Abstract
A major obstacle to stem cell gene therapy is the extremely low efficiency of stem cell transduction. In vivo selection is a strategy for enriching a minor population of genetically modified bone marrow cells through the introduction of a drug resistance gene, followed by subsequent administration of the corresponding cytotoxic drug in vivo. Achieving persistent effects from in vivo selection is expected to require selection at the level of stem cells or, minimally, selection at the level of progenitors. Major limitations to in vivo selection are the nonhematologic toxicities of the cytotoxic drugs used and the resistance of stem cells and progenitors to killing by most cytotoxic agents. Experiments were performed in mice to evaluate whether the drugs used for selection in combination with multiple drug resistance gene 1 (MDR1) could have an enhanced effect on clonogenic progenitors if preceded by administration of the cytokine, stem cell factor (SCF). Single doses of taxol, navelbine, or vinblastine produced 10-fold reductions in the total number of mononuclear cells per femur, indicating a significant depletion of nonclonogenic precursor cells. However, for each of these agents, clonogenic progenitors, assayed as colony-forming unit cells and day-12 spleen colony-forming units, were relatively spared. Administration of SCF before taxol, navelbine, or vinblastine completely abrogated the progenitor-sparing phenomenon, because clonogenic progenitors were depleted as effectively as nonclonogenic precursor cells. Furthermore, the administration of SCF before drug administration allowed the dosages of taxol and vinblastine to be reduced by more than half, while retaining reductions in progenitor numbers that were unachievable using very high doses of the cytotoxic drug alone. Doxorubicin administration resulted in a 30- to 40-fold depletion in progenitors that was not significantly altered by preceding SCF administration. These results suggest that previous observations of in vivo selection using MDR1 gene transfer followed by taxol administration may have resulted from selection at the level of relatively mature, nonclonogenic precursor cells. Furthermore, these data suggest that cytokine prestimulation may be a useful strategy for improving the selection of drug-resistant clonogenic progenitors and, possibly, stem cells in vivo.
INEFFICIENT DNA TRANSFER into the human hematopoietic stem cell presents one of the most difficult obstacles confronting stem cell gene therapy. Recent reports from gene-marking trials suggest that far fewer than 1% of stem cells incorporate the marker gene.1,2 The development of therapeutic applications for stem cell gene transfer hinges on markedly increasing the proportion of genetically corrected stem cells. One strategy for increasing the frequency of modified stem cells is to specifically direct their preferential expansion through a process termed in vivo selection.3 In vivo selection is achieved through the construction of vectors that link the nonselectable therapeutic gene to a second gene conferring drug resistance. After the introduction of these genes into a small population of stem cells, selective pressure is applied through the administration of the corresponding cytotoxic drug in vivo. The success of this strategy requires that the cytotoxic drug exert a proportionally greater toxic effect on the population of unmodified bone marrow (BM) cells relative to their transduced, drug-resistant counterparts. Studies of this approach using the dihydrofolate reductase (DHFR) gene or the multiple drug resistance 1 (MDR1) gene in mice (1) consistently show a significant reduction in drug-induced myelosuppression and lethality4-8 and (2) commonly show an increase in BM cells containing the drug resistance gene after in vivo drug administration.6,8,9 However, permanent increases in the frequency of genetically modified BM cells would require that selection be applied at the level of the hematopoietic stem cell; evidence for selection at this level is limited.6
Significant limitations to the success of in vivo selection are the nonhematologic toxicities of the cytotoxic drugs used for selection and the resistance of hematopoietic stem cells and progenitors to killing by most cytotoxic drugs. The cytokine stem cell factor (SCF ) promotes the redistribution and expansion of early hemopoietic cell populations in vivo.10-15 Experiments by Dexter and colleagues have shown that the preceding administration of SCF markedly accentuates the BM toxicity of 5-fluorouracil.16 Cytokine prestimulation of BM before cytotoxic drug administration has two theoretical advantages for in vivo selection. First, cytokine prestimulation may increase the disparity between the hematologic and nonhematologic toxicities of the cytotoxic drug. Second, cytokines that promote the proliferation of stem cells may render this population susceptible to cytotoxic drug selection. Studies were performed to evaluate the hematologic effects of preceding SCF administration in combination with taxol, navelbine, vinblastine and doxorubicin, drugs to which MDR1 gene transfer confers cellular resistance.17
MATERIALS AND METHODS
Mice.Female B6D2F1 mice (Jackson Labs, Bar Harbor, ME) were housed at the University of Washington Animal Care Facility. Mice were 8 to 10 weeks old and weighed approximately 20 g each. Pegylated recombinant rat SCF (a gift from Amgen, Thousand Oaks, CA) was diluted in phosphate-buffered saline (PBS) with 0.1% bovine serum albumin to a concentration of 5 μg/mL and administered at a dose of 1 mg/injection (approximately 50 mg/kg/injection). Taxol (paclitaxel; Bristol-Myers Squibb Co, Princeton, NJ) was diluted in normal saline to a concentration of 1.2 mg/mL. One milliliter was injected intraperitoneally for a total dose of 1.2 mg (approximately 60 mg/kg/dose). Vinblastine was purchased as a lyophilized powder (Chiron, Emeryville, CA) and diluted to 1 mg/mL in normal saline, and 200 μL was injected via tail vein for a dose of approximately 10 mg/kg. Navelbine (vinorelbine tartrate; Burroughs Wellcome, Research Triangle Park, NC) was diluted in PBS to a concentration of 1 mg/mL and injected via tail vein in a 200-μL volume for a dose of 0.2 mg (approximately 10 mg/kg). Doxorubicin (Chiron), purchased as a lyophilized powder, was diluted in PBS to a concentration of 1.75 mg/mL and injected via tail vein in a 200-μL volume for a total dose of 0.35 mg (approximately 17.5 mg/kg). Two mice were used for each experimental variable. All experiments were performed at least twice except as indicated. Anesthetized mice were killed by cervical dislocation; the spleen and hind limb femurs were transferred to ice-cold PBS containing 2.5% defined calf serum, 0.1% glucose, 50 U/mL penicillin, and 50 mg/mL streptomycin (PBS-2.5% DCS) until culture.
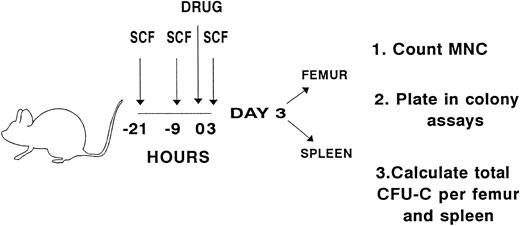
Experimental design for evaluating whether preceding exposure to pegylated, recombinant rat SCF enhances the hematologic effects of taxol, navelbine, vinblastine, or doxorubicin. SCF (50 mg/kg/injection) was administered to B6D2F1 mice subcutaneously every 12 hours for a total of three doses. Three hours before the last dose of SCF, a single intravenous injection of navelbine, vinblastine, or doxorubicin or an intraperitoneal dose of taxol, was administered. Dosages are described in Materials and Methods. Pairs of mice received either cytotoxic drug alone, SCF alone, SCF plus cytotoxic drug, or no treatment. The last dose of SCF was administered on the morning of day 1, and analysis was performed on day 3. Femurs and spleens were separately pooled for each experimental variable. Mononuclear cells (MNCs) were counted and plated in semisolid media. Colonies were scored on day 8 of culture, and the number of CFU-C per femur and spleen was calculated.
Experimental design for evaluating whether preceding exposure to pegylated, recombinant rat SCF enhances the hematologic effects of taxol, navelbine, vinblastine, or doxorubicin. SCF (50 mg/kg/injection) was administered to B6D2F1 mice subcutaneously every 12 hours for a total of three doses. Three hours before the last dose of SCF, a single intravenous injection of navelbine, vinblastine, or doxorubicin or an intraperitoneal dose of taxol, was administered. Dosages are described in Materials and Methods. Pairs of mice received either cytotoxic drug alone, SCF alone, SCF plus cytotoxic drug, or no treatment. The last dose of SCF was administered on the morning of day 1, and analysis was performed on day 3. Femurs and spleens were separately pooled for each experimental variable. Mononuclear cells (MNCs) were counted and plated in semisolid media. Colonies were scored on day 8 of culture, and the number of CFU-C per femur and spleen was calculated.
Culture assay.Spleens and femurs were pooled separately for each experimental variable. BM was gently flushed from the femurs by puncturing either end with an 18-gauge needle, then introducing a sterile 25-gauge needle, and flushing with 2 mL of ice cold PBS-2.5% DCS. Flushing was repeated on either end of the femur until it was macroscopically free of BM cells. Spleen cells were obtained by gentle disaggregation using two 23-gauge needles until the capsule was macroscopically free of cells. Cell suspensions were washed twice with 2 mL of ice-cold PBS-2.5% DCS and resuspended in 1 mL PBS-2.5% DCS. Cells were counted in Turk's solution, diluted to appropriate concentrations in PBS-2.5% DCS, and maintained on ice until culture. Spleen cells were plated at a concentration of 1 × 106 cells/mL, and BM cells were plated at 1 × 105 cells/mL. For some experiments, additional higher plating concentrations were used (spleen, 5 × 106 cells/mL; BM, 5 × 105 cells/mL). All cultures contained the following components: 30% fetal calf serum, 1% bovine serum albumin, 5 × 10−4 mol/L 2-mercaptoethanol (Sigma, St Louis, MO), 5 U/mL recombinant human erythropoietin (Genetics Institute, Cambridge, MA), 10% WEHI-3–conditioned medium, 5% pokeweed mitogen spleen cell-conditioned medium, 50 ng/mL recombinant rat SCF (Amgen), 5 U/mL recombinant murine interleukin-3 (Genzyme, Cambridge, MA), and 1% methylcellulose (Fisher Scientific, Pittsburgh, PA). Cultures were plated in triplicate and incubated in a highly humidified 37°C incubator flushed continuously with 5% CO2 . Colonies were evaluated on day 8.
Day-12 spleen colony-forming unit (CFU-S) assay.B6D2F1 mice were irradiated with 850 cGy, a dose that was determined to consistently prevent the formation of endogenous day-12 CFU-S in control animals; 6 to 10 animals were used for each experimental variable. After irradiation, B6D2F1 donor BM cells were injected via tail vein at doses previously determined to be optimal for the quantitation of day-12 CFU-S. Control animals were irradiated at the same time but did not receive cells to ensure that endogenous day-12 CFU-S did not form. After irradiation, mice were housed in isolator cages and were provided with food and drinking water supplemented with neomycin sulfate (1.1 g/10 mL of water) and polymyxin (1 million U/10 mL of water; both from Sigma). Twelve days after the injection of cells, mice were killed by cervical dislocation; spleens were removed and placed in Bouin's solution (15 parts picric acid, 5 parts formaldehyde, and 1 part glacial acetic acid; all from Sigma) for 10 minutes, then rinsed in 10% formaldehyde. Macroscopic colonies were counted.
RESULTS
Studies were performed to evaluate the hematologic effects of single large doses of taxol, navelbine, vinblastine, or doxorubicin in mice, either alone or after SCF administration. Clonogenic progenitors were assayed as colony-forming unit cells (CFU-C) and day-12 CFU-S. We evaluated effects at the level of clonogenic progenitors based on the following assumption: if minimal drug-mediated killing were observed at the level of progenitors, it is highly unlikely that significant killing would occur at the level of stem cells. However, conditions that produce significant killing at the level of progenitors would require further study to evaluate their effect at the level of the stem cell. Pairs of mice were treated with one of the following regimens (Fig 1): (1) cytotoxic drug alone; (2) pegylated recombinant rat SCF, 50 μg/kg subcutaneously every 12 hours, for 3 doses; (3) SCF as described in regimen no. 2 but with the drug administered 3 hours before the last dose of SCF; or (4) no treatment. On day 3, progenitors were quantitated in the femur and spleen as CFU-C and as day-12 CFU-S.
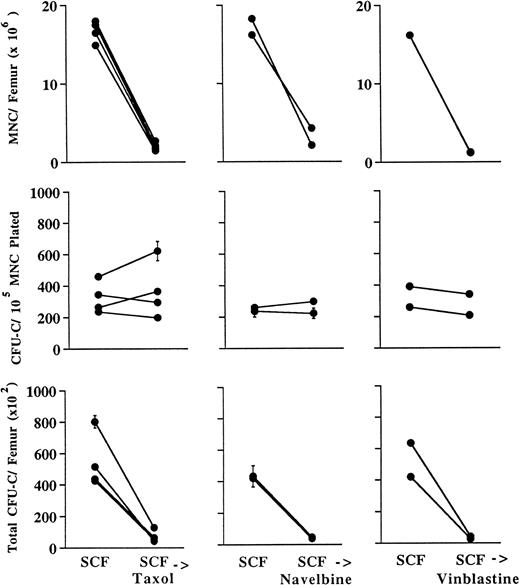
Taxol, navelbine, and vinblastine exert their maximal hematologic effects at the level of the nonclonogenic precursor compartment.Taxol, navelbine, and vinblastine bind to distinct sites on tubulin and act to impair normal microtubule function.18 Mice were treated with either intraperitoneal injections of taxol (60 mg/kg) or intravenous injections of navelbine or vinblastine (both 10 mg/kg). Drug doses used either represented the highest dose attainable based on maximal drug concentration and volume (taxol) or were approximately equivalent to the lethal dose for 10% of animals (LD10) based on available information provided by the manufacturer (navelbine and vinblastine). As shown in Fig 2, these agents appear to exert similar types of effects on BM. Both taxol and navelbine reduced the total number of mononuclear cells (MNCs) per femur by 5.6- to 7.6-fold, whereas vinblastine reduced the MNCs per femur by 10.5- to 12.7-fold (Fig 2, top panels). However, as shown in the middle panels of Fig 2, the plating efficiency of MNCs obtained from the femurs of drug-treated mice was consistently increased compared with that of controls, suggesting that clonogenic progenitor cells were killed with a lower efficiency than were nonclonogenic precursor cells. This increase in the plating efficiency of MNCs from drug-exposed animals reflects a relative sparing of clonogenic progenitors. Thus, as shown in the lower panels of Fig 2, total progenitor numbers decreased only 1.5- to 2.5-fold after a single dose of taxol, 2.6- to 2.8-fold after a single dose of navelbine, and about 3.3- to 3.9-fold after a single dose of vinblastine. Progenitor numbers in the spleen were also evaluated. Taxol produced a 1.9- to 2.6-fold reduction; navelbine, a 2- to 2.1-fold reduction; and vinblastine, a 0.8- to 1.6-fold reduction in total progenitors per spleen (data not shown). Persistent effects from in vivo selection would require that the cytotoxic agent kill a large fraction of nontransduced progenitors and stem cells. Therefore, the progenitor-sparing properties of taxol, navelbine, and vinblastine impose a significant limitation on the value of these drugs for in vivo selection.
Hematologic effects in the femur 48 hours after a single intraperitoneal dose of taxol (60 mg/kg) or intravenous doses of navelbine or vinblastine (both 10 mg/kg). CTL, control mice receiving no drug. Upper panels, total MNCs per femur; middle panels, clonogenic progenitors (CFU-C) per 105 MNCs plated; lower panels, total clonogenic progenitors per femur. Cultures were plated in triplicate; error bars in middle and lower panels denote standard deviations. In the middle panel, note that MNCs obtained from drugtreated animals are enriched for progenitors, reflecting inefficiency in the depletion of progenitors relative to nonclonogenic MNC.
Hematologic effects in the femur 48 hours after a single intraperitoneal dose of taxol (60 mg/kg) or intravenous doses of navelbine or vinblastine (both 10 mg/kg). CTL, control mice receiving no drug. Upper panels, total MNCs per femur; middle panels, clonogenic progenitors (CFU-C) per 105 MNCs plated; lower panels, total clonogenic progenitors per femur. Cultures were plated in triplicate; error bars in middle and lower panels denote standard deviations. In the middle panel, note that MNCs obtained from drugtreated animals are enriched for progenitors, reflecting inefficiency in the depletion of progenitors relative to nonclonogenic MNC.
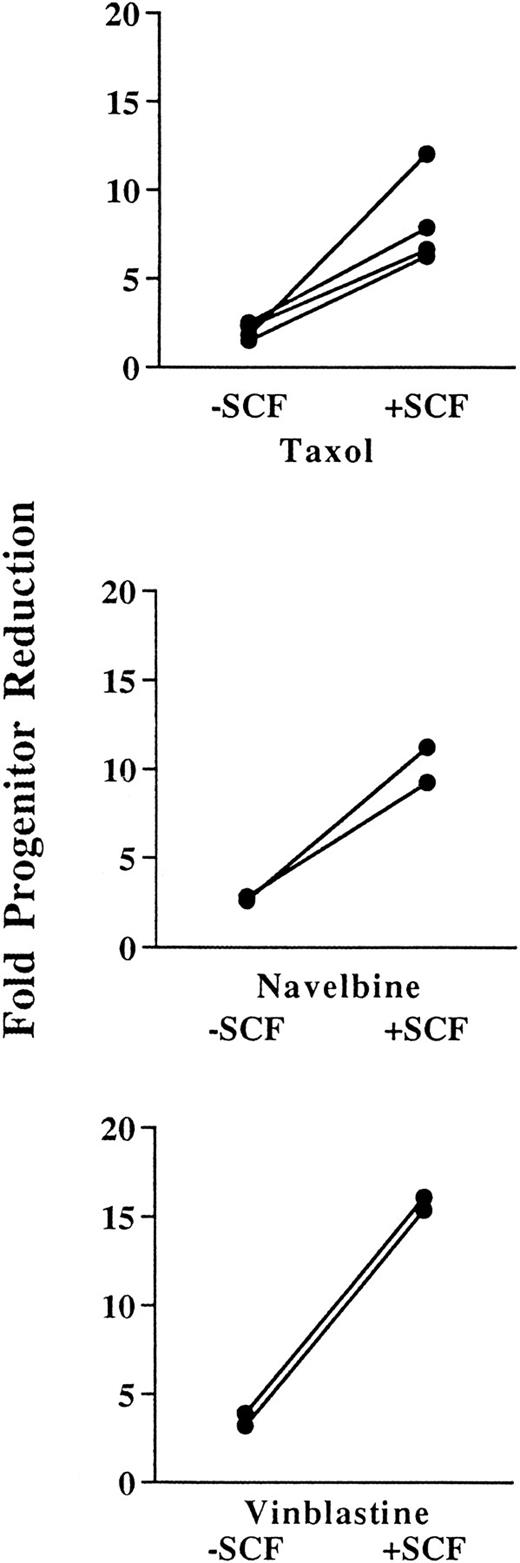
SCF administration sensitizes progenitors to killing by taxol, navelbine, and vinblastine.The effects of preceding SCF administration are shown in Fig 3. SCF in combination with taxol, navelbine, and vinblastine produced 6.6- to 10.3-fold, 3.8- to 8.7-fold, and 6.6- to 10.3-fold reductions in BM MNCs, respectively, as compared with the values for animals receiving SCF alone. These results were not significantly different from those observed in the absence of preceding SCF administration. However, in contrast to results obtained in the absence of SCF, the plating efficiency of the MNCs from chemotherapy-treated animals was approximately the same as that for control animals treated with SCF alone (Fig 3, middle panels). Therefore, preceding SCF administration abrogates the progenitor-sparing effect observed with taxol, navelbine, and vinblastine. As expected, this led to greater reductions in total numbers of progenitors per femur than were observed using cytotoxic drug alone (Fig 3, lower panels). The number of progenitors per femur in control animals divided by the number of progenitors in drug-treated animals reflects the efficiency of progenitor depletion and is described as a “fold reduction” (Fig 4). The fold reduction index reflects the proportion of progenitors killed and is useful for the identification of maneuvers to augment the proportion of drug-resistant progenitors. As shown in the upper panel of Fig 4, taxol alone resulted in an approximate twofold reduction in total progenitor numbers, whereas SCF in combination with taxol reduced total progenitors by sixfold to 12-fold (P < .05). Navelbine produced an approximately threefold reduction in total progenitors, whereas SCF plus navelbine produced a ninefold to 11-fold reduction in total progenitors (P < .1). Vinblastine alone produced a threefold to fourfold reduction in total progenitors, whereas SCF preceding vinblastine resulted in a 15- to 16-fold reduction in total progenitor numbers (P < .01). Thus, preceding SCF administration augments the progenitor-depleting effects of each of these agents by threefold to sixfold in the femur. Preceding SCF administration also augmented cytotoxic drug-mediated progenitor depletion in the spleen. Compared with mice receiving SCF alone, the combination of SCF and taxol produced a 3.3- to 4.5-fold reduction; SCF and navelbine, a 3- to 4.4-fold reduction; and SCF plus vinblastine, a 2.6- to 2.9-fold reduction in total progenitors per spleen (data not shown).
Hematologic effects in the femur 48 hours following SCF in combination with a single intraperitoneal dose of taxol (60 mg/kg) or intravenous doses of navelbine or vinblastine (both 10 mg/kg) as shown in Fig 1. SCF, control mice receiving SCF alone. Upper panels, total MNCs per femur; middle panels, clonogenic progenitors (CFU-C) per 105 MNCs plated; lower panels, total clonogenic progenitors per femur. Cultures were plated in triplicate; error bars in middle and lower panels denote standard deviations. Comparison of the middle panel with Fig 2 shows that sparing of progenitors is markedly reduced or abrogated by preceding SCF administration.
Hematologic effects in the femur 48 hours following SCF in combination with a single intraperitoneal dose of taxol (60 mg/kg) or intravenous doses of navelbine or vinblastine (both 10 mg/kg) as shown in Fig 1. SCF, control mice receiving SCF alone. Upper panels, total MNCs per femur; middle panels, clonogenic progenitors (CFU-C) per 105 MNCs plated; lower panels, total clonogenic progenitors per femur. Cultures were plated in triplicate; error bars in middle and lower panels denote standard deviations. Comparison of the middle panel with Fig 2 shows that sparing of progenitors is markedly reduced or abrogated by preceding SCF administration.
Fold progenitor reduction in the absence (−) or presence (+) of preceding SCF administration for taxol, navelbine, and vinblastine. The fold progenitor reduction was determined by the number of progenitors per femur in control animals divided by the number of progenitors per femur in cytotoxic drug-treated animals. Note that for each drug, preceding SCF administration augments progenitor depletion threefold to sixfold. Using a two-tailed Student's t-test, these results are statistically significant for taxol at P < .05, for navelbine at P < .1, and for vinblastine at P < .005.
Fold progenitor reduction in the absence (−) or presence (+) of preceding SCF administration for taxol, navelbine, and vinblastine. The fold progenitor reduction was determined by the number of progenitors per femur in control animals divided by the number of progenitors per femur in cytotoxic drug-treated animals. Note that for each drug, preceding SCF administration augments progenitor depletion threefold to sixfold. Using a two-tailed Student's t-test, these results are statistically significant for taxol at P < .05, for navelbine at P < .1, and for vinblastine at P < .005.
Doxorubicin administration has a marked effect on progenitor killing that is not augmented by preceding SCF administration.The anthracyclines represent a second class of chemotherapeutic drugs to which overexpression of MDR1 confers cellular resistance.19 Anthracycline-mediated cell killing occurs through free radical formation or topoisomerase-II–dependent DNA damage.20
A dose of doxorubicin equivalent to the LD10 (information from manufacturer), 17.5 mg/kg, was administered as a single intravenous injection, and analysis was performed as described above. Results after the administration of doxorubicin alone are shown in Fig 5A. As observed with taxol, navelbine, and vinblastine, a single dose of doxorubicin produced an approximately 10-fold reduction in MNCs per femur. However, in marked contrast to these other agents, there was a threefold to fourfold decrease in the plating efficiency of doxorubicin-exposed BM MNCs (Fig 5A, middle panel), suggesting a preferential killing of BM progenitors relative to precursors. A decrease in BM MNCs combined with a decrease in their plating efficiency resulted in a marked, 30- to 40-fold reduction in total progenitors per femur, as shown on the left of Fig 5C. As shown in Fig 5B, SCF administration before doxorubicin administration produces results similar to those obtained with doxorubicin alone. Thus, as shown in Fig 5C, preceding SCF administration does not augment the marked effect on progenitor killing exerted by doxorubicin alone. Similarly severe reductions in progenitor numbers occurred in the spleen; however, in contrast to femur progenitors, preceding SCF administration improved doxorubicin-mediated progenitor depletion in the spleen by 1.8- to 3.5-fold (data not shown).
Hematologic effects in the femur 48 hours after the administration of a single intravenous dose of doxorubicin (doxo; 17.5 mg/kg). See legend to Fig 2 for key to other abbreviations. (A) Effects of doxorubicin alone. In the middle panel, note that doxorubicin depletes clonogenic progenitors as efficiently as nonclonogenic BM MNCs, leading to a profound reduction in total progenitor numbers as shown in the lower panel. (B) Preceding SCF administration does not significantly alter doxorubicin-mediated BM toxicity. The inability of SCF to significantly augment doxorubicin's effect on progenitors is shown in (C). Error bars in (A) and (B) middle and lower panels designate standard deviations.
Hematologic effects in the femur 48 hours after the administration of a single intravenous dose of doxorubicin (doxo; 17.5 mg/kg). See legend to Fig 2 for key to other abbreviations. (A) Effects of doxorubicin alone. In the middle panel, note that doxorubicin depletes clonogenic progenitors as efficiently as nonclonogenic BM MNCs, leading to a profound reduction in total progenitor numbers as shown in the lower panel. (B) Preceding SCF administration does not significantly alter doxorubicin-mediated BM toxicity. The inability of SCF to significantly augment doxorubicin's effect on progenitors is shown in (C). Error bars in (A) and (B) middle and lower panels designate standard deviations.
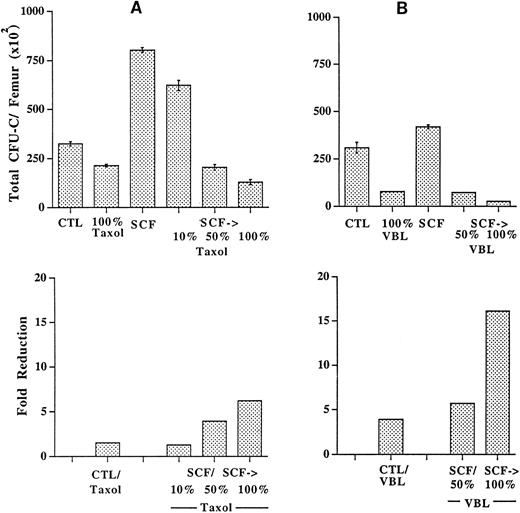
SCF prestimulation permits significant reductions in the dosages of drugs used for in vivo selection.Nonhematologic toxicities markedly reduce the value of cytotoxic agents for in vivo selection. The frequency and severity of most nonhematologic toxicities are directly proportional to drug dose. Experiments were performed to determine whether SCF prestimulation could be used to reduce the dose of the cytotoxic drug used for selection. Mice were treated with taxol, 60 mg/kg, either alone or in combination with SCF as described above (Fig 1). Additional pairs of mice received either SCF in combination with a 50% dose of taxol (30 mg/kg) or SCF combined with a 10% dose of taxol (6 mg/kg). As shown in Fig 6A, a 100% (60 mg/kg) dose of taxol alone produced a 1.5-fold reduction in total progenitors per femur, whereas the same dose of taxol after SCF administration resulted in a 6.2-fold reduction. SCF combined with a 10% dose of taxol produced a 1.3-fold reduction in progenitors per femur, whereas SCF plus a 50% dose of taxol produced a fourfold reduction in progenitors per femur. A similar effect was observed on progenitors in the spleen where, in the setting of prior SCF administration, a 50% dose of taxol produced a greater reduction in progenitor numbers (2.3-fold reduction) than was achieved with a 100% dose of taxol alone (1.9-fold reduction; data not shown). These results, reproduced in a second experiment (data not shown) show that SCF prestimulation permits a significant reduction in taxol dose while maintaining an effect on progenitor killing that is unachievable using maximal doses of taxol alone. CFU-C in culture likely represent a more mature subset of progenitors than do cells with the capacity to form spleen colonies 12 days after infusion into irradiated mice.21 As shown in Table 1, in two experiments, similar results as those observed in CFU-C assays were obtained using the day-12 CFU-S assay.
Preceding SCF administration allows significantly reduced doses of taxol (A) and vinblastine (VBL; B) to achieve levels of progenitor depletion that are not reached with very high doses of drug alone. The upper panels show the total clonogenic progenitors per femur. CTL, control animals receiving no drug; SCF, animals receiving SCF alone; SCF→, preceding SCF administration as shown in Fig 1; 100% taxol, 60 mg/kg; 50% taxol, 30 mg/kg; 10% taxol, 6 mg/kg; 100% VBL, 10 mg/kg; 50% VBL, 5 mg/kg. The lower panels show the fold progenitor reduction as determined by dividing the number of progenitors per femur in animals treated with either no drug (CTL) or SCF alone (SCF) by the number of progenitors per femur in animals treated with drug alone or with preceding SCF administration. Note that preceding SCF administration allows the doses of both taxol and vinblastine to be reduced by more than half, while retaining a level of progenitor depletion that cannot be accomplished using maximal doses of drug alone. Error bars indicate standard deviations.
Preceding SCF administration allows significantly reduced doses of taxol (A) and vinblastine (VBL; B) to achieve levels of progenitor depletion that are not reached with very high doses of drug alone. The upper panels show the total clonogenic progenitors per femur. CTL, control animals receiving no drug; SCF, animals receiving SCF alone; SCF→, preceding SCF administration as shown in Fig 1; 100% taxol, 60 mg/kg; 50% taxol, 30 mg/kg; 10% taxol, 6 mg/kg; 100% VBL, 10 mg/kg; 50% VBL, 5 mg/kg. The lower panels show the fold progenitor reduction as determined by dividing the number of progenitors per femur in animals treated with either no drug (CTL) or SCF alone (SCF) by the number of progenitors per femur in animals treated with drug alone or with preceding SCF administration. Note that preceding SCF administration allows the doses of both taxol and vinblastine to be reduced by more than half, while retaining a level of progenitor depletion that cannot be accomplished using maximal doses of drug alone. Error bars indicate standard deviations.
Effects of Cytokine Prestimulation of Day-12 CFU-S
| . | Day-12 CFU-S . | |
|---|---|---|
| . | Experiment 1 . | Experiment 2 . |
| CFU-S per femur (SD) | ||
| CTL | 5,265 (872) | 4,812 (3,224) |
| TAXOL | 4,320 (584) | 2,680 (573) |
| SCF | 19,286 (3,848) | 15,640 (4,497) |
| SCF → 50% TAXOL | 9,065 (1,807) | 6,397 (485) |
| SCF → 100% TAXOL | 5,904 (764) | 3,359 (663) |
| Fold reduction | ||
| CTL/100% TAXOL | 1.2 | 1.8 |
| SCF/SCF → 50% TAXOL | 2.1 | 2.4 |
| SCF/SCF → 100% TAXOL | 3.3 | 4.7 |
| . | Day-12 CFU-S . | |
|---|---|---|
| . | Experiment 1 . | Experiment 2 . |
| CFU-S per femur (SD) | ||
| CTL | 5,265 (872) | 4,812 (3,224) |
| TAXOL | 4,320 (584) | 2,680 (573) |
| SCF | 19,286 (3,848) | 15,640 (4,497) |
| SCF → 50% TAXOL | 9,065 (1,807) | 6,397 (485) |
| SCF → 100% TAXOL | 5,904 (764) | 3,359 (663) |
| Fold reduction | ||
| CTL/100% TAXOL | 1.2 | 1.8 |
| SCF/SCF → 50% TAXOL | 2.1 | 2.4 |
| SCF/SCF → 100% TAXOL | 3.3 | 4.7 |
A similar experiment using vinblastine confirmed that significant reductions in drug dose are achievable using SCF prestimulation (Fig 6B). A 10-mg/kg dose of vinblastine, shown in Fig 6 as 100%, reduced progenitor numbers in the femur by 4.1-fold. SCF combined with a 100% dose of vinblastine produced a 16.1-fold reduction in femur progenitors, whereas SCF combined with a 50% dose of vinblastine produced a 5.7-fold reduction in femur progenitors. In the spleen, SCF plus a 50% dose of vinblastine resulted in a fourfold reduction in progenitors, whereas a 100% dose of vinblastine alone produced only a 1.6-fold reduction in progenitors (data not shown). Thus, SCF prestimulation allowed the dosage of vinblastine to be reduced by 50% while retaining a level of progenitor depletion greater than that achieved with twice the dose of vinblastine alone.
DISCUSSION
The transfer of drug resistance genes into cells followed by drug selection in vitro readily permits the outgrowth of genetically modified populations. Using retroviral cassettes linking a therapeutic gene to a drug resistance gene, Aran et al22 have shown that drug selection permits the in vitro emergence of a population of cells that coexpress the therapeutic gene. However, extending the use of drug resistance genes to selection in vivo faces important obstacles. First, the cytotoxic agents used for selection uniformly show significant nonhematologic toxicities. Second, stem cells are relatively resistant to most cytotoxic drugs, in large part because of their infrequent entry into the cell cycle.23 Results presented here show that taxol, navelbine, and vinblastine each produce substantial reductions in total BM MNCs; however, with each drug, clonogenic progenitors are less severely affected. The relative abundance of cells in the BM is heavily weighted toward relatively mature, nonclonogenic subsets that comprise approximately 90% of all cells.24 Thus, the reduction in BM MNCs after taxol, navelbine, or vinblastine administration reflects a significant depletion of these relatively mature populations. A plausible explanation for the preferential effect of these cytotoxic agents on relatively mature cell subsets is the high proportion of mitotic, nonclonogenic precursor cells in S phase.25 The significant depletion of relatively mature nonclonogenic precursor cells is compatible with previous observations of taxol administration after MDR1 gene transfer.6,7,9 First, the primary mechanism by which a transferred MDR1 gene confers BM resistance to taxol may be through protection at the level of the nonclonogenic precursor compartment. Selective expansion of mitotic, drug-resistant, nonclonogenic precursor cells after taxol treatment could also produce the increase in MDR1-containing BM MNCs that others have observed.6 Thus, most prior observations of in vivo selection using MDR1 gene transfer followed by taxol administration may be explained on the basis of selection at the level of relatively mature, nonclonogenic cell populations. Because mature nonclonogenic precursor cells are short-lived, the persistent enrichment of genetically modified cells is expected to require selection at the level of stem cells.
Our results may at first appear to be in contrast to those obtained by Hanania et al,7 who showed serial transplantability of MDR1-transduced BM. These investigators showed that MDR1-transduced BM from taxol-treated mice could sustain a maximum of six serial transplants, whereas nontransduced BM from mice not treated with taxol could sustain only a total of three serial transplants. This finding was interpreted by the investigators to suggest that taxol was selecting for MDR1-transduced stem cells. We suggest an alternative explanation based on the fact that mice containing MDR1-transduced BM were treated with taxol between transplants, whereas mice transplanted with nontransduced BM were not treated with taxol. Our data show that taxol treatment is a method for enriching for early hematopoietic cells. Because mice in both groups were transplanted with equivalent cell numbers (1 × 106), we suggest that, in fact, mice in the MDR1 group received transplanted cells that were enriched in stem cells and progenitors and, thus, showed an improved survival. Alternatively, transplantation may have stimulated the cycling of early hematopoietic cells, mimicking the results we observed with preceding SCF administration.
Consistent with previous reports,26 our data show that doxorubicin is highly efficient in depleting clonogenic progenitors. However, this effect is not significantly augmented by SCF prestimulation. Furthermore, the significant nonhematologic toxicity of this drug argues forcefully against its use for in vivo selection.
Data presented here suggest that cytokine prestimulation may be a potentially valuable strategy for the in vivo selection of MDR1-transduced progenitors using taxol, navelbine, or vinblastine. The method used to sensitize BM to the effects of chemotherapy came from that described by Molineux et al,16 who showed that the BM toxicity of 5-fluorouracil is markedly enhanced by the preceding administration of SCF. Preferential sensitization of BM to the effects of cytotoxic drugs has potentially important advantages for in vivo selection. First, cytokine prestimulation may permit a reduction in drug dosage, resulting in fewer toxicities outside the BM. Data presented here suggest that dose reductions by greater than half may be achieved. Furthermore, cytokines that promote the cycling of early hematopoietic progenitors and stem cells may render these normally resistant populations susceptible to the selective cytotoxic drugs. Our data indicate that sensitization occurs in the progenitor compartment. Preceding SCF administration resulted in a threefold to sixfold augmentation of the effects of each of these agents on progenitors. Our observations are consistent with those of a prior report of increased progenitor sensitivity to vinblastine during the period of BM recovery after radiation or busulfan treatment.27 Obviously, the safety as well as the efficacy of cytokine prestimulation will require further evaluation before it is incorporated into clinical trials. Furthermore, whether sensitization also occurs at the level of the stem cell, particularly in view of probable endogenous expression of MDR1,28 must be determined in future studies.
Supported by National Institutes of Health Grants No. HL53750, 3M01 RR00037-35S3, and 5P30 DK47754, and by a Fellowship from the Gottlieb Daimler and Karl Benz Foundation to T.N.
Address reprint requests to C. Anthony Blau, Mail Stop 357720, Health Sciences Building, University of Washington, 1959 Pacific Ave NE, Seattle, WA 98195.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal