Abstract
In this study, we examined the in vitro and in vivo effects of forced expression of Mpl-R (the thrombopoietin receptor) on the progeny of murine hematopoietic stem cells. Bone marrow cells from 5-FU–treated mice were transduced with retroviral vectors containing the human Mpl-R cDNA, or the neomycine gene as a control. After 7 days cocultivation on virus-producer cells, GpE86-Mpl-R or Gp86-Neo, the types of hematopoietic progenitor cells responding to thrombopoietin (TPO) were studied by clonogenic assays. Mpl-R–infected cells gave rise to CFU-GEMM, BFU-E, CFU-MK, but not CFU-GM while Neo-infected cells produced only megakaryocytic colonies. In addition, when nonadherent cells from GpE86-Mpl-R cocultures were grown with TPO as the only stimulus for 7 days, a marked expansion of CFU-GEMM, BFU-E, and CFU-MK was observed, while no change in CFU-GM number was seen. Erythroid and megakaryocytic maturation occurred in the presence of TPO while a block in granulocytic differentiation was observed at the myeloblast stage. The direct effects of TPO on Mpl-R–transduced progenitor cells were demonstrated by single cell cloning experiments. To analyze the effects of the constitutive expression of Mpl-R on the determination of multipotent progenitors (CFU-S) and long-term repopulating stem cells, Mpl-R– or Neo-infected cells were injected into lethally irradiated recipient mice. No difference was seen in (1) the number of committed progenitor cells contained in individual CFU-S12 whether colonies arose from noninfected or Mpl-R–infected CFU-S; (2) the mean numbers of progenitor cells per leg or spleen of mice reconstituted with Mpl-R– or Neo-infected cells, 1 or 7 months after the graft; and (3) the blood parameters of the two groups of animals, with the exception of a 50% reduction in circulating platelet counts after 7 months in mice repopulated with Mpl-R–infected bone marrow cells. These results indicate that retrovirus-mediated expression of Mpl-R in murine stem cells does not modify their ability to reconstitute all myeloid lineages of differentiation and does not result in a preferential commitment toward the megakaryocytic lineage.
THE PROTO-ONCOGENE c-mpl is a member of the hematopoietin receptor superfamily,1-3 and its role in the regulation of megakaryocytopoiesis has been established. c-mpl expression is restricted to human erythroid/megakaryocytic leukemic cell lines, human CD34+-progenitor cells, megakaryocytes, and platelets.4 Methia et al have shown that c-mpl antisense oligodeoxynucleotides inhibited megakaryocyte colony formation, whereas erythroid and granulocyte-macrophage colony formation was not impaired.4 In addition, c-mpl knock-out mice develop a severe but nonlethal thrombocytopenia with a dramatic reduction in megakaryocytes, whereas all the other mature blood cells remain normal.5
The cognate ligand for the c-mpl encoded receptor (Mpl-R) has been purified and cloned from several species.6-10 This novel hematopoietin has been called Mpl ligand (Mpl-L),7 thrombopoietin (TPO),9 megapoietin,8 or megakaryocyte growth and development factor (MGDF ).6 We have chosen the TPO.
We and others have demonstrated that TPO induces the proliferation of megakaryocyte progenitors and their differentiation into large polyploid, platelet-producing megakaryocytes.11-13 Moreover, TPO is essential for the full maturation of megakaryocytes, and acts to enhance platelet production and to speed their recovery after cytoreductive therapies.14-16 However, in addition to its major action on megakaryocytopoiesis and thrombopoiesis, TPO also acts on cells from other hematopoietic lineages. TPO expands erythroid progenitors, increases red cell production, and enhances erythroid recovery after myelosuppressive therapy.17 Furthermore, TPO also acts as a synergistic growth factor for primitive hematopoietic progenitors,18,19 strongly suggesting that primitive stem cells might express Mpl-R.20 This is consistent with the fact that Mpl-R knock-out mice have a reduced number of all hematopoietic progenitor cells in the bone marrow.21 These data imply that Mpl-R may play a role in the regulation of pluripotent hematopoietic cells. The aim of this study was to examine whether a forced expression of Mpl-R in hematopoietic progenitor cells would, first, allow their proliferation and differentiation in the presence of TPO alone, and, second, preferentially modify their differentiation program toward megakaryocytopoiesis. In order to address these questions, we transduced murine bone marrow cells with a retroviral vector encoding the human Mpl-R gene. Our in vitro data show that pluripotent and erythroid progenitor cells expressing Mpl-R were able to proliferate and differentiate in response to TPO. In vivo, pluripotent stem cells constitutively expressing Mpl-R gave rise to normal hematopoiesis in lethally irradiated animals.
MATERIALS AND METHODS
Retroviral infection of bone marrow cells.A retroviral vector encoding Mpl-R was constructed by inserting 1,500 bp of the human Mpl-R cDNA (Sal1-Not1 fragment) in the Xho site of the pBTZenSVNEO vector. This retroviral construct, pBTZen-Mpl-R-SVNEO and the Neo construct, pBTZenSVNEO, were transfected into the packaging cell line GpE86.22 Individual geneticin-resistant clones were derived and their supernatant was tested for viral production on NIH3T3 cells. The Mpl-R or Neo virus-producer clone used in these experiments produced 2 × 105 and 4 × 105 infectious particles/mL, respectively.
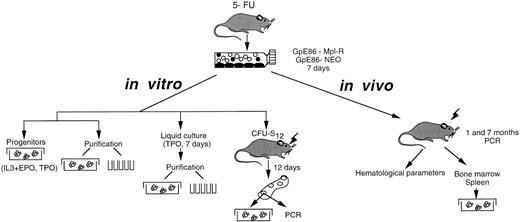
Hematopoietic cells were harvested from the tibiae and femurs of 6- to 10-week-old male CBA/J mice (Janvier, Orléans, France) pretreated with 5-fluorouracil (5-FU, Roche, Paris, France; 150 mg/kg of body weight injected intraperitoneally) 3 days before bone marrow harvest. Monocellular suspensions were prepared in α-MEM (GIBCO, Eragny, France). Cell suspensions were depleted of adherent cells by two successive 90-minute adherence steps in 100 mm tissue-culture–treated Petri dishes (Falcon, Grenoble, France). Nonadherent cells were collected and 9 × 106 cells were cocultured for 7 days at 33°C in 75-cm2 flasks (Falcon) containing subconfluent irradiated (10 Gy) monolayer of either GpE86-Mpl-R or GpE86-Neo virus-producing cells. Coculture medium consisted in α-MEM supplemented with 10% fetal calf serum (FCS, Dutscher, Issy-les-Moulineaux, France), 5% WEHI-3B CM (murine IL-3) and 10−6 mol/L hydrocortisone (Sigma, Saint-Quentin Fallavier, France). At the end of the seventh day, nonadherent cells were analyzed in vitro and in vivo as illustrated in Fig 1.
Experimental protocol. Protocols designed to infect bone marrow cells and to study the in vitro and in vivo effects of the Mpl-R expression on the proliferation and differentiation of murine hematopoietic progenitors are schematically presented.
Experimental protocol. Protocols designed to infect bone marrow cells and to study the in vitro and in vivo effects of the Mpl-R expression on the proliferation and differentiation of murine hematopoietic progenitors are schematically presented.
Progenitor assays.To detect CFU-GEMM, CFU-GM, CFU-M, and BFU-E, cells were plated in methylcellulose medium as described.23 Briefly, nonadherent cells were mixed in 1 mL of 0.8% methylcellulose (Fluka) in α-MEM supplemented with 20% FCS, 7.5 × 10−5 mol/L α-thioglycerol (Sigma) and either 10% WEHI-3B CM (mu IL-3) plus 2 U/mL hu-r-EPO (a gift from Cilag, Paris, France) or 10% conditioned medium from 293 cells transfected with the human TPO cDNA as a source of hu-r-TPO. On titration on the BaF/Mpl-R proliferation assay,11 conditioned medium contained 100 ng/mL of TPO. Cultures were incubated at 37°C in an atmosphere of 5% CO2 in air. Colonies were scored using an inverted microscope at 100 × magnification on day 7 for CFU-GM and CFU-M and on day 9 for pure BFU-E and CFU-GEMM. G418 (GIBCO, Paisley, UK) was used at a concentration of 800 μg/mL.
For CFU-MK, cells were seeded in 0.3% semi-solid agar cultures as described.24 Cultures were stimulated with either 10% (10 ng/mL) conditioned medium from hu-TPO–producing 293 cells or 5% pokeweed mitogen-stimulated mouse spleen lymphocytes (SCM25). Cultures were harvested on day 7 and CFU-MK–derived colonies were visualized by acetylcholinesterase staining.26
At the end of the cocultures, 5 × 104 nonadherent cells were intravenously injected into lethally irradiated (9.5 Gy) syngeneic mice.27 Mice were killed on day 12 and spleen colonies (CFU-S12 ) were isolated. Cells were suspended in α-MEM, and a fraction of the cells (1 × 104) was used to assess retroviral gene integration by PCR. The remaining cells were cultured in methylcellulose medium with mu IL-3 + EPO or in agar medium with hu TPO as described above.
Immunofluorescence labeling and single cell cloning.Cells were collected, washed in serum-free medium, and incubated for 30 minutes at 4°C in the presence of a rat antimouse monoclonal antibody cocktail: Mac-1 (1/3,000), T3 (1/800), Gk-1.5 (1/1,000), Ly-1+ (1/1,000), Gr-1 (1/50), TER-119 (1/100), and B-220 (1/50) (Pharmingen, San Diego, CA). After incubation, cells were washed twice and incubated with sheep antirat IgG-conjugated beads at a 5:1 beads/cell ratio (Dynabeads M-450; Dynal, Oslo, Norway) at 4°C for 45 minutes with gentle agitation. Two rounds of Lin− cell selection were performed. Lineage-depleted cells (Lin− fraction) were stained with a 1/100 diluted mouse antirat kappa chain FITC-conjugated monoclonal antibody (Immunotech, Lumigny, France). After washing, cells were resuspended in medium in the presence of 1 mg/mL of 7 amino actinomycin D (7AAD; Sigma) to visualize dead cells and analyzed on a FACS Vantage flow cytometer (Becton Dickinson, Mountain View, CA) to set up the gates. Cells were gated with respect to light scatter properties of blast cells and the thresholds were set to discriminate between FITC− and FITC+ cell fractions. For limiting dilution experiments, FITC-negative cells were directly sorted into 96-well tissue culture plates (Falcon) using an automatic autoclone apparatus.
Studies of reconstituted mice.Five × 105 to 2 × 106 nonadherent cells from GpE86-Neo or GpE86-Mpl-R cocultures were intravenously injected into lethally irradiated mice. One and 7 months posttransplant, PCR analysis was performed on 50 μL of blood to check for the presence of retrovirally infected cells. Mice were considered as positive when the signals were equal or superior to those obtained with a blood mixture containing at least 5% of cells from Neo transgenic mice28 obtained from H. Blüthmann (Hoffmann La Roche, Basel, Switzerland). Hematologic parameters of positive mice were automatically determined with an STKS counter (Coultronics, Margency, France). The number of clonogenic progenitors in the bone marrow and spleen of mice positive for the retrovirus integration was assessed in clonogenic methylcellulose or agar assays as described above.
Polymerase chain reaction (PCR) analysis.PCR analysis was performed directly on cell pellets. Pellets containing 1 × 104 nucleated cells from individual CFU-S12 were lysed in 20 μL of a lysis buffer (0.45% Tween 20 and 400 μg/mL of proteinase K). Whole blood (50 μL) was washed three times in 500 μL of Tris-EDTA (10 mmol/L Tris HCl pH 7.4; 1 mmol/L EDTA pH 8.0). Pellets were suspended in 100 μL of a lysis buffer containing 0.5% Tween 20 and 100 μg/mL of proteinase K. Incubation was performed at 56°C for 30 minutes and followed by 10 minutes at 95°C. Each sample (10 μL) was analyzed for the presence of the proviral sequences using either Neo-specific primers: 5′-ATGATTGAACAAGATGGATTGCACGC-3′ and 5′-GCTGTGCTCGACGTTGTCACTGAA-3′ or Mpl-R–specific primers: 5-TGGAGATGCAGTGGCACTTG-3′ and 5′-TGATGTCTGGGGTGTCAAGA-3′. As a positive control, the murine β2-microglobulin sequence was amplified using specific primers: 5′-CAGTTCCACCCGCCTCAC-3′ and 5′-CACATGTCTCGATCCCAG-3′ (Genosys, Cambridge, UK). PCR amplification was performed in a commercial buffer (ATGC, Noisy le Grand, France) with 0.2 mmol/L of each dNTP (Pharmacia, Saint-Quentin en Yvelines, France), 1 mmol/L of each primer and 0.5 U of Taq DNA polymerase. After amplification (94°C for 1 minute, 55°C for 2 minutes, and 72°C for 3 minutes, 30 cycles) (Appligene; Crocodile, Illkirch, France), aliquots of amplified DNA samples were analyzed by conventional Southern blotting on a 1.8% agarose gel using 32P-γ-ATP labeled Mpl-R (5′-TTCTACCACAGCAGGGCACG-3′ ) or the β2-microglobulin antisense primer as probe.
RESULTS
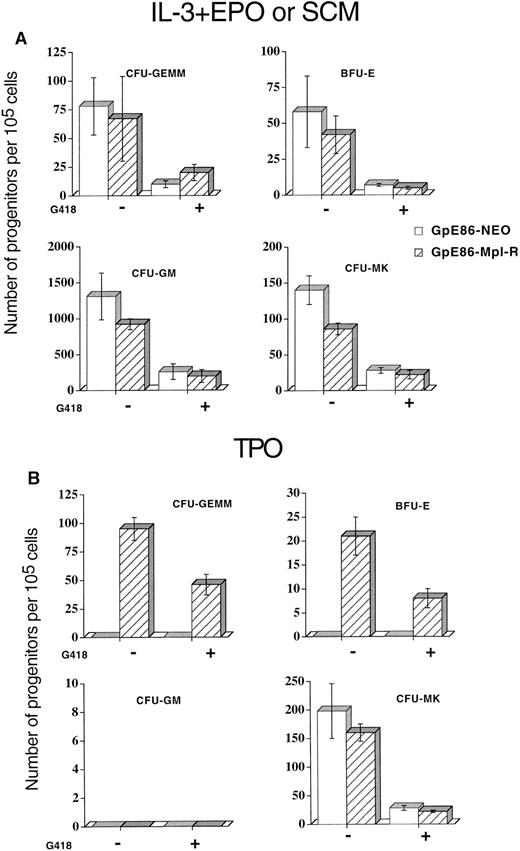
Colony formation of Neo- or Mpl-R–infected progenitor cells.Because transfection might modify the properties of virus-producer cells, we first checked the influence of the adherent cell layer on the total number of nucleated and progenitor cells at the end of the 7-day coculture period. Bone marrow cells from 5-FU–treated mice were cocultured with either GpE86, GpE86-Neo or GpE86-Mpl-R cells. No significant difference in the numbers of nonadherent nucleated cells collected (1.5 ± 0.3, 1.9 ± 0.5, and 1.8 ± 0.4 × 106 cells per flask, respectively) was observed. To determine the number of various progenitor cell types in the nonadherent hematopoietic cell populations from GpE86-Neo and GpE86-Mpl-R cocultures, nonadherent cells were plated in clonogenic assays. Cell growth was stimulated with either a combination of IL-3 and EPO to reveal CFU-GEMM, CFU-GM, and BFU-E or pokeweed mitogen spleen cell stimulated conditioned medium (SCM) to identify CFU-MK. No significant difference was found in the number of CFU-GM–, BFU-E– CFU-MK–, and CFU-GEMM–derived colonies whether cells were infected with Neo or Mpl-R retroviruses (Fig 2A). These results demonstrate that GpE86-Neo or GpE86-Mpl-R virus-producer cells have no influence on the generation of the different types of clonogenic progenitor cells.
Mean number of progenitors ± SEM per 105 marrow cells cocultured on GpE86-Neo or GpE86-Mpl-R cells. Cocultured cells were grown (A) in methylcellulose in the presence of IL-3 + EPO to reveal CFU-GEMM, BFU-E, and CFU-GM or in agar in the presence of pokeweed mitogen spleen cell conditioned medium (SCM) to reveal CFU-MK with (+) or without (−) G418 (n = 4); or (B) in methylcellulose (CFU-GEMM, BFU-E, and CFU-GM) or in agar (CFU-MK) in the presence of TPO (n = 4) with (+) or without (−) G418.
Mean number of progenitors ± SEM per 105 marrow cells cocultured on GpE86-Neo or GpE86-Mpl-R cells. Cocultured cells were grown (A) in methylcellulose in the presence of IL-3 + EPO to reveal CFU-GEMM, BFU-E, and CFU-GM or in agar in the presence of pokeweed mitogen spleen cell conditioned medium (SCM) to reveal CFU-MK with (+) or without (−) G418 (n = 4); or (B) in methylcellulose (CFU-GEMM, BFU-E, and CFU-GM) or in agar (CFU-MK) in the presence of TPO (n = 4) with (+) or without (−) G418.
To evaluate the proportion of infected progenitors after the 7-day coculture period, the same semi-solid cultures were performed in the presence of G418. A similar transduction efficiency for Neo and Mpl-R retroviral genes was observed (Fig 2A).
Effects of r-hu-TPO stimulation on Mpl-R–infected clonogenic progenitor cells.When cells from Neo-infected cultures were plated in semi-solid cutures in the presence of r-hu-TPO, no colonies were seen with the exception of CFU-MK (Fig 2B). In contrast, when the Mpl-R–infected cell populations were stimulated with r-hu-TPO, fully mature CFU-GEMM, BFU-E, and CFU-MK colonies were obtained. In this culture condition, no CFU-GM–derived colonies were ever detected (n = 6) (Fig 2B). These results demonstrate that Mpl-R infected CFU-GEMM and BFU-E, but not CFU-GM, were able to proliferate and differentiate in response to TPO. However, in these culture conditions, an equal number of small CFU-M colonies containing 50 to 100 macrophages was observed in GpE86-Neo and GpE86-Mpl-R cocultures (210 ± 50 and 170 ± 31 colonies per 105 Neo- or Mpl-R–infected cells, respectively).
To ascertain if CFU-GEMM colonies developing in the presence of r-hu-TPO were pluripotent, individual colonies were picked. Cells were either cytocentrifuged to determine the cellular content or replated in methylcellulose in the presence of r-TPO to determine their ability to generate secondary colonies. Thirty colony smears were stained with May Grünwald-Giemsa, benzidine or acetylcholinesterase. In 85% of the colonies, granulocytes, macrophages, erythrocytes, and megakaryocytes were seen, demonstrating the pluripotentiality of the progenitor at the origin of the colony. The remaining 15% were erythroid and megakaryocytic cells. Out of 40 individual replated CFU-GEMM colonies, 17 generated mixed colonies containing 100 to 500 erythroid and megakaryocytic cells, 18 gave rise to CFU-M colonies, and 5 did not produce secondary colonies. These results indicate that CFU-GEMM colonies were generated from a pluripotent progenitor without “self-renewal” ability.
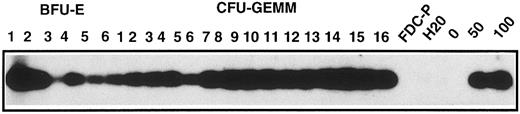
We then compared the percentage of G418-resistant progenitors in nonadherent populations from GpE86-Neo or -Mpl-R cocultures in plating cells in semi-solid medium stimulated with TPO. Although all CFU-GEMM and BFU-E that acquired the capability to respond to TPO should be infected, only 60% of the colonies were able to grow in the presence of G418 (Fig 2B). To understand this discrepancy, PCR analysis was performed on genomic DNA extracted from 40 individual colonies developing in TPO+ G418− medium. The results (Fig 3) showed that all randomly picked CFU-GEMM– and BFU-E–derived colonies were positive for the virally encoded Mpl-R gene. These data strongly suggest that all progenitors giving rise to colonies in response to TPO must have an integrated copy of the retroviral construct. Thus, the decrease in the number of colonies seen in the presence of G418 selection might be due to different levels of expression of the retroviral genes as, in the construct, the Mpl-R gene is under the transcriptional control of the viral LTR, while expression of the Neo gene is driven by the SV40 promoter, which is weaker in hematopoietic cells (our unpublished data).
Transduction of Mpl-R in colonies grown with TPO alone. Representative PCR analysis performed on cell lysates of individual colonies with primers specific for the retroviral Mpl-R gene (6 BFU-E and 16 CFU-GEMM). Colonies were generated from hematopoietic cells cocultured on GpE86-Mpl-R cells and grown in methylcellulose in the presence of TPO without G418. Controls were the cell lysates of uninfected FDC-P1, or a population containing 50% (50) or 100% (100) of Mpl-R–infected FDC-P1 cells.
Transduction of Mpl-R in colonies grown with TPO alone. Representative PCR analysis performed on cell lysates of individual colonies with primers specific for the retroviral Mpl-R gene (6 BFU-E and 16 CFU-GEMM). Colonies were generated from hematopoietic cells cocultured on GpE86-Mpl-R cells and grown in methylcellulose in the presence of TPO without G418. Controls were the cell lysates of uninfected FDC-P1, or a population containing 50% (50) or 100% (100) of Mpl-R–infected FDC-P1 cells.
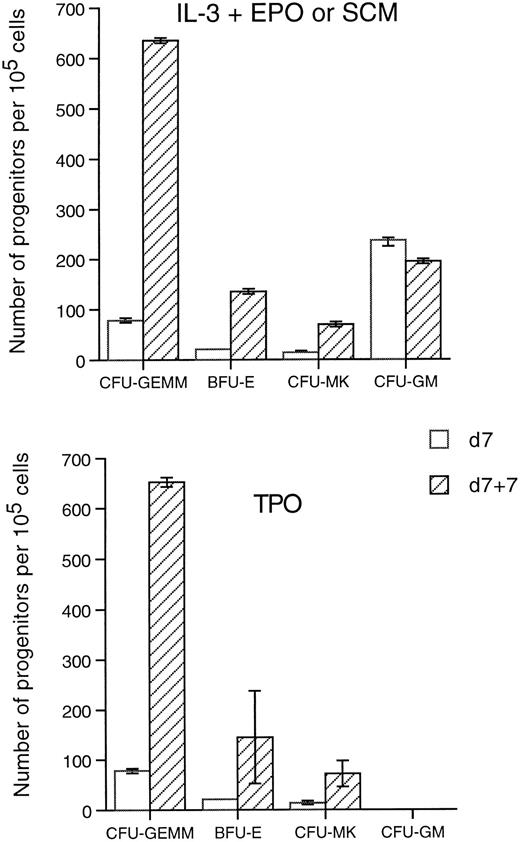
Effects of TPO stimulation on Mpl-R–infected cells in liquid culture.The above results indicate that while TPO is able to induce the proliferation and differentiation of various types of Mpl-R–infected progenitors in semi-solid assays, it induces only megakaryocytic differentiation of Neo-infected progenitors. We therefore tested whether TPO would also amplify the number of clonogenic progenitor cells in liquid cultures. Nonadherent populations were collected after the 7-day coculture with GpE86-Neo and GpE86-Mpl-R producing cells, washed, and transferred into fresh flasks in medium containing r-TPO as the only stimulus for an additional 7 days. The number of nucleated cells was maintained during the liquid culture (78% ± 25% of the input number, n = 6). The TPO-stimulated cells were plated in semi-solid medium with r-hu-TPO, IL-3 + EPO, or SCM to reveal progenitor-derived colonies. Only CFU-MK (observed in agar cultures in the presence of TPO or SCM) were detected from GpE86-Neo–infected cells after 7 days of liquid culture (data not shown). As illustrated in Fig 4, the number of clonogenic progenitors from GpE86-Mpl-R–infected cells either in the presence of TPO, IL-3 + EPO, or SCM, increased after stimulation by TPO in the liquid culture. In contrast, the number of CFU-GM, grown in methylcellulose in the presence of IL-3 + EPO or TPO, did not increase during the liquid culture. The number of CFU-S12 was constant during the 7-day liquid culture with TPO (data not shown). These results show that TPO induces the expansion of several Mpl-R–infected progenitor cells, but not CFU-GM.
Effect of TPO on the expansion of hematopoietic progenitors. Hematopoietic cells cocultured 7 days on GpE86-Mpl-R cells (d7) or cocultured 7 days with GpE86-Mpl-R cells and stimulated with TPO during 7 days in liquid culture (d7 + 7) were plated in methylcellulose or agar in the presence of IL-3 + EPO, SCM, or TPO. The results represent the number ± SEM of progenitors per 105 cells plated (n = 3).
Effect of TPO on the expansion of hematopoietic progenitors. Hematopoietic cells cocultured 7 days on GpE86-Mpl-R cells (d7) or cocultured 7 days with GpE86-Mpl-R cells and stimulated with TPO during 7 days in liquid culture (d7 + 7) were plated in methylcellulose or agar in the presence of IL-3 + EPO, SCM, or TPO. The results represent the number ± SEM of progenitors per 105 cells plated (n = 3).
Putative role of growth factors secreted by accessory cells in the growth and differentiation of pluripotent progenitor cells in response to TPO.Semi-solid cultures of cells infected with the Mpl-R retrovirus demonstrated erythroid maturation in response to TPO. Because our medium contained low amounts of FCS, cultures were performed in the presence of a neutralizing anti-EPO antibody. While the antibody completely abolished the hemoglobinization of BFU-E colonies in control cultures, the number, size, and maturation of colonies containing erythroid cellular elements were not modified in cultures stimulated with TPO (data not shown). Therefore, TPO allows the terminal maturation of Mpl-R–transduced erythroid cells.
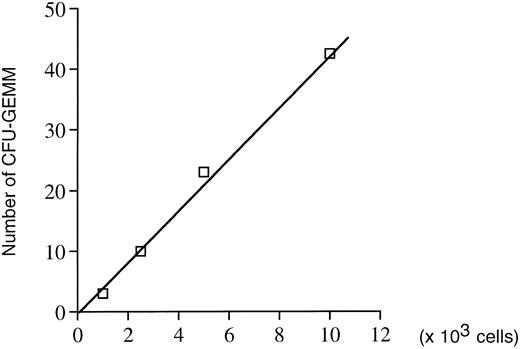
In addition, mature mixed CFU-GEMM colonies containing granulocytes, monocytes, and megakaryocytes were also obtained in the presence of TPO alone. To test whether growth factors secreted by accessory cells were playing a role in the proliferation and/or differentiation of these pluripotent progenitors, two types of experiments were performed. First, the relationship between the number of CFU-GEMM colonies developing and the number of cells plated was established. A linear curve was obtained (Fig 5) strongly suggesting no cooperation between accessory cells and hematopoietic progenitors. Second, Neo- and Mpl-R–infected cells were depleted of mature cells (Lin− depletion). Lin− populations were cloned at one cell per well in the presence of TPO. As expected, no proliferation was seen with the Neo-infected Lin− population with the exception of some wells (11 of 768) containing one or two surviving cells. In sharp contrast, of 768 wells seeded with Mpl-R–infected Lin− population, eight (1%) showed an active cell proliferation (>300 cells), while in the others no cell growth occurred. Cytospin smears prepared from the eight positive wells demonstrated two types of cell growth. Two of the eight clones contained cells with a similar size and phenotype, which were identified as myeloblasts by May Grünwald-Giemsa staining. Addition of GM-CSF to the wells confirmed the nature of these cells as they were able to differentiate into mature granulocytes in 3 to 4 days. Six of the eight clones contained heterogenous populations of cells of various size, which were a mixture of mature megakaryocytes and erythrocytes with or without myeloblasts.
Assessment of the putative role of growth factors secreted by accessory cells in the formation of colonies obtained in methylcellulose in the presence of TPO alone. Correlation between the number of CFU-GEMM progenitors and the number of hematopoietic cells cocultured on GpE86-Mpl-R cells (n = 2).
Assessment of the putative role of growth factors secreted by accessory cells in the formation of colonies obtained in methylcellulose in the presence of TPO alone. Correlation between the number of CFU-GEMM progenitors and the number of hematopoietic cells cocultured on GpE86-Mpl-R cells (n = 2).
Limiting dilution experiments were also performed with Mpl-R–infected Lin− cells collected after the 7 days of r-TPO stimulation in liquid culture. Of 864 wells analyzed, 61 (7%) were positive for cell proliferation, suggesting that an amplification and/or a selection of infected cells had occurred during incubation with r-TPO. Cytospin and May Grünwald-Giemsa staining revealed the same types of growth as in cell cloning performed directly after the cocultures. Fifty percent of the positive wells contained only myeloblasts, while the others contained megakaryocytes and erythrocytes with or without myeloblasts.
These data suggest that r-TPO is able to induce the terminal differentiation of multipotent Mpl-R–infected progenitor cells toward the erythrocytic and megakaryocytic lineages. However, in the granulocytic lineage of differentiation, r-TPO was unable to promote the terminal steps of maturation as cells accumulate at the myeloblast stage. Their subsequent maturation can be induced by GM-CSF.
In vivo effects of Mpl-R–transduction on pluripotent hematopoietic progenitor cells.The effects of the forced expression of Mpl-R in pluripotent progenitor cells were further studied in mice using two types of assays. First, the number and nature of progenitor cells contained in day-12 individual spleen colonies (CFU-S12 ) were analyzed to determine whether an overexpression of Mpl-R would influence the lineage commitment of their progeny. Second, hematopoiesis of irradiated mice reconstituted with infected long-term repopulating cells was studied to evaluate the differentiation capacities of more primitive infected progenitors.
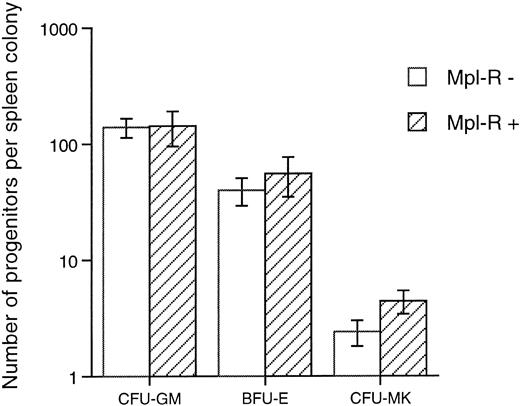
Clonogenic progenitors content of spleen colonies generated by Mpl-R–infected bone marrow cells.Hematopoietic cells cocultured on GpE86-Mpl-R producing cells were injected into lethally irradiated syngeneic recipients. Twelve days postgrafting, individual spleen colonies were isolated. A fraction (1 × 104 cells) was used for PCR analysis; the remaining cells were assessed for their progenitor content as described in the Materials and Methods. Of the 46 colonies studied, 16 were positive for the retroviral transgene. As illustrated in Fig 6, the mean content in CFU-GM, BFU-E + CFU-GEMM, and CFU-MK progenitor cells was not significantly different whether colonies were Mpl-R–infected or not. These data indicate that transduction of Mpl-R in progenitors generating day-12 CFU-S does not significantly modify their commitment capability.
Mean number of progenitors present in individual spleen colonies generated by hematopoietic cells cocultured on GpE86-Mpl-R cells. Cells from each individual spleen colony were grown in methylcellulose in the presence of IL-3 + EPO (CFU-GM and BFU-E) and in agar in the presence of TPO (CFU-MK). Each individual colony was tested for retroviral integration by PCR technique and was determined as negative (Mpl-R−, n = 30) or positive (Mpl-R+, n = 16).
Mean number of progenitors present in individual spleen colonies generated by hematopoietic cells cocultured on GpE86-Mpl-R cells. Cells from each individual spleen colony were grown in methylcellulose in the presence of IL-3 + EPO (CFU-GM and BFU-E) and in agar in the presence of TPO (CFU-MK). Each individual colony was tested for retroviral integration by PCR technique and was determined as negative (Mpl-R−, n = 30) or positive (Mpl-R+, n = 16).
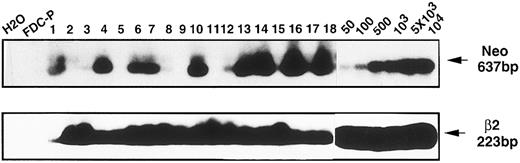
Long-term hematologic reconstitution of mice grafted with Mpl-R–infected cells.The behavior of long-term repopulating pluripotent cells was examined in mice hematologically reconstituted with Neo- or Mpl-R–infected cells, 1 and 7 months after the graft. To test for retroviral integration, semi-quantitative PCR analysis was performed on the mature nucleated blood cells. Mice were considered to be positive when the signal obtained with 50 μL of blood was equal or superior to the signal of samples containing 5% of blood from Neo-transgenic mice (Fig 7). One month after the graft, 21 of 39 (54%) and 10 of 22 (45%) mice were positive for the Mpl-R or Neo transgenes, respectively. Seven months after reconstitution, the number of Mpl-R–positive mice was 16 of 57 (28%) and 13 of 30 (43%) for the Neo transgene.
Presence of transduced Mpl-R cDNA in hematologically reconstituted animals. Examples of PCR analyses performed on blood cells harvested 1 or 7 months after the graft of hematopoietic cells cocultured either on GpE86-Neo or GpE86-Mpl-R cells. Control samples were a mixture of blood cells from normal and Neo transgenic mice (50, 100, 500, 103, 5 × 103 or 104 cells from Neo transgenic mice).
Presence of transduced Mpl-R cDNA in hematologically reconstituted animals. Examples of PCR analyses performed on blood cells harvested 1 or 7 months after the graft of hematopoietic cells cocultured either on GpE86-Neo or GpE86-Mpl-R cells. Control samples were a mixture of blood cells from normal and Neo transgenic mice (50, 100, 500, 103, 5 × 103 or 104 cells from Neo transgenic mice).
Blood parameters of Mpl-R–positive mice (10 positive mice at 1 month and 8 mice at 7 months) were compared with that of Neo-positive mice (10 mice at 1 month and 7 mice at 7 months). No significant difference in the number of nucleated and red blood cells, hematocrit, level of hemoglobin, and mean red cell corpuscular volume was seen in mice reconstituted with Mpl-R– or Neo-infected progenitors or whether these parameters were analyzed 1 or 7 months after the graft (data not shown). The only significant difference observed was the mean number of circulating platelets, which was statistically lower in mice reconstituted with Mpl-R–infected cells at 7 months posttransplantation (347 ± 65 × 103/mL, n = 8) when compared with mice reconstituted with Neo-infected cells (657 ± 59 × 103/mL, n = 13).
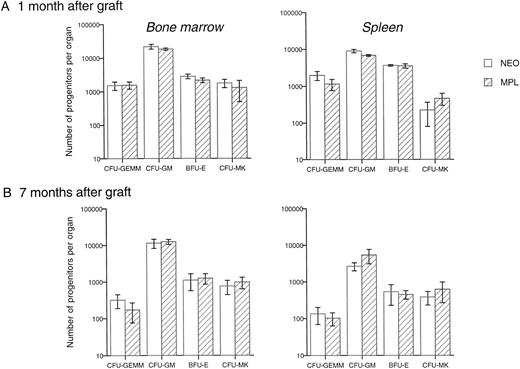
The number of various types of progenitors was also investigated in the bone marrow and the spleen. No significant difference was found (Fig 8), although between 12% and 20% of G418-resistant progenitors were present in the bone marrow and spleen, 1 or 7 months after the graft. These results indicate that the differentiation ability of long-term repopulating stem cells transduced with Mpl-R was not modified.
Transduction of Mpl-R does not modify the progenitor number in reconstituted animals. Mean number of clonogenic progenitors per leg or spleen, grown in methylcellulose in the presence of IL-3 + EPO or in agar in the presence of TPO, one (6 Neo- and 6 Mpl-R-reconstituted mice were studied) and 7 months after the graft (n = 6 for each group) of hematopoietic cells cocultured either on GpE86-Neo (NEO) or on GpE86-Mpl-R (Mpl-R) cells.
Transduction of Mpl-R does not modify the progenitor number in reconstituted animals. Mean number of clonogenic progenitors per leg or spleen, grown in methylcellulose in the presence of IL-3 + EPO or in agar in the presence of TPO, one (6 Neo- and 6 Mpl-R-reconstituted mice were studied) and 7 months after the graft (n = 6 for each group) of hematopoietic cells cocultured either on GpE86-Neo (NEO) or on GpE86-Mpl-R (Mpl-R) cells.
DISCUSSION
Mpl-R expression was initially reported to be restricted to CD34+ cells and relatively mature cells from the megakaryocyte lineage.4 More recently, it was demonstrated that thrombopoietin, the ligand for the Mpl receptor, acts synergistically with stem cell factor and other early acting cytokines in supporting the proliferation of primitive hematopoietic progenitors of mice.18 19 These results suggested that primitive progenitors express Mpl-R and that Mpl-R could play a role in the regulation of stem cell renewal and differentiation. Using retroviral-mediated gene transfer, we forced Mpl-R expression in hematopoietic cells and studied the effects of r-TPO stimulation on Mpl-R–expressing hematopoietic progenitor cells.
Our results demonstrated that several types of Mpl-R–expressing progenitors became capable of proliferation in response to TPO. First, when Mpl-R–infected bone marrow cells were plated in methylcellulose with TPO as the sole stimulus, CFU-GEMM, BFU-E, and CFU-MK but not CFU-GM colonies were observed. Second, the number of CFU-GEMM, BFU-E, and CFU-MK, but not CFU-GM colonies, was significantly increased after 7 days of TPO stimulation in liquid culture. These results indicate that Mpl-R expression efficiently induces proliferation and terminal differentiation of a large variety of progenitor cells. In contrast, although cells transduced with Mpl-R formed CFU-GM colonies in cultures stimulated with IL-3 + EPO, this was not observed in cultures stimulated with TPO. In methylcellulose assay, it was difficult to determine whether the lack of CFU-GM growth was due to an inability of TPO to induce signals for the survival and the first mitoses of these progenitor cells, to promote their terminal differentiation, or both. Indeed, in semi-solid assays, colonies are observed only when a progenitor cell is able to differentiate into mature cells. In contrast, the limiting dilution experiments are more informative as any proliferation from a single cell is detectable. When Mpl-R–infected cells were cloned at one cell per well in the presence of TPO alone, two types of clones were observed. One type contained more than 500 myeloblasts characterized by May Grünwald-Giemsa staining. The myeloblastic nature of these cells was further confirmed by the addition of GM-CSF to the culture medium. Within 4 to 7 days, all cells were induced to terminal granulocytic maturation. These data strongly suggest that TPO was able to induce the initial proliferation and differentiation of CFU-GM to myeloblasts but was unable to induce the terminal differentiation from the myeloblast stage to the fully mature neutrophils. Although not formally demonstrated in our experiments, the observation that CFU-GM could proliferate in response to TPO further strengthened the assumption that the retroviral-encoded Mpl-R is expressed on the surface of CFU-GM progenitor cells. It seems unlikely that the known molecules involved in the signal transduction pathways activated in response to TPO, GM-CSF, or G-CSF are responsible for the block in granulocytic differentiation as these growth factors activate Jak1 or Jak229-33 and Stat 3 and/or Stat 5.34 35
It is noteworthy that in methylcellulose assays performed in the presence of TPO we observed CFU-GEMM–derived colonies that contained mature neutrophils associated with macrophages, erythrocytes, and megakaryocytes. In the culture, accessory cells and/or megakaryocytes may produce cytokines promoting the maturation of polynuclear cells.36 37
In addition, other clones contained mature erythrocytes, and polyploid megakaryocytes with or without myeloblasts. Thus, at a single cell level, Mpl-R activation is sufficient to induce terminal differentiation of both the erythroid and megakaryocyte lineages. Indeed, full erythroid maturation was dependent on TPO, and not on exogenously EPO, as is shown by our clonogenic assays performed in the presence of anti-EPO neutralizing antibodies. These data demonstrate that the ectopic expression of the Mpl-R replaces the Epo-R.
To better evaluate whether the constitutive expression of Mpl-R would modify the fate of multipotential progenitors, we used in vivo assays. The progenitor cell content of 12-day spleen colonies (CFU-S12 ) generated by Mpl-R–infected versus noninfected progenitors, and the hematopoiesis of lethally irradiated mice repopulated with either Neo- or Mpl-R–infected bone marrow cells were analyzed. By the mean number of erythroid, megakaryocytic, or granulo-macrophagic progenitors that were not significantly different in Mpl-R–transduced or untransduced colonies, we conclude that forced expression of Mpl-R does not modify the differentiation fates of the primitive progenitors giving rise to CFU-S12 . When the hematopoietic parameters of animals repopulated with Neo- or Mpl-R–infected cells were compared at 1 or 7 months posttransplant, no significant difference was observed, except for the number of circulating platelets, which was 50% decreased in Mpl-R–reconstituted mice at 7 months following the graft. The regulation of TPO production is not fully established. Several reports have shown that TPO production is not regulated at a transcriptional level in the two major producing organs, namely the liver and the kidneys,38-45 but could be regulated by the platelet mass in the bone marrow.46 Two hypotheses may explain the thrombocytopenia observed in mice reconstituted with Mpl-R–transduced stem cells. The unregulated expression of Mpl-R on various cell types might decrease the plasma TPO level and/or mice may develop antibodies directed against the Mpl-R protein binding site, as in our study in which a human c-mpl cDNA was used. Although we do not have an assay sensitive enough to measure murine TPO plasma levels, we favor the first hypothesis. Indeed, when plasma from our thrombocytopenic mice was tested on the BaF/hu-Mpl-R proliferation assay, no decrease in the levels of thymidine incorporation could be demonstrated, suggesting an absence of antibodies interfering with the hu-TPO binding site (data not shown).
Finally, our results also show that the absolute and relative numbers of progenitors were similar in the bone marrow and spleen of animals grafted with Neo- or Mpl-R–infected cells, whether analyses were performed 1 or 7 months after the graft. It is possible that the low circulating level of murine TPO was not sufficient to activate the human Mpl receptor expressed by the murine hematopoietic progenitors; this explains the absence of modification in the orientation of the differentiation of Mpl-R expressing pluripotent progenitors. However, our results indicate that Mpl-R expression does not modify the orientation of pluripotent stem cells.
Our data show that, when Mpl-R expression is forced by a retroviral infection, TPO induces the proliferation and differentiation of progenitors from several lineages, with the exception of the terminal granulocytic maturation. However, TPO does not induce a preferential differentiation of pluripotent progenitors toward megakaryocytopoiesis. These results are similar to those we47 and McArthur et al48 obtained after bone marrow infection with a retrovirus carrying the EPO-R cDNA. Multipotent progenitors expressing EPO-R were able to proliferate in response to EPO and did not preferentially differentiate toward erythropoiesis. The fact that the effects of Mpl-R and EPO-R constitutive expression in multipotent progenitors are similar is not surprising because, while the distribution of the expression is different from EPO-R and Mpl-R, these receptors belong to the same family, share important homology, and induce activation of the same type of molecules after activation by their respective factors. These results also reinforce the idea that expression of a lineage-specific receptor at the surface of pluripotent progenitors does not modify the orientation of their differentiation.
ACKNOWLEDGMENT
We thank P. Ardouin and A. Rouches for taking care of our animals. We are grateful to Eric Kremer for helpful discussions and critical reading of the manuscript.
Supported by the Association pour la recherche contre le cancer (contrat no. 6277); F.G. is a student of Institut de formation supérieure biomédicale (IFSBM).
Address reprint requests to Dominique Duménil, PhD, INSERM U362, Institut Gustave Roussy, Laboratoire Hématopoı̈èse et Cellules Souches, 39 rue Camille Desmoulins, 94805 Villejuif, France.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal