In this issue of Blood, Maynard et al1 present a compelling study leveraging immunocompetent nonhuman primate (NHP) models to systematically compare chimeric antigen receptor T-cell (CAR-T) designs. Their work addresses a critical gap in preclinical evaluation of CAR-T candidates by moving beyond traditional immunocompromised mouse models, thereby more faithfully recapitulating the complex immunologic milieu in patients.
First-generation CAR-Ts, developed in the 1980s and 1990s, combined the antibody-derived targeting domains with T-cell cytotoxic machinery but showed limited clinical efficacy. Integration of CD28 and, later, 41BB directly into the CAR molecule (second-generation CARs) dramatically enhanced CAR-T persistence, with some patients’ CAR-Ts discoverable in the blood over 10 years after treatment.2 These 2 second-generation costimulatory strategies now underpin all 7 US Food and Drug Administration (FDA)–approved CAR-T therapies. Nevertheless, despite the heroic efforts leading to the development of these highly effective therapies, many cancer patients still relapse following CAR-T therapy, underscoring an unmet clinical need.
Persistent CAR-T engraftment is now recognized as a major determinant of durable clinical responses in multiple disease contexts.3 The field continues to search for novel costimulatory domains capable of further augmenting CAR-T persistence and function. Although in vitro screens for novel CAR-T costimulatory domains have identified promising candidates,4 these approaches often rely on the selection of “hits” through artificial metrics of in vitro success (eg, production of certain cytokines or proliferation in nutrient-enriched culture medium), which may not translate into effective CAR-T products in humans. One recent innovative approach to address the shortcomings of in vitro-based selection strategies is the use of pooled in vivo screens in which a panoply of CAR-T designs are administered simultaneously to mice bearing xenografts, followed by the identification of CAR-Ts that have trafficked to the tumor, revealing those CAR-T candidates with enhanced antitumor potential.5 However, this approach, like most preclinical CAR-T studies, relies on the use of immunocompromised mouse models, which do not recapitulate the complex immunologic niches of humans.
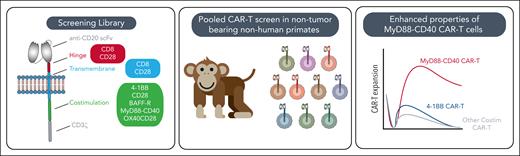
Here, Maynard et al address these limitations by constructing a focused library of 20 CAR-T variants, each featuring distinct hinge, transmembrane, and costimulatory domains, including CD28, 4-1BB, MyD88-CD40, OX40, and BAFF-R, targeting CD20 in NHPs (see figure). This pooled, in vivo screening strategy enables direct, head-to-head comparison of CAR-T performance in a model that closely mirrors human physiology and faithfully reproduces the toxicities seen after cell therapy.
Pooled primate screen schematic and key results. BAFF-R, B-cell activating factor receptor; Costim, costimulatory; scFv, single chain variable fragment.
Pooled primate screen schematic and key results. BAFF-R, B-cell activating factor receptor; Costim, costimulatory; scFv, single chain variable fragment.
MyD88-CD40 costimulated CAR-Ts had enhanced activation, tonic signaling, distinct cytokine production, and resistance to exhaustion on repetitive ex vivo stimulation, outperforming constructs based on FDA-approved designs. These results reinforce previous reports that MyD88-CD40 imparts favorable properties and a hyperactive phenotype6 to CAR-Ts, underscoring the robustness of the findings. Principal component analysis revealed that the costimulatory domain was generally the primary determinant of CAR-T behavior, outweighing the influence of hinge and transmembrane variations. Notably, CARs with the MyD88-CD40 and a CD28 hinge/transmembrane clustered distinctly, consistent with prior evidence that these domains enhance activity and antigen sensitivity.7 The study also challenges the prevailing paradigm that tonic signaling is inherently detrimental8; the clinical activity observed here suggests that this feature may, under certain conditions, contribute to improved therapeutic outcomes. Finally, the unexpected failure of MyD88-CD40 CAR-Ts to expand ex vivo compared to standard 41BB and CD28 CAR-Ts, in contrast to the extreme in vivo expansion of MyD88-CD40 CAR-Ts in NHPs, highlights the limited predictive power of in vitro assays for the behavior of CAR-Ts in complex organisms.
Important caveats must be included with this study’s findings. First, one NHP had uncontrolled MyD88-CD40 CAR-T expansion with hallmarks of cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS) and required euthanasia. This behavior of MyD88-CD40 costimulated CAR-Ts mirror what has been seen clinically in a clinical trial for prostate cancer targeting a different antigen, in which 2 treatment-related deaths and grade 4 CRS and ICANS were seen, leading to trial discontinuation. Second, the degree of proliferation and toxicity in NHPs contrasts with the largely safe experience seen thus far in treating patients for autoimmune conditions whose sole antigen burden is their native B cells; toxicity has been limited, with almost all patients experiencing only grade 1 CRS (fever) and no ICANS.9 The development of such severe toxicity in the absence of any tumor in the NHP raises serious safety concerns regarding the MyD88-CD40 costimulatory domain, although this conceivably could be addressed through cautious dosing or safety switches, among other strategies.
In a timely and relevant publication, a consortium of key stakeholders in the CAR-T field recently advocated for pooled screening approaches in patients to identify optimal CAR-T designs, which conceivably could be disease- or even patient-specific.10 This important study from Maynard et al highlights why such a strategy is warranted and the limitations of our existing preclinical approaches.
Conflict-of-interest disclosure: M.B.L. is a contributor to patent filings on CAR-T technology that are held by the Massachusetts General Hospital and reports consultancy for BioNtech, Cabaletta Bio, and Adaptimmune.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal