Abstract
Acquired immunodeficiency syndrome (AIDS)-related non-Hodgkin's lymphomas (AIDS-NHL), a major source of morbidity and mortality among human immunodeficiency virus (HIV)-infected individuals, are derived from B cells and are classified into two major categories, Burkitt's lymphoma (BL) and diffuse large cell lymphoma (DLCL). Anaplastic large cell lymphoma (ALCL) and body-cavity-based lymphoma (BCBL) represent less frequent AIDS-NHL types. The molecular pathogenesis of AIDS-NHL is characterized by distinct genetic pathways, including chromosomal rearrangements of c-MYC and BCL-6 in AIDS-BL and AIDS-DLCL, respectively. In addition to gross rearrangements, recent evidence has suggested that BCL-6 may also be affected by mutations of the gene 5′ noncoding regions. Here we have investigated the distribution of BCL-6 mutations in a panel representative of all the AIDS-NHL subtypes. Forty-three AIDS-NHL were analyzed for mutations in the first exon-first intron boundary region of BCL-6. Mutations were detected in all categories of AIDS-NHL (25 of 43 cases; 58%), including 12 of 20 AIDS-BL, 10 of 15 AIDS-DLCL, two of three AIDS-ALCL, and one of five of AIDS-BCBL. BCL-6 mutations occurred independent of BCL-6 rearrangements and presence of other genetic lesions frequently associated with AIDS-NHL. These results indicate that mutations of BCL-6 5′ noncoding regions represent the most common genetic alteration presently detectable in AIDS-NHL. The frequency of these mutations, as well as their location in the proximity of BCL-6 regulatory sequences, suggest that they may play a role in AIDS-related lymphomagenesis.
ACQUIRED IMMUNODEFICIENCY syndrome-related non-Hodgkin's lymphomas (AIDS-NHL) represent a major complication of human immunodeficiency virus (HIV) infection and constitute the most frequent AIDS-related neoplasm in some HIV risk groups.1-3 AIDS-NHL consistently derive from B cells and are characterized by the potential for clinical aggression.1,2 Histology of systemic AIDS-NHL is heterogeneous and includes two major categories, namely AIDS-related Burkitt's lymphoma (AIDS-BL) and AIDS-related diffuse large cell lymphoma (AIDS-DLCL).1 2 More rarely, AIDS-NHL may present as AIDS-related anaplastic large cell lymphoma (AIDS-ALCL) or AIDS-related body-cavity–based lymphoma (AIDS-BCBL).4,5
Various studies on the molecular pathogenesis of AIDS-NHL have shown that distinct clinicopathologic variants of AIDS-NHL are associated with different patterns of genetic lesions.6,7 In particular, AIDS-BL is characterized by the consistent activation of c-MYC and the frequent disruption of p53, whereas infection by Epstein-Barr virus (EBV) is restricted to a subset of cases.6-8 AIDS-DLCL are more heterogeneous and are commonly associated with EBV infection and with rearrangements of either c-MYC or BCL-6 in a fraction of samples.6-9 Finally, AIDS-BCBL are consistently associated with the co-infection by human herpesvirus type-8 and EBV.5 10 The genetic features of AIDS-ALCL have not been studied extensively.
The BCL-6 gene was originally cloned from 3q27 breakpoints of NHL of the immunocompetent host.11-15 It encodes for a transcription factor belonging to the family of zinc-finger proteins and is normally expressed in B cells within germinal centers, but not in immature B-cell precursors or in differentiated plasma cells.16 17 The tight regulation of BCL-6 expression throughout B-cell differentiation suggests that the gene is involved in the control of lymphoid organ development. Rearrangements of BCL-6 cluster within a 4-kb region spanning the BCL-6 promoter sequences and the first noncoding exon and associate with 40% of DLCL and 10% of follicular lymphomas (FL) among NHL of the immunocompetent host.11-15,18 Among AIDS-NHL, BCL-6 rearrangements are restricted to 20% of AIDS-DLCL, but are absent in other AIDS-NHL categories.9
Recent studies of NHL of the immunocompetent host have suggested that the BCL-6 gene may be altered by mechanisms other than chromosomal rearrangements, namely somatic mutations clustering within the 5′ noncoding regions of the gene.19 These mutations are often multiple in the same tumor sample, are of somatic origin, are frequently biallelic, and are found in cases displaying either normal or rearranged BCL-6 alleles indicating their independence of chromosomal rearrangements or linkage to immunoglobulin genes.19 The sequences affected by these mutations are adjacent to the BCL-6 promoter region and overlap with the major cluster of chromosomal breakpoints. At present, evidence for the occurrence of mutations of BCL-6 5′ noncoding regions is confined to the case of DLCL and FL of the immunocompetent host, but are consistently negative in several types of nonhematopoietic tumors.19 The characteristics of the mutations in NHL of the immunocompetent host, including frequency, tumor specificity, and location in the proximity of BCL-6 regulatory regions, suggest that these genetic alterations might play a role in lymphomagenesis.
This study was aimed at investigating the presence of mutations of the 5′ noncoding regions of BCL-6 in AIDS-NHL. We report that mutations of BCL-6 occur at high frequency throughout the clinicopathologic spectrum of AIDS-NHL and represent the most common genetic alteration presently detectable in these tumors.
MATERIALS AND METHODS
Tumor biopsies, cell lines, and DNA extraction.Thirty-three AIDS-NHL primary samples and 10 AIDS-NHL cell lines were included in this study. Patients from whom primary samples were derived had been referred to the Centro di Riferimento Oncologico, Aviano, Italy. Primary samples of AIDS-NHL were derived from lymph node, bone marrow, peripheral blood, body cavity effusion, or other involved organs and were obtained in the course of routine diagnostic procedures. In all instances, the specimen was collected at diagnosis before specific therapy. Diagnosis were based on hystopathological and immunophenotypic analysis of cell surface markers and immunogenotypic analysis of antigen receptor gene rearrangement.7,20 The fraction of malignant cells in the pathologic specimen was ≥ 60%, and in most cases ≥ 75%, as determined by morphologic, immunophenotypic and immunogenotypic studies. Primary samples were representative of the distinct clinicopathologic categories of AIDS-NHL and included AIDS-BL (n = 11), AIDS-DLCL (n = 15), AIDS-ALCL (n = 3), and AIDS-BCBL (n = 4). All AIDS-NHL cell lines investigated in this study have been previously characterized in detail.21-27 Nine cell lines were representative of AIDS-BL (HBL-1, HBL-2, HBL-3, EsIII, As283A, PA682, LAM-C3+, BRG-M, BRG-A) and one cell line was representative of AIDS-BCBL (HBL-6). Seven B-cell lymphoblastoid cell lines were also included in the study as negative controls.19 Genomic DNA was purified by cell lysis followed by digestion with proteinase K, “salting out” extraction, and precipitation by ethanol.28
Oligonucleotides.All the oligonucleotides used in this study were synthesized by the solid phase triester method. The sequences of oligonucleotides corresponding to BCL-6 exon 1 (fragments E1.7 and E1.8) and exon 1-intron 1 boundary region (fragments E1.10, E1.11, and E1.12) were19: E1.15, 5′-GGAAAGCAAAGCGCACTC-3′, and E1.16, 5′-CCAAAAGCCCTCAAAGCC-3′ (for fragment E1.7); E1.17, 5′-ACGCTCTGCTTATGAGGA-3′, and E1.18, 5′-CGGCAGCAACAGCAATAA-3′ (for fragment E1.8); E1.21B, 5′-CTCTTGCCAAATGCTTTG-3′, and E1.24, 5′-TAATTCCCCTCCTTCCTC-3′ (for fragment E1.10); E1.23, 5′-AGGAAGGAGGGGAATTAG-3′, and IP1.6, 5′-AAGCAGTTTGCAAGCGAG-3′ (for fragment E1.11); IP1.7, 5′-TTCTCGCTTGCAAACTGC-3′, and E1.26, 5′-CACGATACTTCATCTCATC-3′ (for fragment E1.12). The oligonucleotides used as primers for the mutational analysis of p53 exons 5 through 9 and c-MYC first exon-first intron boundary region have been reported previously.8 The oligonucleotides used as primers for the analysis of EBV DNA sequences have also been described.29
Polymerase chain reaction-single strand conformation polymorphism (PCR-SSCP).PCR-SSCP was performed as previously reported.8 Briefly, 100 ng of genomic DNA, 10 pmol of each primer, 2.5 μmol/L dNTPs, 1 μCi of [α-32 P]dCTP (Amersham, Amersham, UK; specific activity, 3,000 Ci/mmol; 1 Ci = 37 GBq), 10 mmol/L Tris-HCl (pH 8.8), 50 mmol/L KCL, 1 mmol/L MgCl2 , 0.01% gelatin, 0.5 U AmpliTaq polymerase (Perkin-Elmer, Norwalk, CT) were mixed in a final volume of 10 μL. Thirty cycles of denaturation (94°C), annealing (annealing temperatures were optimized for each pair of primers), and extension (72°C) were performed in a temperature controller (DNA Thermal Cycler; Perkin-Elmer). Samples were heated at 95°C for 5 minutes, chilled on ice, and immediately loaded (3 μL) onto a 6% acrylamide-Tris-borate-EDTA (TBE) gel containing 10% glycerol. Gels were run at 8 W for 12 to 15 hours at room temperature, fixed in 10% acetic acid, air dried, and analyzed by autoradiography using an intensifying screen for 6 to 24 hours. By using the conditions described above, reconstruction experiments have shown that the sensitivity of the PCR-SSCP method allows the detection of mutations present in 10% of the cell populations tested (sensitivity limit, 10%). In selected experiments, the PCR conditions of the PCR-SSCP technique were altered to decrease the sensitivity of the method. In such modified PCR-SSCP method, the PCR mixture contained 25 μmol/L (instead of 2.5 μmol/L) dNTPs and PCR cycles were reduced to 25 (instead of 30). The sensitivity limit of this modified PCR-SSCP is 30%, as assessed by reconstruction experiments.
DNA sequencing procedures.For DNA sequencing of BCL-6 5′ noncoding regions, a unique PCR product encompassing fragments E1.10, E1.11, and E1.12 (nucleotides +404 to +1142) and amplified by primers E1.21B and E1.26, was subcloned into the pGEM-T vector (Promega, Madison, WI). For each case of AIDS-NHL subjected to DNA sequencing, the DNA obtained from at least 20 different subclones was mixed in equal proportions to ensure a clonal representation similar to that of the genomic DNA derived from primary samples or cell lines. Subsequently, the DNA mixture was analyzed by DNA sequencing using forward and reverse primers, including universal primers SP6 and T7, as well as the BCL-6–specific primers listed above. DNA sequencing procedures were performed as recommended by the manufacturer (Sequenase version 2, USB/Amersham, UK). DNA sequencing analysis of p53 and c-MYC was performed as previously reported.8
Southern blot analysis and DNA probes.For Southern blot analysis,30 6 to 10 μg of genomic DNA were digested with the appropriate restriction endonuclease, electrophoresed in a 0.8% agarose gel, denatured, neutralized, transferred to Hybond C+ filters, and hybridized to probes that had been 32P-labeled by the random priming extension method.31 Filters were washed in 0.2× SSC (NaCl/Na citrate)/0.5% sodium dodecyl sulfate (SDS) for 2 hours at 60°C and then autoradiographed using intensifying screens (Quanta III; Dupont-NEN, Boston, MA). The organization of the BCL-6 locus was analyzed by hybridization of BamHI and Xba I digested DNA to the human BCL-6 probes Sac4.0 and Sac0.8, which detect the cluster of BCL-6 rearrangements of NHL.9,11,12 The organization of the c-MYC locus was analyzed by hybridization of EcoRI and HindIII digested DNA to the human c-MYC probe MC413RC, representative of the third exon of the c-MYC gene.32 The presence of the EBV genome was investigated with a probe specific for the EBV genomic termini (5.2-kb BamHI-EcoRI fragment isolated from the fused BamHI terminal fragment NJ-het).33
In situ hybridization studies.In situ hybridization studies of the EBV encoded EBER transcripts were performed as previously reported.4
RESULTS
A panel of 43 cases of AIDS-NHL were included in this study. Cases were representative of the different clinicopathologic categories of the disease, including 20 AIDS-BL (11 primary specimens and 9 cell lines), 15 AIDS-DLCL, 3 AIDS-ALCL, and 5 AIDS-BCBL (4 primary specimens and 1 cell line). All cases of AIDS-NHL displayed a major monoclonal B-cell population based on immunogenotypic analysis and/or light chain restriction immunohistochemical studies (data not shown).
Mutations in the 5′ noncoding sequences of BCL-6 in all AIDS-NHL subtypes.All 43 samples of AIDS-NHL were subjected to PCR-SSCP analysis of five partially overlapping PCR fragments encompassing the BCL-6 first noncoding exon (fragments E1.7 and E1.8) and a 740-bp region within BCL-6 intron 1 (fragments E1.10, E1.11, E1.12). The selection of these five PCR fragments for the mutational analysis of BCL-6 in AIDS-NHL was based on recent evidence derived from NHL of the immunocompetent host showing that these sequences are consistently mutated in all cases of NHL of the immunocompetent host carrying mutations of the BCL-6 gene.19
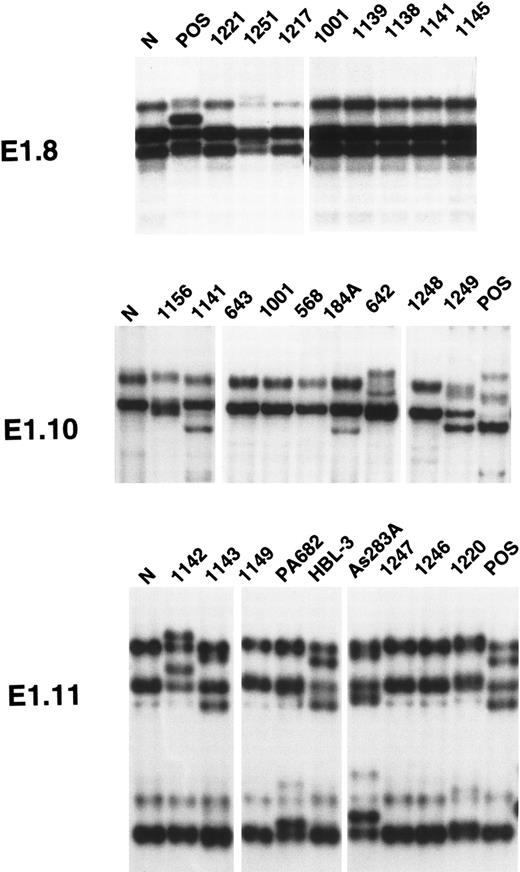
The AIDS-NHL panel tested in this study displayed a total of 45 PCR-SSCP variants, which were unique to individual tumor DNAs (see Fig 1 for representative results). Additional, recurrent PCR-SSCP variants were detected in fragments E1.11 and E1.12, consistent with the reported presence of population polymorphisms in this region of BCL-6.19 Cases of AIDS-NHL were scored positive for mutation when one or more PCR-SSCP fragments displayed a variant pattern, which could not be attributed to a population polymorphism. Mutations of 5′ noncoding regions of BCL-6 were detected in 25 of 43 (58.1%) AIDS-NHL samples and, in particular, in 12 of 20 (60.0%) AIDS-BL, 10 of 15 (66.6%) AIDS-DLCL, two of three (66.6%) AIDS-ALCL, and 1 of 5 (20.0%) AIDS-BCBL (Table 1). Among AIDS-BL, mutations occurred both in primary specimens (6 of 11; 54.5%) and in tumor cell lines (6 of 9; 66.6%). Among AIDS-BCBL, the only case scored positive was represented by a tumor cell line, whereas all primary specimens scored negative. All (n = 7) B-cell lymphoblastoid cell lines, which are known to be devoid of BCL-6 mutations,19 scored consistently negative in all experiments.
PCR-SSCP analysis of mutations of the 5′ noncoding regions of BCL-6 in AIDS-NHL. Representative results obtained for PCR products E1.8 (located within BCL-6 exon 1), E1.10, and E1.11 (both located within BCL-6 intron 1) are shown. Each AIDS-NHL sample is indicated at the top of each lane by a numbered code or, in the case of AIDS-NHL cell lines, by its conventional denomination. A positive control (POS), represented by a tumor sample known to harbor BCL-6 mutations,19 as well as a normal (N) sample, represented by a B-lymphoblastoid cell line, are also included for each PCR-SSCP fragment shown. Samples were scored positive when their migration pattern differed from the normal control (N) and their migration abnormalities could not be ascribed to population polymorphisms. Among the AIDS-NHL samples shown in the figure, cases scored as positive included cases 1251 (for PCR product E1.8), 1141, 184A, 642, 1249 (for PCR product E1.10), 1142, 1143, PA682, HBL-3, As283A and 1220 (for PCR product E1.11).
PCR-SSCP analysis of mutations of the 5′ noncoding regions of BCL-6 in AIDS-NHL. Representative results obtained for PCR products E1.8 (located within BCL-6 exon 1), E1.10, and E1.11 (both located within BCL-6 intron 1) are shown. Each AIDS-NHL sample is indicated at the top of each lane by a numbered code or, in the case of AIDS-NHL cell lines, by its conventional denomination. A positive control (POS), represented by a tumor sample known to harbor BCL-6 mutations,19 as well as a normal (N) sample, represented by a B-lymphoblastoid cell line, are also included for each PCR-SSCP fragment shown. Samples were scored positive when their migration pattern differed from the normal control (N) and their migration abnormalities could not be ascribed to population polymorphisms. Among the AIDS-NHL samples shown in the figure, cases scored as positive included cases 1251 (for PCR product E1.8), 1141, 184A, 642, 1249 (for PCR product E1.10), 1142, 1143, PA682, HBL-3, As283A and 1220 (for PCR product E1.11).
Distribution of Mutations in the 5′ Noncoding Regions and Gross Rearrangements of BCL-6 in Systemic AIDS-NHL
| Histology . | BCL-6 Mutations . | BCL-6 Rearrangements . |
|---|---|---|
| . | (positive/tested) . | (positive/tested) . |
| AIDS-BL | 12/20* | 0/16 |
| AIDS-DLCL | 10/15 | 3/12 |
| AIDS-ALCL | 2/3 | 0/3 |
| AIDS-BCBL | 1/5† | 0/1 |
| Histology . | BCL-6 Mutations . | BCL-6 Rearrangements . |
|---|---|---|
| . | (positive/tested) . | (positive/tested) . |
| AIDS-BL | 12/20* | 0/16 |
| AIDS-DLCL | 10/15 | 3/12 |
| AIDS-ALCL | 2/3 | 0/3 |
| AIDS-BCBL | 1/5† | 0/1 |
Abbreviations: AIDS-BL, AIDS-related Burkitt's lymphoma; AIDS-DLCL, AIDS-related diffuse large cell lymphoma; AIDS-ALCL, AIDS-related anaplastic large cell lymphoma; AIDS-BCBL, AIDS-related body-cavity-based lymphoma.
AIDS-BL cases harboring mutations of the 5′ non-coding regions of BCL-6 included 6 of 11 biopsy specimens and 6 of 9 tumor cell lines.
The case of AIDS-BCBL carrying mutations of the 5′ noncoding regions of BCL-6 is represented by a cell line.
Distribution of PCR-SSCP Variants Within the 5′ Noncoding Regions of BCL-6 in AIDS-NHL
| Sample . | Diagnosis . | R BCL-6* . | PCR-SSCP Variants† . | ||||
|---|---|---|---|---|---|---|---|
| . | . | . | E1.7 . | E1.8 . | E1.10 . | E1.11 . | E1.12 . |
| 642 | AIDS-BL | ND | • | • | |||
| 1142 | AIDS-BL | − | • | • | |||
| 1143 | AIDS-BL | − | • | • | |||
| 1220 | AIDS-BL | − | • | • | • | ||
| 1247 | AIDS-BL | − | • | ||||
| 1249 | AIDS-BL | − | • | • | |||
| HBL-2 | AIDS-BL | − | • | ||||
| HBL-3 | AIDS-BL | − | • | • | |||
| PA682 | AIDS-BL | − | • | • | |||
| As283A | AIDS-BL | − | • | ||||
| BRG-A | AIDS-BL | − | • | ||||
| LAM-C3+ | AIDS-BL | − | • | ||||
| 110 | AIDS-DLCL | + | • | • | • | ||
| 116 | AIDS-DLCL | + | • | • | • | ||
| 120 | AIDS-DLCL | + | • | • | |||
| 184A | AIDS-DLCL | ND | • | ||||
| 568 | AIDS-DLCL | − | • | ||||
| 674 | AIDS-DLCL | − | • | ||||
| 1139 | AIDS-DLCL | − | • | • | |||
| 1141 | AIDS-DLCL | − | • | • | • | ||
| 1145 | AIDS-DLCL | − | • | ||||
| 1156 | AIDS-DLCL | ND | • | • | • | ||
| 1221 | AIDS-ALCL | − | • | ||||
| 1251 | AIDS-ALCL | − | • | • | |||
| HBL-6 | AIDS-BCBL | − | • | • | |||
| Sample . | Diagnosis . | R BCL-6* . | PCR-SSCP Variants† . | ||||
|---|---|---|---|---|---|---|---|
| . | . | . | E1.7 . | E1.8 . | E1.10 . | E1.11 . | E1.12 . |
| 642 | AIDS-BL | ND | • | • | |||
| 1142 | AIDS-BL | − | • | • | |||
| 1143 | AIDS-BL | − | • | • | |||
| 1220 | AIDS-BL | − | • | • | • | ||
| 1247 | AIDS-BL | − | • | ||||
| 1249 | AIDS-BL | − | • | • | |||
| HBL-2 | AIDS-BL | − | • | ||||
| HBL-3 | AIDS-BL | − | • | • | |||
| PA682 | AIDS-BL | − | • | • | |||
| As283A | AIDS-BL | − | • | ||||
| BRG-A | AIDS-BL | − | • | ||||
| LAM-C3+ | AIDS-BL | − | • | ||||
| 110 | AIDS-DLCL | + | • | • | • | ||
| 116 | AIDS-DLCL | + | • | • | • | ||
| 120 | AIDS-DLCL | + | • | • | |||
| 184A | AIDS-DLCL | ND | • | ||||
| 568 | AIDS-DLCL | − | • | ||||
| 674 | AIDS-DLCL | − | • | ||||
| 1139 | AIDS-DLCL | − | • | • | |||
| 1141 | AIDS-DLCL | − | • | • | • | ||
| 1145 | AIDS-DLCL | − | • | ||||
| 1156 | AIDS-DLCL | ND | • | • | • | ||
| 1221 | AIDS-ALCL | − | • | ||||
| 1251 | AIDS-ALCL | − | • | • | |||
| HBL-6 | AIDS-BCBL | − | • | • | |||
Abbreviations: AIDS-BL, AIDS-related Burkitt's lymphoma; AIDS-DLCL, AIDS-related diffuse large cell lymphoma; AIDS-ALCL, AIDS-related anaplastic large cell lymphoma; AIDS-BCBL, AIDS-related body-cavity-based lymphoma; ND, not done.
Rearrangements (R) of BCL-6: −, negative; +, positive.
For each sample, PCR-SSCP variants scored positive are indicated by dots.
Table 2 summarizes the distribution of PCR-SSCP variants (with the exception of those attributed to population polymorphisms) in the AIDS-NHL analyzed. The majority of cases displayed ≥ 2 variant fragments. Of the 45 PCR-SSCP variants detected, 43 clustered within a 740-bp region located ≈100 bp downstream the first noncoding exon and corresponding to PCR fragments E1.10, E1.11, E1.12, whereas only two PCR-SSCP variants were detected in the BCL-6 first exon (fragments E1.7 and E1.8).
In selected cases (n = 9), mutational analysis was performed in parallel by the standard PCR-SSCP assay (sensitivity 10%) and by a modified PCR-SSCP assay (see Materials and Methods; sensitivity 30%). The results of the two assays were superimposable (not shown). These data suggest that BCL-6 mutations, when present, occur in > 30% of cells within the total cell population, although we cannot formally prove that mutations occurred in each single tumor cell present in the sample tested.
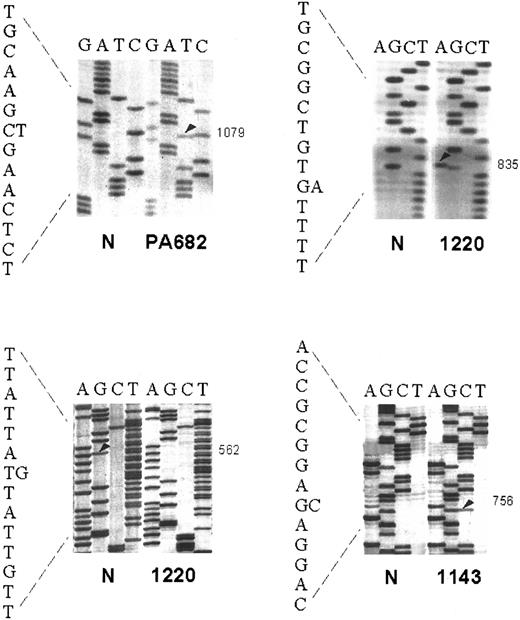
Sequence analysis of BCL-6 mutations.To confirm and characterize the mutations affecting the 5′ noncoding regions of the BCL-6 gene in AIDS-NHL, we performed a sequence analysis in 11 cases representative of the different clinicopathologic variants of the disease. In all cases tested, the sequence analysis involved all the fragments displaying an abnormally variant pattern by PCR-SSCP. For each tumor case studied, all abnormal PCR-SSCP fragments were found to contain ≥ 1 mutation on DNA sequencing. As previously discussed,19 the following criteria validate the presence of mutations in the tested samples: (1) concordance between PCR-SSCP and sequencing performed on independent PCR reactions; (2) consistent negativity of normal DNAs known to be devoid of BCL-6 5′ mutations (ie, B-cell lymphoblastoid cell lines) in PCR-SSCP experiments; (3) clustering of mutations with NHL vis a vis ≈ 200 negative solid tumor cases19; (4) presence of mutations in tumor, but not normal, DNA from the same patient.19
The characteristics of the mutations detected in AIDS-NHL are reported in Table 3. Representative examples of mutations are shown in Fig 2. A total of 29 alterations was scored in the AIDS-NHL panel studied. All sequence alterations were unique to individual AIDS-NHL cases. The mutations observed included single bp substitutions (n = 23), point deletions (n = 3), point insertions (n = 2), and insertion of an 11-bp DNA stretch (n = 1). No bias in the type of single bp substitutions was detected (not shown), although the number of mutations studied may not be sufficient for statistically informative analysis. All mutations detected in AIDS-NHL occurred in the presence of the germline sequence of the BCL-6 gene. However, with respect to AIDS-NHL primary specimens, it could not be discriminated whether mutations were truly heterozygous or whether the germline sequence was contributed by reactive normal cells that frequently contaminate the tissue biopsies of these lymphomas. Finally, previous studies of NHL of the immunocompetent host have shown that BCL-6 mutations may occur in a biallelic fashion.19 However, the allelic distribution of BCL-6 mutations in AIDS-NHL could not be defined because of the sequencing strategy adopted in this study.
Characteristics of Mutations in the 5′ Noncoding Region of BCL-6 in AIDS-NHL
| Sample . | Histology . | Mutation (position)*† . |
|---|---|---|
| 1142 | AIDS-BL | +G (706), Δ‡ T (1098) |
| 1143 | AIDS-BL | T→C (705), T→A (717), G→C (756), ΔT (1104) |
| 1220 | AIDS-BL | T→G (562), G→A (835), +T (1104) |
| PA682 | AIDS-BL | C→A (742), C→T (1079) |
| HBL-2 | AIDS-BL | T→G (469), ΔA (494) |
| BRG-A | AIDS-BL | A→T (658) |
| 674 | AIDS-DLCL | T→G (465), A→G (468) |
| 1139 | AIDS-DLCL | T→A (418), C→A (425), G→A (503), C→A (601), G→A (678) |
| 1141 | AIDS-DLCL | G→A (481), T→C (631), +[TCTTTGGGTTT] (1083) |
| 1156 | AIDS-DLCL | C→A (522), C→A (610), T→G (628), G→A (672) |
| 1221 | AIDS-ALCL | C→A (526) |
| Sample . | Histology . | Mutation (position)*† . |
|---|---|---|
| 1142 | AIDS-BL | +G (706), Δ‡ T (1098) |
| 1143 | AIDS-BL | T→C (705), T→A (717), G→C (756), ΔT (1104) |
| 1220 | AIDS-BL | T→G (562), G→A (835), +T (1104) |
| PA682 | AIDS-BL | C→A (742), C→T (1079) |
| HBL-2 | AIDS-BL | T→G (469), ΔA (494) |
| BRG-A | AIDS-BL | A→T (658) |
| 674 | AIDS-DLCL | T→G (465), A→G (468) |
| 1139 | AIDS-DLCL | T→A (418), C→A (425), G→A (503), C→A (601), G→A (678) |
| 1141 | AIDS-DLCL | G→A (481), T→C (631), +[TCTTTGGGTTT] (1083) |
| 1156 | AIDS-DLCL | C→A (522), C→A (610), T→G (628), G→A (672) |
| 1221 | AIDS-ALCL | C→A (526) |
Abbreviations: AIDS-BL, AIDS-related Burkitt's lymphoma; AIDS-DLCL, AIDS-related diffuse large cell lymphoma; AIDS-ALCL, AIDS-related anaplastic large cell lymphoma.
The first nucleotide of the BCL-6 cDNA is arbitrarily chosen as position +1.
The allelic distribution of mutations could not be defined because of the sequencing strategy adopted.
Δ, deletion.
Nucleotide sequencing analyses of mutations of the 5′ noncoding regions of BCL-6 in AIDS-NHL. For each sample, a mixture of ≥ 20 subclones was subjected to nucleotide sequencing (see Materials and Methods). The sequence of each AIDS-NHL case shown in the figure is matched to the sequence of a normal control (N) displaying germline BCL-6 alleles. The position of mutations is indicated by the nucleotide number of the corresponding BCL-6 germline sequence (the first nucleotide of the BCL-6 cDNA was arbitrarily chosen as position +1). Arrowheads point to bands corresponding to mutations.
Nucleotide sequencing analyses of mutations of the 5′ noncoding regions of BCL-6 in AIDS-NHL. For each sample, a mixture of ≥ 20 subclones was subjected to nucleotide sequencing (see Materials and Methods). The sequence of each AIDS-NHL case shown in the figure is matched to the sequence of a normal control (N) displaying germline BCL-6 alleles. The position of mutations is indicated by the nucleotide number of the corresponding BCL-6 germline sequence (the first nucleotide of the BCL-6 cDNA was arbitrarily chosen as position +1). Arrowheads point to bands corresponding to mutations.
Relationship between BCL-6 mutations and rearrangements.In 32 cases of AIDS-NHL, the configuration of the BCL-6 gene was tested by Southern blot analysis to define the presence of gross rearrangements and their relationship to mutations of the 5′ noncoding regions of the gene. As expected,9 rearrangements of BCL-6 were restricted to a fraction of AIDS-DLCL (3 of 12; 25.0%), but were absent in cases belonging to other clinicopathologic categories (Table 4). Consequently, the overwhelming majority of AIDS-NHL carrying mutations of BCL-6 were devoid of BCL-6 rearrangements (Tables 2 and 4). Conversely, all three AIDS-DLCL cases carrying BCL-6 rearrangements also carried mutations of the 5′ noncoding regions of the gene. Overall, these data indicate that BCL-6 mutations can occur independent of the concomitant presence of BCL-6 rearrangements in all clinicopathologic categories of AIDS-NHL. For cases harboring both rearrangements and mutations of BCL-6, it could not be defined whether BCL-6 mutations affected the rearranged allele, the germline allele, or both.
Frequency of Genetic Lesions in AIDS-NHL With and Without Mutations of the 5′ Noncoding Regions of BCL-6
| Histology . | BCL-6 . | c-MYC*† . | p53* . | EBV4-150 . |
|---|---|---|---|---|
| . | Rearrangements4-150 . | . | . | . |
| AIDS-BL | ||||
| With BCL-6 5′ mutations | 0/11 | 12/12 | 5/12 | 4/12 |
| Without BCL-6 5′ mutations | 0/5 | 5/5 | 3/5 | 3/8 |
| AIDS-DLCL | ||||
| With BCL-6 5′ mutations | 3/8 | 0/10 | 1/10 | 5/10 |
| Without BCL-6 5′ mutations | 0/4 | 2/5 | 0/5 | 3/5 |
| AIDS-ALCL | ||||
| With BCL-6 5′ mutations | 0/2 | 0/2 | 0/2 | 2/2 |
| Without BCL-6 5′ mutations | 0/1 | 0/1 | 0/1 | 1/1 |
| AIDS-BCBL | ||||
| With BCL-6 5′ mutations | 0/1 | 0/1 | 0/1 | 1.1 |
| Without BCL-6 5′ mutations | ND | 0/4 | 0/4 | 3/4 |
| Histology . | BCL-6 . | c-MYC*† . | p53* . | EBV4-150 . |
|---|---|---|---|---|
| . | Rearrangements4-150 . | . | . | . |
| AIDS-BL | ||||
| With BCL-6 5′ mutations | 0/11 | 12/12 | 5/12 | 4/12 |
| Without BCL-6 5′ mutations | 0/5 | 5/5 | 3/5 | 3/8 |
| AIDS-DLCL | ||||
| With BCL-6 5′ mutations | 3/8 | 0/10 | 1/10 | 5/10 |
| Without BCL-6 5′ mutations | 0/4 | 2/5 | 0/5 | 3/5 |
| AIDS-ALCL | ||||
| With BCL-6 5′ mutations | 0/2 | 0/2 | 0/2 | 2/2 |
| Without BCL-6 5′ mutations | 0/1 | 0/1 | 0/1 | 1/1 |
| AIDS-BCBL | ||||
| With BCL-6 5′ mutations | 0/1 | 0/1 | 0/1 | 1.1 |
| Without BCL-6 5′ mutations | ND | 0/4 | 0/4 | 3/4 |
Abbreviations: AIDS-BL, AIDS-related Burkitt's lymphoma; AIDS-DLCL, AIDS-related diffuse large cell lymphoma; AIDS-ALCL, AIDS-related anaplastic large cell lymphoma; AIDS-BCBL, AIDS-related body-cavity-based lymphoma; ND, not done.
Positive/tested.
Genetic lesions of c-MYC include both gross rearrangements and mutations of c-MYC first exon-first intron border. Gross rearrangements were detected in 13 AIDS-BL and 2 AIDS-DLCL; mutations of c-MYC first exon-first intron border were detected in 5 AIDS-BL. Cases scored as positive harbored c-MYC gross rearrangements or mutations of c-MYC first exon-first intron border or both. Cases scored as negative were devoid of both c-MYC gross rearrangements and mutations of c-MYC first exon-first intron border.
Relationship between BCL-6 mutations and c-MYC alterations in AIDS-NHL.Alterations of c-MYC were investigated using a previously reported strategy, which includes Southern blot analysis using a c-MYC third exon probe on HindIII and EcoRI digests,32 as well as mutational analysis of two PCR-SSCP fragments (fragments F and G) spanning the c-MYC first exon-first intron border.8 This combined strategy allows the molecular detection of c-MYC lesions characteristic of both the sporadic and the endemic type of BL and yields a positive result (by Southern blot or by mutational analysis) in virtually all cases of BL.34
By applying this combined strategy to the AIDS-NHL panel included in the study, alterations of c-MYC were detected in 17 of 17 (100%) AIDS-BL and two of 15 (13.3%) AIDS-DLCL (Table 4; data not shown). No c-MYC alterations were detected in cases of AIDS-ALCL or AIDS-BCBL (Table 4). Among AIDS-NHL cases carrying c-MYC alterations, 13 AIDS-BL and two AIDS-DLCL harbored c-MYC rearrangements detectable by Southern blot analysis. In addition, five cases of AIDS-BL harbored mutations within the first exon-first intron border of the c-MYC gene. Overall, the distribution of rearrangements and first exon-first intron border mutations of c-MYC observed in this report is consistent with that observed in studies of other AIDS-NHL panels.8 35
Comparison of the distribution of BCL-6 5′ mutations with that of c-MYC alterations showed that mutations of BCL-6 5′ noncoding regions occurred both in cases harboring c-MYC rearrangements or mutations, as well as in cases devoid of any genetic alteration of c-MYC (Table 4).
Relationship between BCL-6 mutations and other genetic lesions in AIDS-NHL.Mutations of p53 and infection by EBV were also tested in the AIDS-NHL panel included in this study. The data are summarized in Table 4. Mutations of p53 occurred in eight of 17 (47.0%) AIDS-BL and one of 15 (6.6%) AIDS-DLCL, but were absent in AIDS-ALCL and AIDS-BCBL. Infection of the tumor clone by EBV, tested by multiple approaches as previously reported,10 was present in seven of 20 (35.0%) AIDS-BL, eight of 15 (53.3%) AIDS-DLCL, three of three (100.0%) AIDS-ALCL, and four of five (80.0%) AIDS-BCBL.
Comparative analysis of the distribution of mutations of the 5′ noncoding regions of BCL-6 with that of EBV infection and p53 mutations defined that BCL-6 mutations may occur both in the presence and in the absence of such genetic lesions (Table 4).
DISCUSSION
This study reports that mutations of the 5′ noncoding regions of the BCL-6 gene are common in the major clinicopathologic categories of systemic AIDS-NHL, including AIDS-BL, AIDS-DLCL, and AIDS-ALCL. These mutations affect BCL-6 sequences that are located in proximity of the gene promoter and that presumably contain regulatory elements implicated in the control of BCL-6 expression36. Overall, the occurrence of mutations of the 5′ noncoding regions of BCL-6 in AIDS-NHL appears to be independent of the concomitant presence of BCL-6 rearrangements, infection by EBV, c-MYC alterations, and p53 mutations.
Our analysis of the different clinicopathologic variants of AIDS-NHL expands the spectrum of B-cell NHL types associated with mutations of the 5′ noncoding regions of BCL-6. The detection of BCL-6 mutations in B-cell NHL clinicopathologic categories as different as BL, ALCL, and BCBL (this study), as well as FL and DLCL,19 suggests that BCL-6 mutations may represent a general phenomenon in mature B-cell neoplasia. With respect to DLCL, however, it should be noted that the cumulative frequency of BCL-6 alterations, including chromosomal rearrangements and mutations, is apparently lower in cases related to AIDS (66.6%; this study) than in cases arising in the immunocompetent host (90%).19 The implications of this observation are presently unknown.
The detection of mutations of BCL-6 5′ noncoding regions may shed some light on the histogenesis of the AIDS-NHL categories associated with these mutations. Among NHL of the immunocompetent host, mutations of BCL-6 5′ noncoding regions are regarded as a marker of germinal center (GC) or post-GC B cells, based on the evidence that mutations cluster with lymphoid malignancies, such as FL and DLCL, that derive from GC or post-GC B cells, but are rare or absent in lymphoid tumors deriving from B-cell precursors or virgin B cells19 (and Migliazza et al, unpublished observations, August 1996). Thus, it is conceivable that also AIDS-BL, AIDS-DLCL, and AIDS-ALCL, which frequently harbor BCL-6 mutations, may originate from GC or post-GC B cells, at least in a fraction of cases. This hypothesis is further corroborated by the frequent association of AIDS-NHL with other biologic features typical of GC or post-GC B cells, including expression of the BCL-6 protein and accumulation of somatic mutations within the immunoglobulin gene hypervariable regions.26 37-39
The high rate of mutations of the 5′ noncoding regions of BCL-6 in AIDS-NHL raises the issue of their possible functional significance. The frequency of these mutations and their location in the proximity of the BCL-6 promoter, a region also consistently affected by BCL-6 rearrangements,11-15 suggest that these mutations may have been selected during tumorigenesis based on their potential ability to alter BCL-6 regulatory regions. In this respect, data obtained in our laboratory with the transfection of one single multiply mutated BCL-6 allele indicate that BCL-6 5′ mutations are associated with deregulated transcriptional activity of BCL-6 and that deregulation is caused by a single mutation (Migliazza and Dalla-Favera, unpublished observation, September 1996). Thus, given the extreme heterogeneity of the BCL-6 mutations detected in lymphomas, these studies must be extended to multiple BCL-6 mutant alleles before the pathogenetic significance of these mutations can be comprehensively assessed.
Mutations of proto-oncogenes involved in lymphomagenesis have been previously described in the case of translocated c-MYC and BCL-2 alleles. Mutations of c-MYC may involve both the 5′ noncoding sequences of the gene and the c-MYC protein transactivation domain.40,41 Mutations of 5′ regulatory sequences of c-MYC have been shown to alter the function of c-MYC regulatory sequences in vitro,40,42 although such mutations are not sufficient for activating the oncogenic potential of c-MYC alleles (Dalla-Favera et al, unpublished observation, April 1995). Mutations of the c-MYC transactivation domain abrogate the binding of the c-MYC protein to p107, thus altering the physiologic mechanisms of c-MYC regulation.43 Finally, the biologic consequences of mutations of translocated BCL-2 alleles are not totally clarified,44,45 although these mutations may be involved in transformation of follicular lymphoma to high grade histologies.46
The genesis of mutations of BCL-6 appears to substantially differ from that of mutations of c-MYC and BCL-2. In the case of c-MYC and BCL-2, mutations were attributed to the Ig variable gene somatic hypermutation mechanism acting on sequences that had become linked to the Ig locus following chromosomal translocation.34,44 45 In contrast with these observations, BCL-6 5′ mutations occur independently of translocation to Ig loci and in the absence of any recognizable rearrangement of BCL-619 (and this report).
Independent of the precise pathogenetic relevance of mutations of BCL-6 5′ noncoding regions, it is notable that these mutations constitute the most common genetic alteration presently detectable throughout the clinicopathologic spectrum of AIDS-NHL. In this respect, mutations of the 5′ noncoding sequences of BCL-6 may prove useful as clonal markers for monitoring minimal residual disease.
ACKNOWLEDGMENT
The authors are indebted to Dr A. Ganser for the gift of the EsIII cell line.
Supported by grants from IX Progetto AIDS, I.S.S., Rome, Italy (Grants No. 9404-33 and 9404-04), from National Institutes of Health, Bethesda, MD, (Grant No. CA 44029), and from “Fondazione Piera, Pietro e Giovanni Ferrero”, Alba, Italy. C.P. is being supported by a fellowship from A.I.R.C., Milan, Italy.
Address reprint requests to Gianluca Gaidano, MD, PhD, Division of Internal Medicine, Department of Medical Sciences, University of Torino at Novara, 28100 Novara, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal