Abstract
Erythropoietin (EP) and stem cell factor (SCF ) are essential growth factors for erythroid progenitor cell proliferation and differentiation in serum-free culture. It has been previously shown that burst-forming units-erythroid and colony-forming units-erythroid from patients with polycythemia vera (PV) have enhanced sensitivity to EP and SCF compared with normal erythroid progenitors, but little is known about the mechanism for this difference. In the present investigation, the effect of EP and SCF on protein tyrosine phosphorylation in day-8 normal and PV erythroid colony-forming cells, which give rise to colonies of 2-49 hemoglobinized cells, was studied. EP rapidly induced tyrosine phosphorylation of the EP receptor, whereas the most prominent phosphorylated protein induced by SCF was identified as the SCF receptor. No additional phosphorylated proteins were evident when PV cells were compared with normal cells. Culture of normal erythroid progenitors with orthovanadate, an inhibitor of protein tyrosine phosphatases, resulted in an increased number of erythroid colonies and enhanced protein tyrosine phosphorylation. However, in contrast, little enhancement was evident with PV cells. These results indicate that, although vanadate may be acting in normal erythroid progenitors as a phosphatase inhibitor that potentiates the kinase activity induced by SCF and EP, this function is diminished in PV cells. Because erythropoiesis is regulated by a balance between protein tyrosine kinase activity and protein tyrosine phosphatase activity, PV patients may have an abnormal phosphatase activity allowing increased cell proliferation.
PROLIFERATION and differentiation of erythroid progenitor cells is regulated by erythropoietin (EP), stem cell factor (SCF ), interleukin-3 (IL-3), and insulin-like growth factor I (IGF-I).1 EP is the principal regulator of erythropoiesis and promotes erythroid differentiation by preventing apoptosis, while having very little effect on erythroid cell replication.2 SCF is a potent costimulating growth factor that also retards apoptosis,3-6 but in addition markedly stimulates DNA synthesis by a process independent of programmed cell death.2-7 Using serum-free medium, we have shown that highly purified human blood burst-forming units-erythroid (BFU-E) require SCF and IL-3 initially for BFU-E growth and cell viability,8,9 whereas late erythroid progenitors, day-5 to day-8 erythroid colony-forming cells (ECFC), including colony-forming units-erythroid (CFU-E), are dependent on EP, SCF, and IGF-I for maximum differentiation and proliferation.8,10 SCF is not necessary for CFU-E development in the presence of high concentrations of EP (1 U/mL), but still stimulates CFU-E proliferation at a lower EP concentration (0.1 U/mL) and reduces apoptosis of these cells.6 11
Although knowledge of the complete transduction system for each of the above-mentioned growth factors is still incomplete, many investigators have reported that EP and SCF rapidly stimulate tyrosine phosphorylation of a number of proteins in human and murine cell lines.12-15 However, little work has been reported with primary human progenitor cells, particularly highly purified erythroid progenitors.
Polycythemia vera (PV) is a clonal myeloproliferative disease characterized by a trilineage marrow hyperplasia with increased production of red blood cells, granulocytes, and platelets.16,17 We have previously shown that PV blood BFU-E are hypersensitive to IL-3, EP, and SCF,11,18,19 and others have shown a similar hypersensitivity to IGF-I as well as a hypersensitive tyrosine phosphorylation of the IGF-I receptor β subunit.20,21 However, binding experiments and autoradiographic analyses showed no significant differences in specific binding of EP or IL-3 between PV and normal blood BFU-E,19 and SCF receptor numbers, dissociation constants, and internalization rates were similar for day-8 ECFC from normal and PV individuals.22 PV CFU-E also have been shown to have a normal number of low-affinity EP receptors but to lack high-affinity EP receptors, which indicated that enhanced EP sensitivity is not related to enhanced EP binding to these cells.23 Therefore, the hypersensitivity of PV erythroid progenitor cells to the wide variety of growth factors that control normal erythropoiesis must reside in an as yet unrecognized central, internal cellular molecular abnormality.
In this study, we have investigated EP- and SCF-inducible protein tyrosine phosphorylation in highly purified human day-8 erythroid progenitor cells from normal individuals and PV patients and compared the effect of orthovanadate on tyrosine phosphorylation in each group.
MATERIALS AND METHODS
Reagents.Recombinant human SCF (rhSCF ) was a gift from Amgen, Inc (Thousand Oaks, CA) and rhEP was a gift from Ortho Biotech, Inc (Raritan, NJ). Antiphosphotyrosine (anti-pTyr) murine IgG monoclonal antibody, 4G10, was purchased from Upstate Biotechnology, Inc (UBI; Lake Placid, NY) and polyclonal IgG rabbit antibody to phosphotyrosine was purchased from ZYMED Laboratories, Inc (South San Francisco, CA). Affinity-purified rabbit polyclonal antibody to EP receptor (EPR) was produced by Bryan Varnum and supplied by Steve Elliott (both of Amgen). Protein A-purified rabbit polyclonal IgG antihuman c-kit protein antibody was kindly provided by Amgen and affinity-purified, monospecific IgG rabbit antibody to the murine EP receptor was prepared as previously described.24 The enhanced chemiluminescence (ECL) reagents were purchased from Amersham Corp (Arlington Heights, IL).
Protein tyrosine phosphorylation in normal and PV ECFC after incubation with EP or SCF. Day-8 cells were incubated at 37°C with medium, rhEP at 20 U/mL, rhSCF at 100 ng/mL, or rhEP plus rhSCF for 10 minutes. The cells were then lysed and immunoprecipitated with anti-pTyr antibody for SDS-PAGE. -P h o s p h o t y r o s i n e - c o n t a i n i n g -proteins were identified by immunoblotting with anti-pTyr antibody. The purity of the ECFC was 74% ± 5% (normal) and 77% ± 4% (PV).
Protein tyrosine phosphorylation in normal and PV ECFC after incubation with EP or SCF. Day-8 cells were incubated at 37°C with medium, rhEP at 20 U/mL, rhSCF at 100 ng/mL, or rhEP plus rhSCF for 10 minutes. The cells were then lysed and immunoprecipitated with anti-pTyr antibody for SDS-PAGE. -P h o s p h o t y r o s i n e - c o n t a i n i n g -proteins were identified by immunoblotting with anti-pTyr antibody. The purity of the ECFC was 74% ± 5% (normal) and 77% ± 4% (PV).
Generation of ECFC.Four hundred milliliters of blood in 20 U/mL heparin was obtained from normal volunteers or 4 patients treated only with phlebotomy and meeting the established criteria for PV.25 One additional PV patient had received 5.1 mCu of 32P 4 years before the study, but had a subsequent relapse and only had phlebotomies for 4 years. The study was approved by the Vanderbilt Committee for the Protection of Human Subjects and the Nashville Department of Veterans Affairs Research and Development Committee. The cell preparation method has been previously described in detail by Sawada et al26 and was slightly modified for this study. Light-density mononuclear cells were separated over Ficoll-Hypaque (FH; 1.077 g/mL; Pharmacia Fine Chemicals, Piscataway, NJ; Sanofi Winthrop Pharmaceuticals, New York, NY) and were then centrifuged through 10% bovine serum albumin (BSA; Intergen Co, Purchase, NY) to deplete platelets. Lymphocyte depletion and adherent cell depletion were performed by sheep erythrocyte rosetting and overnight incubation in polystyrene tissue culture flasks at 37°C. Negative panning with CD11b/OKM*1, CD2/OKT*11, CD45R/MY11, and CD16/My23 was then used to further enrich the ECFC. These cells were cultured in a mixture containing 30% fetal calf serum (FCS), 1% deionized BSA, 50 U/mL rhIL-3, 2 U/mL of rhEP, 10 μg/mL insulin, 10−4 mol/L 2-mercaptoethanol, 500 U/mL penicillin, 40 μg/mL streptomycin, and 0.9% methylcellulose in Iscove's modified Dulbecco's medium (IMDM) at 37°C in a high humidity 5% CO2 , 95% air incubator.
On day 7 of BFU-E culture, the cells were collected and ECFC were further purified by adherence and centrifugation over FH. The day-7 cells were then incubated in a liquid mixture containing 15% FCS, 15% heat-inactivated pooled human AB serum, 0.5% deionized human serum albumin (HSA), 1 U/mL rhEP, penicillin, and streptomycin at 37°C overnight (day-8 cells).
To test the response of BFU-E to vanadate, highly purified BFU-E were prepared and cultured as previously described.9 Data are presented as the mean ± SD and significance was calculated using the t-test.
Immunoprecipitation and immunoblotting.Day-8 cells were washed twice and resuspended at 3 to 6 × 106/mL in IMDM containing 0.1% BSA. The cells were incubated for 1 hour at 37°C and were then washed again to further remove exogenous protein before incubation for an additional 2 hours at 37°C. In some experiments, sodium orthovanadate was added for the last 1 hour of the culture period. After growth factor starvation, the cells were exposed to various growth factors at optimum, plateau concentrations for 10 to 40 minutes at 37°C.11,18,26 These are concentrations that completely saturate all EP and SCF receptors of normal and PV cells.22,27 28 The cells were then washed with ice cold phosphate-buffered saline (PBS) and extracts were prepared in lysis buffer (1% Triton X-100, 20 mmol/L Tris-HCl, pH 7.5, with 10% glycerol, 140 mmol/L NaCl, 100 mmol/L sodium fluoride, 10 mmol/L EDTA, 2 mmol/L vanadate, 0.2 mmol/L phenylmethylsulfonyl fluoride, and 0.15 U/mL aprotinin) at 4°C for 20 minutes. Insoluble materials were removed by centrifugation for 20 minutes at 14,000g and 4°C.
The supernatants (200 μL) were incubated with 2 μg of the appropriate antibody (Zymed anti-pTyr, anti–c-kit protein, or antimurine EP receptor) for 4 hours at 4°C. Protein A-agarose (Calbiochem, La Jolla, CA) was added for an additional 2 hours at 4°C to collect the antigen-antibody complexes and the beads were then washed four times with lysis buffer. Bound proteins were eluted by boiling for 5 minutes in sodium dodecyl sulfate (SDS) sample buffer before 8% SDS-polyacrylamide gel electrophoresis (SDS-PAGE). After SDS-PAGE, proteins were electrophoretically transferred from the gels to nitrocellulose membranes in a buffer containing 25 mmol/L Tris, 192 mmol/L glycine, and 20% methanol. Residual binding sites on the membranes were blocked by incubation in Tris-buffered saline (TBS; PBS, 20 mmol/L Tris-HCl, 130 mmol/L NaCl) containing 2% BSA plus 10% skim-milk powder for 2 hours at 26°C. Immunoblots were then washed in TBS with 0.05% Tween-20 (TBST) and incubated for 2 hours at 26°C or overnight at 4°C in TBST with the appropriate antibody (1 μg/mL UBI anti-pTyr; 40 μg/mL anti–c-kit protein) or 26°C overnight (0.2 μg/mL anti-EPR). The membranes were then washed in TBST and probed with a 1:2,000 dilution of horseradish peroxidase-labeled sheep antimouse or donkey antirabbit antibody for 2 hours at 26°C. After washing, the immunoblots were placed in ECL substrate solution and exposed to Kodak X-film (Eastman Kodak, Rochester, NY) to visualize immunoreactive bands. In some experiments, immunoblots were stripped by incubation in a buffer containing 2% SDS, 78 mmol/L Tris-HCl, and 100 mmol/L β-mercaptoethanol for 30 minutes at 50°C and were then reblocked and reprobed. Scanning densitometry was performed using the LKB 2222-010 UltroScan XL Laser Densitometer (LKB Produkter AB, Bromma, Sweden).
Clot cultures and enumeration of day-8 ECFC.Day-8 cells were cultured at a concentration of 103 cells/mL in clots with 15% FCS, 15% human AB serum, 0.5% HSA, 1.5 mmol/L aminocaproic acid, 1 U/mL rhEP, penicillin, streptomycin, 2 mg/mL fibrinogen, and 0.2 U/mL bovine thrombin.10,26 The clots were incubated at 37°C in a high-humidity 5% CO2 , 95% air incubator for 7 days and were then fixed and stained with benzidine-hematoxylin.29 ECFC gave rise to colonies of 2-49 hemoglobinized cells, whereas CFU-E gave rise to colonies of 8-49 cells.26
RESULTS
Tyrosine phosphorylation induced by EP and SCF.In experiments not shown, growth factor-dependent phosphorylation was maximum within 10 minutes of treatment. When day-8 normal human erythroid progenitors were incubated with rhEP for 10 minutes at 37°C, enhanced tyrosine phosphorylation of a 78-kD band was clearly evident (Fig 1). Phosphorylation of this protein was specific for rhEP because incubation of the cells with rhSCF did not produce this effect. rhEP also enhanced tyrosine phosphorylation of several additional bands, but this was not regularly detected in all samples. rhSCF always rapidly induced tyrosine phosphorylation of a 155-kD protein, and it enhanced tyrosine phosphorylation of 4 bands with molecular weights of 65, 55, 42, and 31 kD in some experiments. Very little tyrosine phosphorylation of the EPR was evident when the cells were treated with SCF alone. Simultaneous stimulation with rhEP and rhSCF did not produce any additional phosphorylated bands and over several experiments no qualitative differences in the patterns were found when normal ECFC were compared with PV ECFC (Fig 1). However, in some patients, both p78 and p155 from PV cells with a similar number of ECFC appeared to show an increased density compared with the bands from normal cells.
Tyrosine phosphorylation of EPR in response to EP.The predominant tyrosine phosphorylated protein band with a molecular weight of 78 kD, detected after the cells were exposed to EP, appeared to be the EPR because a 78-kD protein has been identified as the functional form of the EPR in murine erythroid progenitor cells.24 To establish the identity of the EPR protein with this band, we initially used murine erythroid progenitor cells infected with the Friend virus that produces anemia (FVA cells) as a control because a satisfactory precipitating antibody to the human EPR was not available. The human cells incubated with rhEP or medium were lysed and immunoprecipitated with anti-pTyr antibody, whereas FVA cells were immunoprecipitated with antimurine EP receptor antibody. The samples were then immunoblotted with anti-pTyr antibody. The mouse EP receptor protein was strongly tyrosine phosphorylated in response to rhEp and comigrated with the EP-dependent band from the human cells (Fig 2A). When a rabbit polyclonal antibody that reacted with the human EPR became available, immunoprecipitation of human cell lysates with anti-pTyr antibody, followed by Western blotting with the anti-EPR, confirmed that this 78-kD protein was the human EPR (Fig 2B).
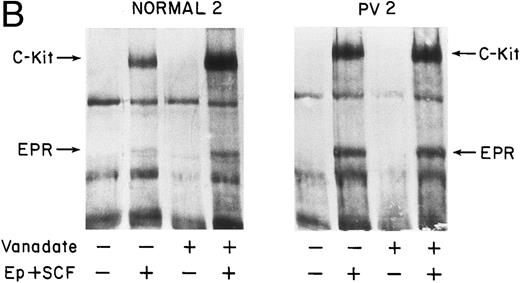
Tyrosine phosphorylation of EPR in normal and PV ECFC. Day-8 cells or FVA cells were incubated with rhEP (20 U/mL in [A] and 50 U/mL in [B]) or medium for 10 minutes (A) or 20 minutes (B) at 37°C before lysis. Human cell lysates were immunoprecipitated with anti-pTyr antibody, whereas FVA cell lysates were immunoprecipitated with antimurine EP receptor antibody. Immunoprecipitates were separated by 8% SDS-PAGE and immunoblotted with anti-pTyr antibody (A) or anti-EPR (B). The purity of the ECFC was 83% ± 2% (normal) and 65% ± 7% (PV) in (A) and 81% ± 6% (normal) in (B).
Tyrosine phosphorylation of EPR in normal and PV ECFC. Day-8 cells or FVA cells were incubated with rhEP (20 U/mL in [A] and 50 U/mL in [B]) or medium for 10 minutes (A) or 20 minutes (B) at 37°C before lysis. Human cell lysates were immunoprecipitated with anti-pTyr antibody, whereas FVA cell lysates were immunoprecipitated with antimurine EP receptor antibody. Immunoprecipitates were separated by 8% SDS-PAGE and immunoblotted with anti-pTyr antibody (A) or anti-EPR (B). The purity of the ECFC was 83% ± 2% (normal) and 65% ± 7% (PV) in (A) and 81% ± 6% (normal) in (B).
Tyrosine phosphorylation of c-kit protein, the SCF receptor (SCFR), in response to SCF.The 155-kD band is one of the most prominent phosphorylated bands induced after SCF stimulation of human ECFC. This protein appeared to be the SCFR because the molecular weight of the c-kit protein has been reported to be 145 to 160 kD.14 30 To further characterize the phosphorylation of the SCFR after treatment with rhSCF, the lysates of untreated or rhSCF-treated normal and PV ECFC were immunoprecipitated and immunoblotted with anti-pTyr antibody. p155 tyrosine phosphorylation was only evident in rhSCF-treated cells. Reprobing the blot with anti–c-kit protein antibody showed that the phosphorylated p155 was the c-kit protein in both the normal and PV cells (Fig 3).
SCF-induced tyrosine phosphorylation of c-kit in normal and PV ECFC. Day-8 cells were incubated at 37°C for 10 minutes in medium or in medium with rhSCF at 100 ng/mL. The cells were then lysed and immunoprecipitated with anti-pTyr antibody. Western blotting was performed with anti-pTyr antibody (A) and the immunoblots were reprobed with antibody to c-kit (B). The purity of the ECFC was 69% ± 12% (normal) and 77% ± 10% (PV).
SCF-induced tyrosine phosphorylation of c-kit in normal and PV ECFC. Day-8 cells were incubated at 37°C for 10 minutes in medium or in medium with rhSCF at 100 ng/mL. The cells were then lysed and immunoprecipitated with anti-pTyr antibody. Western blotting was performed with anti-pTyr antibody (A) and the immunoblots were reprobed with antibody to c-kit (B). The purity of the ECFC was 69% ± 12% (normal) and 77% ± 10% (PV).
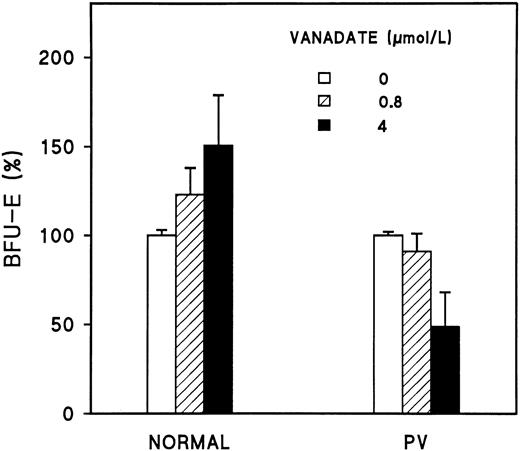
Effect of vanadate on erythroid colony formation.In a previous study,31 which was confirmed by additional experiments here, the addition of vanadate to highly purified normal human BFU-E in vitro markedly enhanced the size and number (P < .03) of the BFU-E colonies (Fig 4). However, when we added vanadate to the BFU-E of 3 PV patients, no stimulation by vanadate was observed (Fig 4). Because the number of BFU-E that we obtained was insufficient for protein phosphorylation studies, day-8 ECFC were used as target cells in the present study to test the effect of vanadate on normal and PV tyrosine phosphorylation.
Reduced response of PV BFU-E to vanadate. Day-1 BFU-E were prepared as previously described9 and were plated at 100 to 200 cells/mL in plasma clots with serum medium, 2 U/mL rhEP, 50 U/mL rhIL-3, and increasing concentrations of vanadate. After 14 days of culture, the clots were fixed and stained with benzidine-hematoxylin.29 Each value is the mean ± SD of four (normal) or three (PV) experiments expressed as a percentage of the controls without vanadate, which were set at 100. The purity of the normal BFU-E was 48% ± 6%, 31% ± 4%, 49% ± 5%, and 59% ± 3%, whereas the purity of the PV BFUE was 43% ± 4%, 38% ± 4%, and 50% ± 8%, respectively.
Reduced response of PV BFU-E to vanadate. Day-1 BFU-E were prepared as previously described9 and were plated at 100 to 200 cells/mL in plasma clots with serum medium, 2 U/mL rhEP, 50 U/mL rhIL-3, and increasing concentrations of vanadate. After 14 days of culture, the clots were fixed and stained with benzidine-hematoxylin.29 Each value is the mean ± SD of four (normal) or three (PV) experiments expressed as a percentage of the controls without vanadate, which were set at 100. The purity of the normal BFU-E was 48% ± 6%, 31% ± 4%, 49% ± 5%, and 59% ± 3%, whereas the purity of the PV BFUE was 43% ± 4%, 38% ± 4%, and 50% ± 8%, respectively.
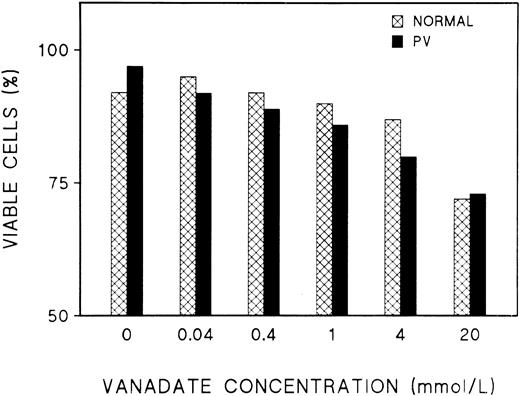
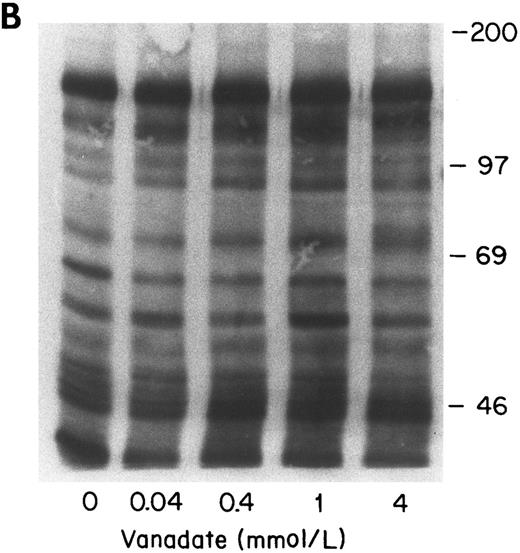
Effect of vanadate on growth factor-induced tyrosine phosphorylation of normal ECFC.The effect of vanadate on cell viability was evaluated after culturing normal and PV cells with increasing concentrations of vanadate for 1 hour (Fig 5). Cell viability did not show any significant difference when untreated cells were compared with treated cells at concentrations of 0.04 to 1 mmol/L. When the effect of vanadate on EP- and SCF-induced protein tyrosine phosphorylation was tested, it was evident that pretreatment of normal day-8 ECFC with vanadate (Fig 6A) significantly enhanced EP- and SCF-induced tyrosine phosphorylation of almost all of the observed proteins and that this increase occurred in a dose-dependent manner. However, a similar dose-response relationship of vanadate and tyrosine phosphorylation was not evident with PV cells (Fig 6B), and this was confirmed by densitometry of all the distinct bands (Dai and Krantz, unpublished data). Further experiments were performed with 0.4 mmol/L vanadate because this concentration led to increased tyrosine phosphorylation that was in the middle of the dose-response curve and because it had no adverse effect on cell viability (Fig 4).
Effect of increasing concentrations of vanadate on cell viability. Day-8 normal and PV cells were incubated with vanadate at 0 to 20 mmol/L at 37°C for 1 hour. The cells were then collected and stained with trypan blue.
Effect of increasing concentrations of vanadate on cell viability. Day-8 normal and PV cells were incubated with vanadate at 0 to 20 mmol/L at 37°C for 1 hour. The cells were then collected and stained with trypan blue.
Effect of increasing concentrations of vanadate on growth factor-induced protein tyrosine phosphorylation in normal (A) and PV (B) ECFC. Day-8 cells were incubated with increasing concentrations of vanadate for 1 hour at 37°C and were then exposed to rhEP at 20 U/mL and rhSCF at 100 ng/mL for 10 minutes at 37°C. The cells were lysed and then immunoprecipitated and immunoblotted with anti-pTyr antibody. The purity of the normal ECFC was 73% ± 2% and the purity of the PV ECFC was 78% ± 5%.
Effect of increasing concentrations of vanadate on growth factor-induced protein tyrosine phosphorylation in normal (A) and PV (B) ECFC. Day-8 cells were incubated with increasing concentrations of vanadate for 1 hour at 37°C and were then exposed to rhEP at 20 U/mL and rhSCF at 100 ng/mL for 10 minutes at 37°C. The cells were lysed and then immunoprecipitated and immunoblotted with anti-pTyr antibody. The purity of the normal ECFC was 73% ± 2% and the purity of the PV ECFC was 78% ± 5%.
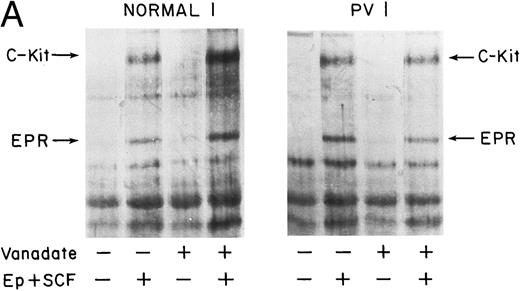
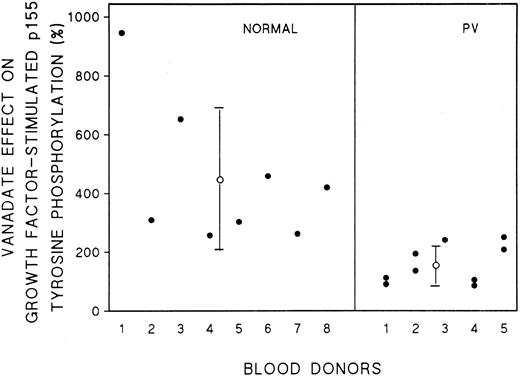
Effect of vanadate on growth factor-induced tyrosine phosphorylation of PV ECFC.Treatment of normal and PV ECFC with vanadate by itself did not appear to enhance tyrosine phosphorylation of the SCFR or related proteins (Fig 7A and B) and similar qualitative patterns of protein tyrosine phosphorylation in response to rhEP and rhSCF were observed in normal and PV day 8 cells (Fig 7A and B). Treatment of the normal cells with 0.4 mmol/L vanadate for 1 hour before stimulation with growth factors resulted in a marked increase of tyrosine phosphorylation of most of the observed proteins. An average 451% increase in tyrosine phosphorylation of p155 occurred with vanadate pretreatment of ECFC from 8 normal donors compared with the same protein in cells pretreated with medium alone (Fig 8). In contrast, pretreatment of cells from 5 PV patients with vanadate produced a markedly reduced enhancement of rhSCF-stimulated tyrosine phosphorylation of 158% (Fig 7A and B and Fig 8) that was significantly different from that observed with normal cells (P < .004). In addition, Fig 7A and B shows reduced enhancement of rhEP-stimulated tyrosine phosphorylation of the EPR in the PV cells, which is also evident in the vanadate dose-response curves performed with a third PV patient (Fig 6A and B). Lower concentrations of vanadate had little effect and higher concentrations produced cell lysis. Two of five PV patients had cells that displayed no evidence of vanadate effect on growth factor-induced tyrosine phosphorylation of p155, and only a very small increase of intensity of p155 was observed in the cells from the other three patients. Repeat studies several months later on cells from four of five of the PV patients clearly showed no significant difference compared with previous results.
Effect of vanadate on growth factorinduced protein tyrosine phosphorylation in PV and normal ECFC. Day-8 cells were incubated with rhEP at 20 U/mL and rhSCF at 100 ng/mL, or medium, for 10 minutes at 37°C after incubation at 37°C with vanadate, 0.4 mmol/L, or medium alone for 1 hour. Cell lysates were immunoprecipitated and immunoblotted with anti-pTyr antibody. The purity of the normal ECFC was 66% ± 8% (A) and 50% ± 6% (B), whereas the purity of the PV ECFC was 63% ± 3% (A) and 47% ± 6% (B).
Effect of vanadate on growth factorinduced protein tyrosine phosphorylation in PV and normal ECFC. Day-8 cells were incubated with rhEP at 20 U/mL and rhSCF at 100 ng/mL, or medium, for 10 minutes at 37°C after incubation at 37°C with vanadate, 0.4 mmol/L, or medium alone for 1 hour. Cell lysates were immunoprecipitated and immunoblotted with anti-pTyr antibody. The purity of the normal ECFC was 66% ± 8% (A) and 50% ± 6% (B), whereas the purity of the PV ECFC was 63% ± 3% (A) and 47% ± 6% (B).
Response of normal and PV SCFR tyrosine phosphorylation intensity to treatment with vanadate. Cell lysates from 8 normal donors and 5 PV patients were treated as described in the legend for Fig 7. Scanning densitometry was performed using the LKB 2222-010 UltroScan XL Laser Densitometer. The intensity measured in the controls not treated with vanadate was taken as 100%. The purity of the PV ECFC from 5 patients, including repeat cultures, ranged from 39% ± 8% to 79% ± 6% and from 8 normal donors ranged from 34% ± 4% to 79% ± 2%.
Response of normal and PV SCFR tyrosine phosphorylation intensity to treatment with vanadate. Cell lysates from 8 normal donors and 5 PV patients were treated as described in the legend for Fig 7. Scanning densitometry was performed using the LKB 2222-010 UltroScan XL Laser Densitometer. The intensity measured in the controls not treated with vanadate was taken as 100%. The purity of the PV ECFC from 5 patients, including repeat cultures, ranged from 39% ± 8% to 79% ± 6% and from 8 normal donors ranged from 34% ± 4% to 79% ± 2%.
DISCUSSION
In this study, we investigated Ep- and SCF-inducible tyrosine phosphorylation in highly purified human erythroid progenitor cells from normal and PV patients by Western blotting with antibody specific for phosphotyrosine. We also compared the effect of vanadate on protein tyrosine phosphorylation in these cells. A prominent tyrosine phosphorylated 78-kD protein was rapidly enhanced after EP stimulation of both normal and PV erythroid progenitor cells. This protein was tentatively identified as the EPR by showing that the tyrosine phosphorylated murine EP receptor comigrated with this band and by using anti-EPR antibody. SCF stimulation induced or enhanced 5 tyrosine phosphorylated proteins with molecular weights of 155, 65, 55, 42, and 31 kD in normal human and PV day-8 cells and p155, the most prominent phosphorylated protein, was identified as the c-kit product (SCFR) using anti–c-kit antibody. Simultaneous stimulation with both Ep and SCF did not induce any additional protein phosphorylation and no additional, different proteins were tyrosine phosphorylated by either EP or SCF stimulation of PV cells compared with phosphorylations in normal cells.
Vanadate is a phosphatase inhibitor that has been widely used to investigate the effects of protein tyrosine phosphatases in signal transduction,32 and we have previously shown that vanadate markedly enhanced both the number and size of normal erythroid bursts in vitro, mimicking the effect of SCF.31 In this study, vanadate treatment of normal human erythroid progenitor cells had a profound effect on protein tyrosine phosphorylation induced by either EP or SCF, but little effect on tyrosine phosphorylation in the absence of these stimulating factors. Densitometry measurement of the intensity of p155 phosphorylation indicated a 4.5-fold increase after vanadate exposure. It has been previously shown that the hematopoietic protein tyrosine phosphatase PTP1C (also called SHPTP1, HCP, and SHP) acts as a negative regulator of erythropoiesis by associating through its SH2 domains with the tyrosine phosphorylated EP receptor.33 This interaction leads to the dephosphorylation plus inactivation of JAK2, and cells expressing an EP receptor unable to bind PTP1C are hypersensitive to EP.33 PTP1C has also been reported to associate with the IL-3 receptor β chain and the SCFR.34,35 Thus, PTP1C appears to downregulate the function of several receptor kinase complexes, and vanadate, as a phosphatase inhibitor, may be acting to reverse the downregulation of receptor phosphorylation in normal ECFC. This would explain the action of vanadate in stimulating the number and size of human BFU-E in vitro.31 In contrast, PV patients' cells treated with vanadate before stimulation by EP and SCF did not show much enhancement of receptor tyrosine phosphorylation and the effect of vanadate on PV erythroid proliferation was also greatly reduced.
Past studies have shown that PV erythroid progenitor cells are hypersensitive to a variety of growth factors.11,18-20,36 It is now clear that orthovanadate, a tyrosine phosphatase inhibitor that enhances normal erythropoiesis in vitro and normal growth factor induced SCFR and EPR tyrosine phosphorylation, has a markedly reduced effect on PV ECFC. In addition, at least one phosphatase, PTP1C, has been shown to downregulate erythropoiesis and mutations of this phosphatase produce a viable moth-eaten (mev/mev) phenotype in mice with marked splenomegaly and increased numbers of splenic CFU-E that are hypersensitive to EP.37 Thus, a likely cause for the enhanced erythropoiesis of PV may be an abnormal phosphatase, such as PTP1C, which might produce increased granulocyte and platelet production as well. However, orthovanadate may have other effects, such as inhibition of protein degradation38 or inhibition of other negative regulators. Tyrosine phosphatases other than PTPIC may also have similar roles as negative regulators of erythropoiesis and a mutation of one of these tyrosine phosphatases could be equally important in producing PV. Because some protein tyrosine phosphatases have positive roles in signal transduction,39 it is also possible that one of these phosphatases has become fixed by mutation in a stimulatory mode and unresponsive to orthovanadate. Nevertheless, these studies appear to indicate that production of a defective tyrosine phosphatase, due to a gene mutation in this clonal disease, may be involved in the production of PV. Further investigations are necessary to define the state of the tyrosine phosphatases in this disease.
ACKNOWLEDGMENT
The authors are grateful for the generous gifts of rhSCF and antibody to c-kit from Amgen and rhEP from Ortho Biotech, Inc. We thank Kathleen Kollar, Ella Stitt, and Amanda Hodges for their excellent technical help; Pat Hofmann for her kind assistance with typing; and Dr Harriet Gilbert for her gracious provision of some of the PV blood.
Supported by a Veterans Health Administration Merit Review Grant (S.B.K.) and by Grants No. DK-15555 (S.B.K.), DK-39781 (S.T.S.), and 5 T32-DK07186 (S.B.K.) from the National Institutes of Health.
Address reprint requests to Sanford B. Krantz, MD, Department of Medicine/Hematology, Vanderbilt University Medical School, MRB II, Room 547, Nashville, TN 37232-6305.


![Fig. 2. Tyrosine phosphorylation of EPR in normal and PV ECFC. Day-8 cells or FVA cells were incubated with rhEP (20 U/mL in [A] and 50 U/mL in [B]) or medium for 10 minutes (A) or 20 minutes (B) at 37°C before lysis. Human cell lysates were immunoprecipitated with anti-pTyr antibody, whereas FVA cell lysates were immunoprecipitated with antimurine EP receptor antibody. Immunoprecipitates were separated by 8% SDS-PAGE and immunoblotted with anti-pTyr antibody (A) or anti-EPR (B). The purity of the ECFC was 83% ± 2% (normal) and 65% ± 7% (PV) in (A) and 81% ± 6% (normal) in (B).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/10/10.1182_blood.v89.10.3574/3/m_bl_0027f2a.jpeg?Expires=1765917574&Signature=Q3DYffYjAn5CV5uHVfYW0F0sRc87SnJ68edTRVkV33LqbInQJ8JpVPVD5KAsv0QeWTxpesSI42myUJlwIHT2MiMYmQz0Bph3ZbEE8kZWzvFchpN4pO97hJve7dfH4Q4NvIeNnTtZ4yuLOWVZWahgOvyvhnTlrARAsPz5Rka2TDCAfTFCankR1kgoSl66PeWihR2sW3kMW0H929JqxNTJcStxTsQf2dj8EvvBEcX1-mqJEv~UtWvcKlqXkBCl31j2Gtl82LexXrCnD3VD~iRmBvH5GDqaET7j1T67RPInyXjsarEfPXwgVYt7IJ2iGfQXmNnvO5JAjYu-cLvh7~xGgA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Tyrosine phosphorylation of EPR in normal and PV ECFC. Day-8 cells or FVA cells were incubated with rhEP (20 U/mL in [A] and 50 U/mL in [B]) or medium for 10 minutes (A) or 20 minutes (B) at 37°C before lysis. Human cell lysates were immunoprecipitated with anti-pTyr antibody, whereas FVA cell lysates were immunoprecipitated with antimurine EP receptor antibody. Immunoprecipitates were separated by 8% SDS-PAGE and immunoblotted with anti-pTyr antibody (A) or anti-EPR (B). The purity of the ECFC was 83% ± 2% (normal) and 65% ± 7% (PV) in (A) and 81% ± 6% (normal) in (B).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/10/10.1182_blood.v89.10.3574/3/m_bl_0027f2b.jpeg?Expires=1765917574&Signature=gksyCQIf26EslgATCSgXBmEVDi26dFVrfZbVGyjUkXWgNj15GRk4fjup9Bn4h-mIuNcmH1qeKYxiCDu-dIPszDn5mBbI6~eNJkG0LA3kYdCQVFEW0P-9d9WWNIGoaYwdTCNVIl55E~0BdKdZPVTYH-DgmOeAb2M5Oi8OJF1kc9EXk1x-FtkMT4ssPlpqPPpZ-RXWRdra074Fz~PxeCvFmZrVxV6K8We3P61wO01ae~oGsAryRs6~zSGlme65R9bZ2D05CRTjm1KXc~ly0etd-z5AyN~4gUKTIlj04geNbk~Z1fgL~Jp48y65HosaLjCVJf7XYdTg~C8Jzapt26pPfA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal