In this issue of Blood, Arellano et al1 report that type 2 CALR mutation (CALRins5)–mediated activation of activating transcription factor 6 (ATF6) leads to upregulation of the antiapoptotic protein B-cell lymphoma-extra large (BCL-xL), revealing a potential therapeutic vulnerability in calreticulin (CALR)-mutant myeloproliferative neoplasms (MPNs) (see figure).
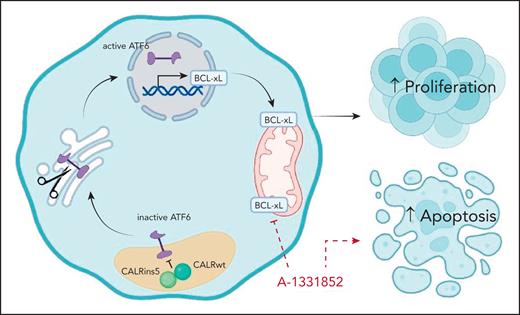
CALRins5 drives ATF6 activation and BCL-xL–mediated cell survival in MPNs. In CALRins5-mutant cells, CALRins5 disrupts the chaperone function of wild-type CALR, leading to activation of the ATF6 pathway. Active ATF6 translocates to the nucleus and induces the transcription of prosurvival genes, including BCL-xL. Elevated BCL-xL expression promotes cell survival and proliferation while inhibiting apoptosis. The BCL-xL–specific inhibitor A-1331852 disrupts this ATF6/BCL-xL axis, restoring apoptotic sensitivity in CALRins5-mutant MPNs. Figure created with BioRender.com. He, F. (2025) https://BioRender.com/obwugjo.
CALRins5 drives ATF6 activation and BCL-xL–mediated cell survival in MPNs. In CALRins5-mutant cells, CALRins5 disrupts the chaperone function of wild-type CALR, leading to activation of the ATF6 pathway. Active ATF6 translocates to the nucleus and induces the transcription of prosurvival genes, including BCL-xL. Elevated BCL-xL expression promotes cell survival and proliferation while inhibiting apoptosis. The BCL-xL–specific inhibitor A-1331852 disrupts this ATF6/BCL-xL axis, restoring apoptotic sensitivity in CALRins5-mutant MPNs. Figure created with BioRender.com. He, F. (2025) https://BioRender.com/obwugjo.
MPNs are driven by different disease-causing mutations, including those in JAK2, CALR, and MPL. CALR is a calcium (Ca2+)-binding chaperone protein, and most CALR mutations occur in the C-terminal domain, resulting in a neomorphic function that enables binding to and activation of the thrombopoietin receptor MPL, thereby leading to constitutive JAK-STAT signaling and uncontrolled cell proliferation.2 Despite the established critical role of mutant CALR in MPN pathogenesis, no targeted therapies currently exist for patients with CALR-mutant disease.
Several experimental strategies are being explored to address this therapeutic gap. These include antibody- and chimeric antigen receptor-T–based strategies targeting the mutant CALR neoepitope,3,4 as well as inhibition of N-glycosylation required for oncogenic CALR-MPL interaction.5 Recent work has also highlighted the unfolded protein response (UPR) as a mechanistic vulnerability.6,7 Type 1 CALR mutations have specifically been shown to activate the inositol-requiring enzyme α (IRE1α)/X-box binding protein 1 (XBP1) arm of the UPR because of impaired calcium homeostasis, which promotes cell survival via upregulation of BCL-2, an antiapoptotic protein.7
Building on this line of inquiry, Arellano et al now show that CALRins5 activates the distinct ATF6 branch of the UPR across multiple models, including cell lines, patient-derived cells, and knock-in mouse models. Notably, they comprehensively dissect the mechanism of ATF6 activation, demonstrating through structural modeling and recombinant protein analysis that the activation arises from loss of CALR chaperone function, caused by a dominant-negative interaction between mutant and wild-type CALR proteins. This loss of function complements the mutant protein's gain of function in MPL activation, highlighting the complex roles of mutant CALR in MPN biology.
The authors further identify BCL-xL, a canonical antiapoptotic member of the BCL-2 family, as a direct transcriptional target of ATF6 in CALRins5-expressing cells. BCL-xL inhibits the intrinsic apoptotic pathway and is known to confer resistance to therapy across a range of hematologic malignancies.8 In this study, its upregulation downstream of ATF6 appears to be essential for MPN cell survival. Importantly, CALRins5-mutant cells demonstrate heightened sensitivity to BCL-xL inhibition, uncovering a potential therapeutic dependency not previously appreciated. This work refines our understanding of CALR mutation biology by demonstrating that type 1 and type 2 variants activate distinct branches of the UPR, IRE1α/XBP1, and ATF6, respectively, each driving unique downstream survival programs.
Overall, this study uncovers a previously unrecognized mechanism of prosurvival signaling in CALRins5-driven MPN and provides a compelling rationale for targeted therapy against ATF6 or BCL-xL. However, several important translational questions remain. The absence of a robust CALRins5 mouse model with a strong and durable MPN phenotype poses a challenge for preclinical validation. Moreover, the essential role of BCL-xL in maintaining normal hematopoietic stem cell survival and differentiation raises concerns regarding potential hematologic toxicity,9 and the thrombocytopenia risk associated with BCL-xL inhibition further complicates its clinical development.10 Altogether, this study provides new insights into the distinct biology of specific CALR mutations, with important implications for the development of new therapeutic approaches.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal