fluorescence microscopy, and photographed. Shown are photographs of typical single cells centered within the frame such that the perimeter of the cell approximately reaches the edge of the photograph.
Comparison of daunorubicin and Lysosensor yellow/blue DND-160 distribution in U-937 cells. Parental U-937 cells (A and B) and U-A10 cells (C and D) were exposed to 500 ng/mL of daunorubicin for 1 hour, and the cells were washed, visualized by fluorescence microscopy, and photographed. Parental U-937 cells (E and F ) and U-A10 cells (G and H) were exposed to Lysosensor yellow/blue DND-160 (10 μmol/L) for 2 hours, and the cells were washed, visualized by fluorescence microscopy, and photographed. Shown are photographs of typical single cells centered within the frame such that the perimeter of the cell approximately reaches the edge of the photograph.
Statistical analyses.Standard error bars are presented for each value on the drug accumulation graphs. Student's paired t-test was used to compare differences in accumulation between parental and drug-resistant sublines as indicated. All statistics were calculated with software programs from StatView (Brainpower Inc, Calabasas, CA).
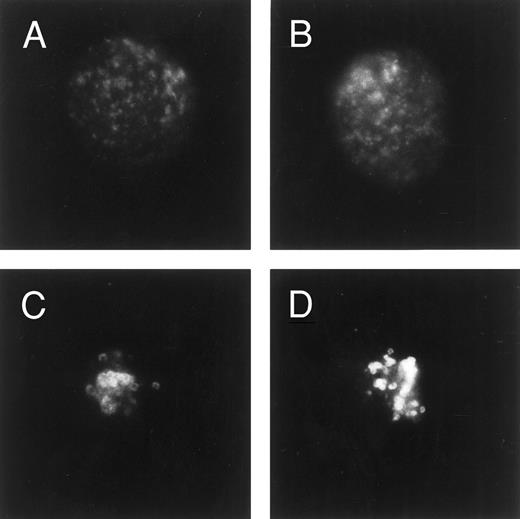
Immunofluorescence analysis of LAMP-1 expression. Cells were fixed/permeabilized in ethanol before blocking in goat serum, incubating with LAMP-1 MoAb, and then exposing to an FITC second step. Cells were examined by fluorescence microscopy and photographed. Shown are photographs of typical single cells (centered within the frame) from parental U-937 cells (A and B) or U-A10 cells (C and D).
Immunofluorescence analysis of LAMP-1 expression. Cells were fixed/permeabilized in ethanol before blocking in goat serum, incubating with LAMP-1 MoAb, and then exposing to an FITC second step. Cells were examined by fluorescence microscopy and photographed. Shown are photographs of typical single cells (centered within the frame) from parental U-937 cells (A and B) or U-A10 cells (C and D).
RESULTS
The U-A10 cell line was derived by selecting the human myeloid leukemia cell line, U-937, in 10 ng/mL of doxorubicin.12 Studies using 3[H]-daunorubicin showed that U-A10 cells accumulate net levels of daunorubicin that are almost equal to that observed in parental U-937 cells. However, fluorescence microscopy of U-937 and U-A10 cells after 1 hour of exposure to 500 ng/mL of daunorubicin showed marked differences in the subcellular distribution of drug between the two cell lines (Fig 1). In U-937 cells, drug-associated nuclear fluorescence predominated, although a faint, punctate cytoplasmic accumulation of daunorubicin was observed (Fig 1A and B). By contrast, in U-A10 cells, daunorubicin was sequestered into expanded cytoplasmic vesicles, generally located to one side of the nucleus (Fig 1C and D). U-A10 cells have markedly less drug-associated nuclear fluorescence than the parental cell line.
To examine for the presence of acidic vesicles and to characterize further the site of vesicular anthracycline accumulation, the pH-sensitive vital stain, Lysosensor yellow/blue DND-160, was used. This indicator fluoresces yellow in highly acidic organelles. After 2 hours of accumulation of dye, a distinct punctate pattern of yellow fluorescence consistent with vesicular dye accumulation was observed in parental U-937 cells (Fig 1E and F ). The vesicles were distributed uniformly throughout the cell. By contrast, U-A10 cells were observed to accumulate dye into large expanded clustered vesicles that were ecentrically placed in the cells (Fig 1G and H). The intense yellow fluorescence confirmed the acidic nature of these organelles. With the exception of a lack of nuclear staining, the pattern of dye accumulation in U-937 and U-A10 cells was essentially identical to that observed with daunorubicin.
The current studies as well as previous data have suggested that the anthracyclines accumulate into lysosomes.20,26 To define further this compartment, the expression of the lysosomal-associated membrane proteins, LAMP-1 and LAMP-2, was examined by immunofluorescence.18 In parental U-937 cells, staining for LAMP-1 shows the protein to be expressed in a fine, punctate pattern throughout the cytoplasm of fixed, permeabilized cells (Fig 2A and B). However, staining of U-A10 cells showed enlarged ring-like structures that were tightly clustered in the perinuclear area (Fig 2C and D). The staining for LAMP-1 closely resembled the vesicular compartment that accumulates anthracyclines or the LysoSensor dye in these cells. When U-A10 cells were grown in the presence of doxorubicin (10 ng/mL for 30 days), there was no appreciable difference in the staining for LAMP-1 as assessed by immunofluorescence (data not shown). Staining for LAMP-2 appeared identical to that for LAMP-1 in both cell lines (data not shown).
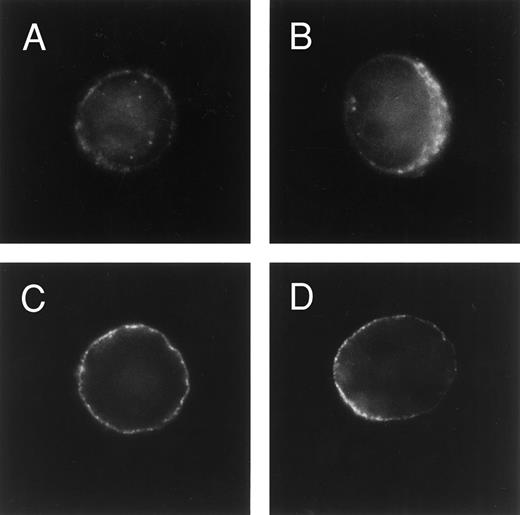
To examine the contribution of MRP and the P-glycoprotein to vesicular anthracycline accumulation in the drug-resistant cells, the cells were fixed, permeabilized, stained for MRP or P-glycoprotein, and observed by fluorescence microscopy. Parental U-937 cells showed extremely faint MRP staining (data not shown). Expression of the P-glycoprotein could not be detected in parental cells by this method. When examined for MRP staining with either polyclonal antisera 6KQ or MoAbs QCRL-1 and QCRL-3, MRP expression was observed primarily along the perimeter of the cell (Fig 3A and B). Likewise, the P-glycoprotein was observed to be expressed primarily in the cytoplasmic membrane (Fig 3C and D). Taken together, these studies show that U-A10 cells express MRP and the P-glycoprotein largely independently of the lysosomal compartment in a pattern consistent with a plasma membrane location.
Immunofluorescence analysis of MRP and P-glycoprotein expression. U-A10 cells were fixed/permeabilized in ethanol, blocked with goat serum, incubated with polyclonal antibody MRP-6KQ (A and B) or MoAb C219 (C and D), and then exposed to an FITC-conjugated second step. Cells were examined by fluorescence microscopy and photographed. Shown are photographs of typical single cells that are centered within the frame.
Immunofluorescence analysis of MRP and P-glycoprotein expression. U-A10 cells were fixed/permeabilized in ethanol, blocked with goat serum, incubated with polyclonal antibody MRP-6KQ (A and B) or MoAb C219 (C and D), and then exposed to an FITC-conjugated second step. Cells were examined by fluorescence microscopy and photographed. Shown are photographs of typical single cells that are centered within the frame.
To assess more thoroughly how the expanded lysosomal compartment observed in U-A10 cells affected subcellular daunorubicin distribution, anthracycline accumulation was quantified in various compartments using 3[H]-daunorubicin. To measure drug in the nucleus, cells were exposed to 3[H]-daunorubicin (0 to 500 ng/mL) for 1 hour, the nuclei were then isolated, and nuclear-associated radioactivity was determined. The results demonstrate that nuclei from U-A10 cells, as compared with U-937 cells, contain 2.5-fold to threefold less daunorubicin at each extracellular daunorubicin concentration (Fig 4A). The observed differences were statistically significant (eg, at 500 ng/mL, P < .005).
Assessment of nuclear daunorubicin accumulation after exposure of cells or isolated nuclei to 3[H]-daunorubicin. (A) (⊡) U-937 and (♦) U-A10 cells were exposed to 0 to 500 ng/mL of 3[H]-daunorubicin for 1 hour, and the cells were washed, ruptured, and nuclei purified by pelleting through a sucrose cushion. All isolation steps were performed at 4°C to minimize nuclear drug loss. Nuclear-associated radioactivity was determined by scintillation counting. Shown is a plot of the mean (± the standard error) nuclear daunorubicin accumulation versus extracellular drug concentration for three experiments each performed in duplicate. The differences were statistically significant at each concentration tested (P < .05 for 50 ng/mL; P < .005 for 200 and 500 ng/mL). (B) Nuclei were first isolated by lysing cells in a hypotonic, NP-40–containing buffer and pelleting by centrifugation. They were then exposed to 500 ng/mL of daunorubicin for 15 minutes before sedimenting through silicone oil and radioactivity determined by scintillation counting. Shown are the mean (± the standard error) of three experiments performed in duplicate.
Assessment of nuclear daunorubicin accumulation after exposure of cells or isolated nuclei to 3[H]-daunorubicin. (A) (⊡) U-937 and (♦) U-A10 cells were exposed to 0 to 500 ng/mL of 3[H]-daunorubicin for 1 hour, and the cells were washed, ruptured, and nuclei purified by pelleting through a sucrose cushion. All isolation steps were performed at 4°C to minimize nuclear drug loss. Nuclear-associated radioactivity was determined by scintillation counting. Shown is a plot of the mean (± the standard error) nuclear daunorubicin accumulation versus extracellular drug concentration for three experiments each performed in duplicate. The differences were statistically significant at each concentration tested (P < .05 for 50 ng/mL; P < .005 for 200 and 500 ng/mL). (B) Nuclei were first isolated by lysing cells in a hypotonic, NP-40–containing buffer and pelleting by centrifugation. They were then exposed to 500 ng/mL of daunorubicin for 15 minutes before sedimenting through silicone oil and radioactivity determined by scintillation counting. Shown are the mean (± the standard error) of three experiments performed in duplicate.
To define further the differences in nuclear drug accumulation, nuclei were first isolated from cells and then exposed to drug for 15 minutes. Over this time, essentially no difference could be detected in the net nuclear accumulation of daunorubicin (Fig 4B). Longer periods of drug exposure were not possible due to nuclear degeneration at 37°C. These results suggested that the diminished nuclear drug accumulation observed when whole cells were incubated in daunorubicin was due to extranuclear factors, specifically cytoplasmic sequestration.
Because net daunorubicin accumulation is essentially equal in U-937 and U-A10 cells,12 but net nuclear accumulation in U-A10 cells is about twofold to threefold less than in U-937 cells, then drug accumulation into the cytoplasmic compartment must be greater in U-A10 cells. To measure cytoplasmic drug accumulation, enucleated cells or cytoplasts were prepared. Controls for these experiments included fluorescence microscopy of cytoplasts exposed to 500 ng/mL of daunorubicin (Fig 5). In cytoplasts from parental U-937 cells, fine punctate granules were seen to accumulate daunorubicin (Fig 5A and B), whereas in cytoplasts from U-A10 cells large expanded vesicles were observed to accumulate drug (Fig 5C and D). The finding that accumulation of tritiated water is nearly identical in cytoplasts from U-937 and U-A10 cells supports the presence of similar aqueous volumes (data not shown). Incubation of U-937 or U-A10 cells with cytochalasin B for 1 hour (10 μg/mL) did not affect daunorubicin accumulation in intact cells (<5% difference), suggesting that this agent did not affect anthracycline accumulation in these cell lines.
Daunorubicin distribution in cytoplasts prepared from U-937 and U-A10 cells. Cytoplasts (enucleated cells) were prepared from U-937 cells (A and B) and from U-A10 cells (C and D) by treating cells with cytochalasin B and then pelleting through a discontinuous Ficoll gradient. Cytoplasts were exposed to daunorubicin at 500 ng/mL for 1 hour before being washed, visualized by fluorescence microscopy, and photographed. Shown are photographs of typical single cytoplasts with the images centered within the frame.
Daunorubicin distribution in cytoplasts prepared from U-937 and U-A10 cells. Cytoplasts (enucleated cells) were prepared from U-937 cells (A and B) and from U-A10 cells (C and D) by treating cells with cytochalasin B and then pelleting through a discontinuous Ficoll gradient. Cytoplasts were exposed to daunorubicin at 500 ng/mL for 1 hour before being washed, visualized by fluorescence microscopy, and photographed. Shown are photographs of typical single cytoplasts with the images centered within the frame.
Exposure of cytoplasts from U-937 cells to increasing concentrations of 3[H]-daunorubicin for 1 hour resulted in a nonlinear drug accumulation into cytoplasts (Fig 6). By contrast, cytoplasts from U-A10 cells accumulated 3[H]-daunorubicin in a linear fashion over a broad concentration range (0 to 750 ng/mL). At extracellular drug concentrations greater than 100 ng/mL, cytoplasts from U-A10 cells accumulated more daunorubicin than cytoplasts from parental cells. With exposure to higher daunorubicin concentrations, the differences in net drug accumulation became more pronounced and statistically significant (at 500 ng/mL, P < .005; Fig 6).
Accumulation of 3[H]-daunorubicin into cytoplasts prepared from (⊡) U-A10 and (♦) U-937 cells. Cytoplasts were exposed to 0 to 750 ng/mL of 3[H]-daunorubicin for 1 hour before terminating drug accumulation by pelleting through silicone oil. Shown is a plot of the mean (± the standard error) daunorubicin accumulation into cytoplasts versus external drug concentration for three experiments each performed in duplicate. The differences were statistically significant at 500 (P < .005) and 750 ng/mL (P < .01) of daunorubicin.
Accumulation of 3[H]-daunorubicin into cytoplasts prepared from (⊡) U-A10 and (♦) U-937 cells. Cytoplasts were exposed to 0 to 750 ng/mL of 3[H]-daunorubicin for 1 hour before terminating drug accumulation by pelleting through silicone oil. Shown is a plot of the mean (± the standard error) daunorubicin accumulation into cytoplasts versus external drug concentration for three experiments each performed in duplicate. The differences were statistically significant at 500 (P < .005) and 750 ng/mL (P < .01) of daunorubicin.
The finding of increased daunorubicin accumulation into a functionally expanded vesicular or lysosomal compartment suggested that U-A10 cells may preferentially accumulate alkaline lysosomotropic agents, such as chloroquine. Cells were exposed to various chloroquine concentrations for 1 hour, the cells were lysed, the chloroquine was extracted, and the amount was quantified by HPLC analysis. U-A10 cells accumulated 1.5-fold to twofold more chloroquine compared with U-937 cells at extracellular chloroquine concentrations of 10 and 50 μmol/L (P < .005 and P < .05, respectively; Fig 7). The finding of increased chloroquine accumulation suggested that U-A10 cells may demonstrate enhanced sensitivity to this agent as compared with parental U-937 cells. Cells were exposed to increasing amounts of this agent for 96 hours and the concentration that produced 50% decreased cell survival was determined in an MTT assay (Table 1). These studies showed that U-A10 cells are approximately twofold more sensitive to chloroquine than the U-937 cells.
Cellular accumulation of chloroquine. U-937 and U-A10 cells were exposed to chloroquine (0 to 50 μmol/L) for 2 hours before cells were washed and lysed and chloroquine was extracted. The amount of chloroquine was measured by HPLC analysis using 8-hydroxyquinoline as an internal standard. Shown is the mean (± the standard error) net cellular chloroquine accumulation when cells were exposed to 10 or 50 μmol/L chloroquine. The differences between U-937 and U-A10 cells were statistically significant at (▨) 10 (P < .005) or (▨) 50 μmol/L (P < .05) of chloroquine.
Cellular accumulation of chloroquine. U-937 and U-A10 cells were exposed to chloroquine (0 to 50 μmol/L) for 2 hours before cells were washed and lysed and chloroquine was extracted. The amount of chloroquine was measured by HPLC analysis using 8-hydroxyquinoline as an internal standard. Shown is the mean (± the standard error) net cellular chloroquine accumulation when cells were exposed to 10 or 50 μmol/L chloroquine. The differences between U-937 and U-A10 cells were statistically significant at (▨) 10 (P < .005) or (▨) 50 μmol/L (P < .05) of chloroquine.
Effect of Chloroquine on Anthracycline Sensitivity in U-937 and U-A10 Cells
| Drug . | U-937 (IC50) . | U-A10 (IC50) . | Fold Resistance . |
|---|---|---|---|
| Chloroquine (μmol/L) | 56.5 ± 8.2 | 23.2 ± 4.6 | 0.41 |
| Daunorubicin (ng/mL) | 8.4 ± 0.5 | 293 ± 23 | 35 |
| Daunorubicin/chloroquine | 9.8 ± 0.6 | 11.9 ± 2.4 | 1.2 |
| Doxorubicin (ng/mL) | 9.5 ± 0.8 | 683 ± 89 | 72 |
| Doxorubicin/chloroquine | 11.1 ± 2.0 | 66.7 ± 2.9 | 6.0 |
| Drug . | U-937 (IC50) . | U-A10 (IC50) . | Fold Resistance . |
|---|---|---|---|
| Chloroquine (μmol/L) | 56.5 ± 8.2 | 23.2 ± 4.6 | 0.41 |
| Daunorubicin (ng/mL) | 8.4 ± 0.5 | 293 ± 23 | 35 |
| Daunorubicin/chloroquine | 9.8 ± 0.6 | 11.9 ± 2.4 | 1.2 |
| Doxorubicin (ng/mL) | 9.5 ± 0.8 | 683 ± 89 | 72 |
| Doxorubicin/chloroquine | 11.1 ± 2.0 | 66.7 ± 2.9 | 6.0 |
The sensitivity of the parental U-937 and the doxorubicin-selected U-A10 cell lines to chloroquine, daunorubicin, and doxorubicin was determined in a standard MTT assay after 96 hours of drug exposure. Shown is the average (±the standard mean) IC50 value from three experiments each performed in quadruplicate. The effect of chloroquine on anthracycline cytotoxicity was determined by adding the lysosomotropic agent to a final concentration of 10 μmol/L just before adding the anthracycline. The fold resistance is calculated from the ratio of the IC50 of U-A10 cells/the IC50 of U-937 cells.
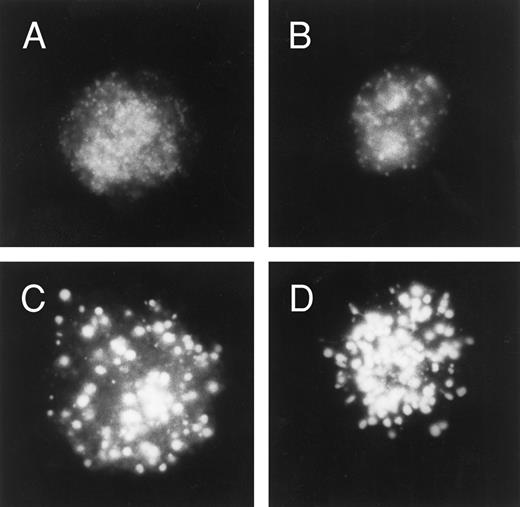
The ability of chloroquine to modulate resistance to daunorubicin and to doxorubicin in parental and U-A10 cells was also examined. U-A10 cells were exposed to chloroquine for 2 hours before 1 hour of treatment with daunorubicin. Chloroquine at 10 μmol/L caused enlargement of daunorubicin-containing vesicles and greater anthracyclineassociated nuclear fluorescence (Fig 8A and B). Higher chloroquine concentrations (100 μmol/L) also resulted in enlargement of vesicles, however, with diminution of daunorubicin-associated fluorescence in the vesicles (Fig 8C and D). There was also an accompanying greater anthracycline-associated nuclear fluorescence. That chloroquine was affecting lysosomal pH was demonstrated by the elimination of yellow fluorescence associated with Lysosensor yellow/blue when cells were treated with 10 μmol/L chloroquine (data not shown). When used at a noncytotoxic concentration of 10 μmol/L, chloroquine did not increase the cytotoxicity of either daunorubicin or doxorubicin in U-937 cells as determined by the MTT assay (Table 1). However, the same concentration of chloroquine markedly sensitized U-A10 cells to daunorubicin and to doxorubicin. For example, sensitivity to daunorubicin, measured as 35-fold resistant, was restored to nearly the same level as in parental cells (Table 1).
Effect of chloroquine on cellular daunorubicin distribution. U-A10 cells were exposed to chloroquine (0 to 100 μmol/L) for 2 hours before the addition of daunorubicin to a final concentration of 500 ng/mL for 1 hour. The cells were then washed, visualized by fluorescence microscopy, and photographed. Shown are photographs of typical single cells (with images centered within the frame) after treatment with 10 μmol/L chloroquine (A and B) or 100 μmol/L chloroquine (C and D) before daunorubicin exposure.
Effect of chloroquine on cellular daunorubicin distribution. U-A10 cells were exposed to chloroquine (0 to 100 μmol/L) for 2 hours before the addition of daunorubicin to a final concentration of 500 ng/mL for 1 hour. The cells were then washed, visualized by fluorescence microscopy, and photographed. Shown are photographs of typical single cells (with images centered within the frame) after treatment with 10 μmol/L chloroquine (A and B) or 100 μmol/L chloroquine (C and D) before daunorubicin exposure.
DISCUSSION
The studies presented here provide clear evidence that the lysosomal compartment actively participates in the acquired drug-resistance phenotype associated with the doxorubicin-selected U-A10 cell line. Fluorescence microscopy studies have shown that the U-A10 cell line exhibits an enhanced vesicular accumulation of anthracyclines and diminished nuclear drug uptake (Fig 1). Although similar observations have been reported for other drug-selected cell lines,7-13 the identification of this compartment has remained unclear. Examination for the expression of the LAMP-1 and LAMP-2 antigens showed that these vesicles were part of the lysosomal compartment (Fig 2). LAMPs are transmembrane proteins whose expression is restricted primarily to lysosomes.27 Dramatic differences in the staining of this protein between parental U-937 and doxorubicin-selected cell lines were observed. Immunofluorescence showed that both LAMP-1 and LAMP-2 stained enlarged vesicles that were present in a perinuclear distribution in U-A10 cells. Parental U-937 cells exhibited a pattern of staining distinct from that observed in U-A10 cells. The vesicular compartment observed in the drug-resistant cell lines is continuously present because there was no difference in the general staining pattern for LAMP-1 expression in the presence or absence of doxorubicin. Because staining for LAMP-1 requires cell permeabilization and fixation, colocalization studies with antibody and drug were not possible.
Drug redistribution into individual cellular compartments using radiolabeled daunorubicin was assessed to understand further the contribution of the lysosomal compartment to the resistance phenotype. Redistribution of anthracyclines was shown to result from a process present primarily in the cytoplasmic, not the nuclear, compartment. When the cells were exposed to daunorubicin and the nuclei isolated, the nuclei from U-A10 cells consistently accumulated significantly less daunorubicin than nuclei from U-937 cells when assayed over a broad concentration range of extracellular daunorubicin (Fig 4A). Other studies in cells with vesicular anthracycline sequestration have suggested that reduced nuclear drug accumulation may result in part from outward drug transport from the nuclear compartment.10 We examined daunorubicin accumulation into freshly isolated nuclei and found little difference in net drug accumulation (Fig 4B). These results suggest that in U-A10 cells the diminished net nuclear daunorubicin accumulation observed in whole cells was due to cytoplasmic sequestration.
To quantify accumulation into the cytoplasmic vesicular compartment, we prepared cytoplasts from parental and drug-resistant cell lines (Figs 5 and 6). Cytoplasts from U-937 cells accumulate daunorubicin in a nonlinear manner; as the cytoplasts are exposed to higher extracellular daunorubicin concentrations, they accumulated proportionally less drug. A similar phenomenon was demonstrated with cytoplasts prepared from the human leukemia cell line K562; when exposed to increasing daunorubicin concentrations, drug was shown to accumulate in a saturable fashion into an ionophore-sensitive compartment.20 The cytoplasmic vesicular compartment in either of these myeloid leukemia cells appears to have a limited capacity for daunorubicin accumulation and can, in effect, become saturated. In the absence of a nucleus, the capacity for anthracycline accumulation into the cytoplast diminishes once the vesicular compartment has become loaded with daunorubicin. In marked contrast, the capacity for daunorubicin accumulation into the cytoplasmic compartment from U-A10 cells is greatly increased and cannot be saturated at external concentrations of up to 1,000 ng/mL. Cytoplasts from U-A10 cells accumulate daunorubicin in a linear manner over a broad concentration range (Fig 6). Because the cytotoxic target of the anthracyclines is topoisomerase II, which resides in the nucleus, this enhanced cytoplasmic drug accumulation may directly affect the sensitivity of the cells to the anthracyclines. In parental cells, saturation of the vesicular compartment at pharmacologic drug concentrations permits nuclear drug accumulation with resultant cytotoxicity. In resistant cells, the expanded vesicular compartment is not saturable at pharmacologically relevant drug concentrations (<1,000 ng/mL).
The mechanism by which the anthracyclines traverse the lysosomal membrane is unknown; however, substantial data suggest that these agents cross the plasma membrane by passive diffusion.28-30 Although active or facilitated transport into the lysosome cannot be excluded, our studies, which did not detect appreciable amounts of MRP or P-glycoprotein on the vesicles, suggest these transporters may not be involved in an inward vesicular drug transport in U-A10 cells. Once inside the acidic lysosomal environment, the drug will become protonated. The amino group present on the glycone moiety of daunorubicin has a pKa of ∼8.2; thus, at a pH < 5, more than 99% of the drug will be ionized.31 The lysosome will retain drug solely on the basis of differential permeability between the neutral and protonated forms. Indeed, when U-A10 cells have been observed 2 to 3 days after 1 hour of daunorubicin exposure, drug is readily observed to be retained only in vesicles, strongly suggesting that the anthracycline is not in free equilibrium with the cytoplasmic compartment (data not shown).
The presence of an enhanced acidic vesicular compartment that traps and retains anthracyclines in drug-resistant cells raised the possibility that this compartment could be targeted by lysosomotropic agents, such as chloroquine. Chloroquine has two amino groups with estimated pKa values of 8.1 and 9.9. It will preferentially accumulate into acidic vesicular compartments and raise the intralumenal pH above a critical value with resultant cellular cytotoxicity.25,32 In contrast to the anthracyclines, the cytotoxic target of chloroquine resides in the cytoplasm. Our studies indicated that U-A10 cells accumulate more chloroquine and exhibit enhanced cytotoxicity to this agent compared with parental cells (Fig 7 and Table 1). In addition, chloroquine effectively modulates daunorubicin and doxorubicin resistance in the U-A10 cell line. Although using drug-selected cell lines, chloroquine has been reported to be a P-glycoprotein–modulating agent; we do not believe that is its primary mode of action here.33-35 Previous studies showed that verapamil, another P-glycoprotein–modulating agent, did not affect daunorubicin vesicular accumulation in U-A10 cells.12 U-A10 cells express very low levels of P-glycoprotein, primarily in a plasma membrane distribution. Accordingly, U-A10 cells accumulate more chloroquine, not less, than parental U-937 cells. Moreover, fluorescence microscopy demonstrates that chloroquine directly affects the vesicular compartment that accumulates anthracyclines with an associated increase in daunorubicin nuclear fluorescence (Fig 8). Additionally, chloroquine obliterates the yellow fluorescence associated with Lysosensor yellow/blue (data not shown).
The molecular basis for this phenotype remains incompletely understood. Transfection studies of the P-glycoprotein and MRP have shown that these proteins are expressed at the plasma membrane and function as energy dependent efflux pumps.4,5,36-38 Although anthracycline distribution studies have reported conflicting results regarding the association of MRP expression and this phenotype,39,40 our studies suggested MRP and P-glycoprotein are expressed in U-A10 cells independently of the lysosomal compartment. LRP, the human homolog of the major rat vault protein, also has been shown to be overexpressed in drug-resistant cells that possess an anthracycline sequestration phenotype.41 However, transfection studies have shown that this protein does not confer drug resistance.42 Further studies of cell lines displaying this phenotype will likely result in a more thorough understanding of this acquired drug-resistance phenotype.
When examined together, these studies have shown that the U-A10 cell line contains constitutively expanded vesicles that are part of the lysosomal compartment. Staining for LAMP-1 or LAMP-2 permits this compartment to be identified by immunologic methods. Similar studies using clinical specimens will help determine the relevance of this phenotype in patients with hematologic malignancies who are refractory to treatment with the anthracyclines.
ACKNOWLEDGMENT
We are indebted to Dr Donald W. Kufe for his generous support.
Supported in part by Clinical Investigator Award CA-01613 from the National Cancer Institute (C.A.S.).
Address reprint requests to Christopher A. Slapak, MD, Lilly Research Laboratories, Lilly Corporate Center DC #2133, Indianapolis, IN 46285.

![Fig. 4. Assessment of nuclear daunorubicin accumulation after exposure of cells or isolated nuclei to 3[H]-daunorubicin. (A) (⊡) U-937 and (♦) U-A10 cells were exposed to 0 to 500 ng/mL of 3[H]-daunorubicin for 1 hour, and the cells were washed, ruptured, and nuclei purified by pelleting through a sucrose cushion. All isolation steps were performed at 4°C to minimize nuclear drug loss. Nuclear-associated radioactivity was determined by scintillation counting. Shown is a plot of the mean (± the standard error) nuclear daunorubicin accumulation versus extracellular drug concentration for three experiments each performed in duplicate. The differences were statistically significant at each concentration tested (P < .05 for 50 ng/mL; P < .005 for 200 and 500 ng/mL). (B) Nuclei were first isolated by lysing cells in a hypotonic, NP-40–containing buffer and pelleting by centrifugation. They were then exposed to 500 ng/mL of daunorubicin for 15 minutes before sedimenting through silicone oil and radioactivity determined by scintillation counting. Shown are the mean (± the standard error) of three experiments performed in duplicate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/10/10.1182_blood.v89.10.3745/3/m_bl_0051f4a.jpeg?Expires=1765916331&Signature=Wk4TuM4hJa1BqeGP2djBCTlmOlS-7ZNCGvziTBEfMfZXQnwwllcAklMfLpskiuOB2e9Bu7tzXDqw9Tmuk~0O1PDJ09DV8bCoBmWo9DPrJZer4tQ7TdVt-uzq9v~tBITo84pjj7E8S7Tgfjqa2dTImrCithnA~DR1Vo-SrX4WLjBNg4Yfo4A-EQBQKuoWpz7sk62a5izJk~dgWLJI6LLZfwx0jIe5~ZCpq-Fp-tbmDCu~MK89mgLliVypZJmsuCn0IQT3uAIYDq4jJa76bM9kUOURJ93UZLev85wY-vac2qbjr4clGqQHHqpJ1DfvkVgSysTdAN84lMIVcLn4fetwBQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Assessment of nuclear daunorubicin accumulation after exposure of cells or isolated nuclei to 3[H]-daunorubicin. (A) (⊡) U-937 and (♦) U-A10 cells were exposed to 0 to 500 ng/mL of 3[H]-daunorubicin for 1 hour, and the cells were washed, ruptured, and nuclei purified by pelleting through a sucrose cushion. All isolation steps were performed at 4°C to minimize nuclear drug loss. Nuclear-associated radioactivity was determined by scintillation counting. Shown is a plot of the mean (± the standard error) nuclear daunorubicin accumulation versus extracellular drug concentration for three experiments each performed in duplicate. The differences were statistically significant at each concentration tested (P < .05 for 50 ng/mL; P < .005 for 200 and 500 ng/mL). (B) Nuclei were first isolated by lysing cells in a hypotonic, NP-40–containing buffer and pelleting by centrifugation. They were then exposed to 500 ng/mL of daunorubicin for 15 minutes before sedimenting through silicone oil and radioactivity determined by scintillation counting. Shown are the mean (± the standard error) of three experiments performed in duplicate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/10/10.1182_blood.v89.10.3745/3/m_bl_0051f4b.jpeg?Expires=1765916331&Signature=s7I285CkWCil398ql9DoH5mdD3M7pq3Bt0Fo9Ol06Mr3gkezuzCr1BchKGy3KiVmUGGVCCUDgCm6OIBQhfBUSB~D1W9qVuRZykOTmpw38rrZqNb4iUvZHOc1R2LvUppU69nyfgN0Fn2cucNz3Sepaf4IkkBQPTbmja6eTvCGZ1wMZMlmK~xoXWWBNVYlYEv02HvR7cEUBaMsp-6JWbapwcpp-0mPzl5jowyJNx3bAczjiBGyugaaxo3dKprT~5DPVQcHkhiTQMgdxOSlw3AAedNhsxVaK1Ymo3uBJ457W4Ca16CY7IVw0YSCRf1r-h0Z1W5MqMzGUWCcRblRIguqxw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Accumulation of 3[H]-daunorubicin into cytoplasts prepared from (⊡) U-A10 and (♦) U-937 cells. Cytoplasts were exposed to 0 to 750 ng/mL of 3[H]-daunorubicin for 1 hour before terminating drug accumulation by pelleting through silicone oil. Shown is a plot of the mean (± the standard error) daunorubicin accumulation into cytoplasts versus external drug concentration for three experiments each performed in duplicate. The differences were statistically significant at 500 (P < .005) and 750 ng/mL (P < .01) of daunorubicin.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/10/10.1182_blood.v89.10.3745/3/m_bl_0051f6.jpeg?Expires=1765916331&Signature=Wvtd3dUEWYmD2dhKozuq03Zu9AkdxR7~dDgR9n3vzGsZau3HQTtlaLXQESfCW27~C-jyxsBf1Lzeu5a-65mutLqp1cOrXny3nssFoyghmO-uRRp~DMbZ-JTZ9sa~9~CGgebc1aP3M9lETEBonUqPS1r8nXDiB30Jd3cOwxX1vyIv4sJcvoy~JNeEeLCBSNJ-rLW4k6audvMJJtqLcS6aDi6F4rEyxqAZY6pPs1mkNXR900xvSeopiFSfEfUcEMcbEJ8AXV63BBl9cy-eoOXJj3uLnboerNckKto8jyq8jCWL81RFHLGrOcCuxIFzbyhTyiC4b-Ln0fhqIgC26BuFEg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal