In this issue of Blood, Bruins et al1 show evidence supporting that immune age is a clinically relevant composite metric that better reflects the immune status of patients with multiple myeloma (MM) than their calendar age. As such, immune-age metrics may guide clinical decision-making to reduce the risk of infection, treatment discontinuation, and inferior survival.
Immune health metrics have been developed to identify age-independent derivations from normal immunological performance status in diverse conditions to empower precision medicine.2 In MM, there is emerging evidence that calendar age is not a major determinant of immunotherapy efficacy.3 Here, Bruins et al provide the first proof-of concept that immune clocks trained solely on high-dimensional T-cell data are feasible and can be clinically meaningful. One of the most interesting and novel findings of the study was that, after assigning a specific immune age to each patient, approximately one-third showed ≥±10 years difference between immune and calendar age. Importantly, the correlation with the ability of CD8+ T cells to produce IFN-ɣ was higher with immune vs calendar age.
Virtually all immune cell types are affected by aging.4 There is increasing abundance of neutrophils and nonclassical monocytes together with decreased numbers and function of plasmacytoid dendritic cells, B cells, MAIT, and γδ, CD4, and CD8 T cells. Most of these immune alterations have been described in MM,5 but the association between patients’ age and altered immune profiles remained largely unknown. Bruins et al unveiled that among 305 metaclusters of T and natural killer (NK) cells in bone marrow, only 37 (12%) showed significant differential abundance between age groups. These results suggest that MM expansion and progression over time—a process that may occur for more than 20 years since the first genetic alteration to the development of clinical symtpoms6—may accelerate immune aging to a degree that supersedes that of chronological age.
Another interesting output from the Bruins et al study was that 15% of patients enrolled in the phase 2 HOVON-143 trial, which was specifically designed for newly-diagnosed patients with MM older than 65 years and classified as intermediate fit or frail according to the International Myeloma Working Group (IMWG) frailty index, had an immune profile more typically found in patients younger than 65 enrolled in CASSIOPEIA/HOVON-131 trial. Several T-cell characteristics were associated with a younger immune age such as the expansion of naïve CD8+ T cells and lower frequencies of terminally differentiated, senescent, or exhausted T cells. Surprisingly, the metacluster showing the highest coefficient value in both the age-dichotomized and continuous immune clocks was defined by double-negative naïve T cells (see Figure 5A,B and supplemental Table 4 by Bruins et al). These cells have been off-the-radar in recent MM studies investigating T-cell determinants of response and resistance to immunotherapy,7 but may be important in defining “younger” vs “older” immune profiles linked to clinical outcomes.
Bruins et al investigated the association between patients’ immune age and survival outcomes in the HOVON-143 trial, which consisted of 9 induction cycles of daratumumab-ixazomib-dexamethasone followed by maintenance with daratumumab-ixazomib for a maximum of 2 years.8 On the intent-to-treat population, median progression-free survival (PFS) was 13.8 months and 12-month overall survival (OS) was 78%.8 Notably, patients having “younger” immune profiles showed a median PFS nearing 36 months, a median PFS2 not reached and 12-month OS close to 100%.1 These associations retained significance after adjusting for World Health Organization (WHO) performance status, international staging system and the IMWG frailty index in multivariate Cox regression analysis. Cytogenetic risk was not included in the primary multivariable models due to limited patient numbers, but in the exploratory analysis including cytogenetic risk, the immune-age score lost significance (see supplemental Table 6 by Bruins et al). Although the independent prognostic value of immune-age metrics in peripheral blood remains undefined, the authors convincingly showed that immune age is associated with survival outcomes in older MM patients whereas the calendar age was not.
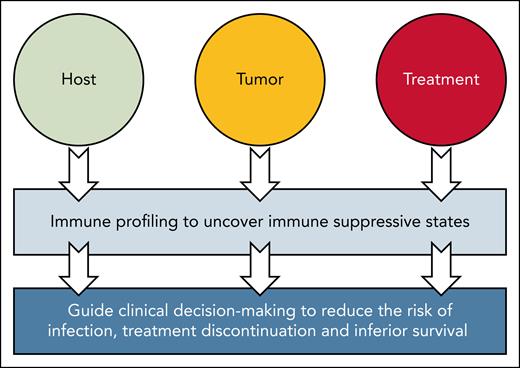
The fact that immune age not associated with depth of response can be partially related to the fact that, although daratumumab relies predominantly in NK-cell function to eradicate MM, immune profiles were developed using a T-cell panel specifically designed to assess key immune features associated with aging, T-cell differentiation, senescence, and exhaustion.1 NK-cell panels were not used to develop immune profiles, probably because age-related differences were more prominent in the T-cell compartment. Thus, one possibility to explain the association with survival is that T-cell immune age is a proxy for overall biological health given its association with comorbidities, WHO performance status and IMWG frailty index.1 Interestingly, patients with a younger immune profile showed borderline significant lower risk of treatment discontinuation and lower mean infection rate.1 This is where immune profiling, particularly in peripheral blood, may become a powerful surrogate of immune suppressive states resulting from host, tumor, and treatment-related factors. In such a case, immune profiling could potentially predict survival and other relevant clinical outcomes such as infection risk (see figure).
iMMune profiling comes of age. The study by Bruins et al provides evidence that immune profiling, even when solely focused in a single subset such as T cells, can be a proxy for overall biological health and uncover immune suppressive states resulting from host, tumor, and treatment-related factors. The authors show further evidence that when immune profiling is converted into a composite metric of immune age, it can help predict the risk of infection, treatment discontinuation, and survival better than the patients’ calendar age.
iMMune profiling comes of age. The study by Bruins et al provides evidence that immune profiling, even when solely focused in a single subset such as T cells, can be a proxy for overall biological health and uncover immune suppressive states resulting from host, tumor, and treatment-related factors. The authors show further evidence that when immune profiling is converted into a composite metric of immune age, it can help predict the risk of infection, treatment discontinuation, and survival better than the patients’ calendar age.
The breakthrough of immunotherapy started by prolonging survival of patients with MM with relapsed disease, is currently being investigated in the newly-diagnosed setting with the aim of achieving operational cure, and there is ambition to use immunotherapy in patients with precursor conditions to change the natural history of the disease by preventing its progression and the development of end-organ damage. Thus, the pioneering study of Bruins et al should motivate this and other groups to build upon the findings in older/frail patients onto other disease settings. Because there is a growing number of approved drugs that use different immune subsets to deploy their mode of action, there should be efforts to enhance immune age metrics by incorporating other cell types that are relevant in MM pathogenesis (eg, myeloid-derived suppressor cells9) and are critical in the mode of action of approved drugs (eg, NK cells in the context of anti-CD38 monoclonal antibodies10). Finally, to define the methods and create user-friendly tools for standardized and routine assessment of immune age in routine practice. The authors should be commended for taking the first step in such a relevant patient population!
Conflict-of-interest disclosure: B.P. reports honoraria for lectures from and participation on advisory boards for Adaptive Biotech, Amgen, Bristol Myers Squibb (BMS)/Celgene, Gilead, GlaxoSmithKline (GSK), Janssen, Oncopeptides, Roche, Sanofi, Takeda, and The Binding Site; has received unrestricted grants from BMS/Celgene, EngMab, GSK, Roche, Sanofi, and Takeda; and has acted as a consultant for BMS/Celgene, Janssen, and Sanofi. A.Z. reports no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal