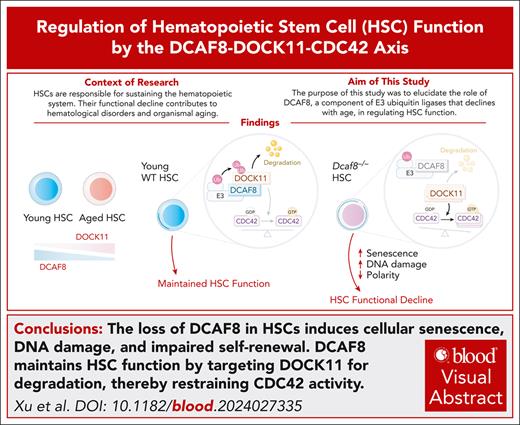
Key Points
Age-related decline of DCAF8 expression in HSCs leads to functional defects.
DCAF8 modulates CDC42 activity via the degradation of DOCK11, which leads to HSC cellular senescence.
Visual Abstract
Hematopoietic stem cells (HSCs) are responsible for sustaining the hematopoietic system throughout life, and their functional decline contributes to hematological disorders and organismal aging. Understanding the molecular mechanisms that govern HSC function is critical for developing interventions for treating and preventing aging-related diseases. Here, we show that DCAF8, a substrate recognition component of Cullin-RING E3 ubiquitin ligases, is highly expressed in HSCs and undergoes a progressive decline with age. Loss of DCAF8 in mice results in impaired function in HSCs, characterized by increased number yet decreased self-renewal capacity, which associates with cellular senescence and elevated DNA damage. Mechanistically, DCAF8 mediates the degradation of dedicator of cytokinesis 11 (DOCK11), a guanine nucleotide exchange factor for CDC42. In the absence of DCAF8, DOCK11 accumulates, leading to elevated CDC42 activity and consequential loss of polarity of HSCs. Knocking out Dock11 mitigates the senescence, DNA damage, and self-renewal defects of Dcaf8−/− HSCs. This study highlights a critical role of DCAF8 in preventing HSC senescence via the DOCK11-CDC42 axis and suggests potential therapeutic targets for preventing functional decline in HSCs.
Introduction
Hematopoietic stem cells (HSCs) are responsible for the lifelong production of blood and immune cells through their ability to self-renew. Nevertheless, HSCs undergo a progressive functional decline during aging, leading to impaired adaptive immunity, increased incidence of anemia, and higher risk of hematological malignancies.1 The accumulation of somatic mutations during HSC aging also contributes to clonal hematopoiesis, which is linked to chronic inflammation, increased risk of cardiovascular diseases, type 2 diabetes, and increased risk of all-cause mortality.2,3 Aged HSCs exhibit reduced self-renewal and regenerative potential, increased frequency, and myeloid-skewed differentiation. Numerous cellular defects, such as DNA damage, cellular senescence, and loss of cell polarity, have been reported to contribute to this age-associated decline in HSC function.4,5
The functional maintenance of HSCs throughout the lifetime depends on a predominantly quiescent state while preserving their ability to respond, which relies on strictly regulated protein homeostasis (proteostasis).6,7 Consequently, HSC aging is accompanied by deregulated proteostasis.1 As a canonical pathway for protein degradation, ubiquitin proteasome system (UPS) maintains cellular proteostasis and HSC function by regulating the levels of key proteins,7,8 such as phosphorylated STAT5 (p-STAT5),9 c-Myc,10 and NOTCH1.11
Cullin-RING ligases (CRLs), the largest class of ubiquitin ligases, play a central role in protein degradation because of their diverse substrate-receptor subunits.12 DCAF8 (DDB1- and Cul4-associated factor 8) acts as a substrate adaptor in the Cul4-RING E3 ubiquitin ligase (CRL4),13 and has been associated with senescence and hematopoiesis. In human hepatocytes, DCAF8 deficiency has been associated with cellular senescence.14 DCAF8 also mediates the degradation of lymphoid-specific helicase (LSH),15 myeloid leukemia factor 2 (MLF2),16 and DNMT3A proteins,17 which play critical roles in hematopoiesis and cancer.
In this study, we show that DCAF8, whose expression declines with age in HSCs, regulates HSC function by preventing cellular senescence through targeting dedicator of cytokinesis 11 (DOCK11) for degradation, highlighting the DCAF8-DOCK11-CDC42 axis as a key regulator of HSC function.
Methods
Mice
Dcaf8−/− mice were generated and genotyped as previously described,18 and maintained on a C57BL/6J (CD45.2) genetic background. Dock11−/− mice (GemPharmatech T030854) were generated by deleting exons 2 to 52, genotyped by polymerase chain reaction (PCR) using primers listed in the supplemental Table (available on the Blood website) and maintained on a C57BL/6J (CD45.2) genetic background.
Flow cytometry and cell sorting
Bone marrow (BM), peripheral blood (PB), and spleen cells were stained using lineage and stem/progenitor marker panels before analysis and cell sorting. Additional staining protocols are provided in supplemental Figure 1D.
BMT assays
For BM transplantation (BMT) assays, recipient mice were irradiated with 9 Gy split into 2 doses, and injected intravenously with 5 × 105 or 1 × 106 BM cells, or 300 fluorescence-activated cell (FAC)-sorted long-term (LT) HSCs, with or without competitor cells, as indicated.
Immunofluorescence staining
FAC-sorted LT-HSCs were fixed with paraformaldehyde, permeabilized with Triton X-100, and blocked with goat serum. Images were acquired using confocal microscopy, and fluorescence intensity were analyzed using ImageJ.
Transcriptomic analysis of LT-HSCs
LT-HSCs were isolated by FAC sorting and subjected to full-length messenger RNA amplification followed by library construction and RNA sequencing, as detailed in the supplemental Methods.
Proteomic and ubiquitin proteomic analysis
Proteomic profiling of lineage-negative (Lin−) hematopoietic stem and progenitor cells (HSPCs) and enriched ubiquitinated peptides from whole BM cells was performed by mass spectrometry. Downregulated ubiquitination sites were identified by comparing wild-type (WT) and Dcaf8−/− samples. Full experimental and analytical procedures are described in the supplemental Methods.
All animal experiments were approved by the institutional animal care and use committee of Shanghai Jiao Tong University School of Medicine, under approval number JUMC2023-046-B.
Results
DCAF8 is highly expressed in HSCs and decreases with age
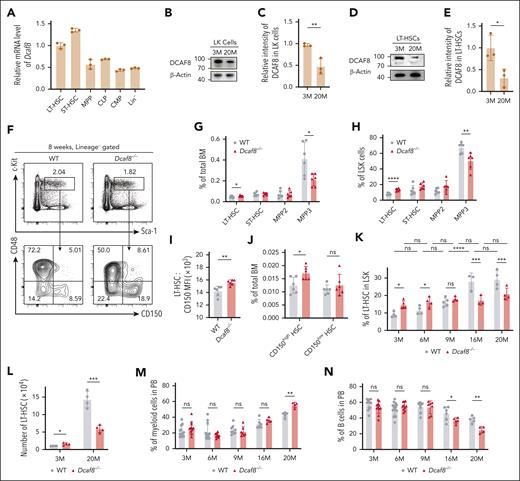
To explore the possibility that DCAF8 plays a role in hematopoiesis, we measured Dcaf8 messenger RNA levels in HSPCs from mice using quantitative PCR (qPCR). The results showed that Dcaf8 expression is markedly higher in LT-HSCs (Lin−Sca-1+c-Kit+CD150+CD48−) and short-term HSCs (Lin−Sca-1+c-Kit+CD150−CD48−) than multipotent progenitors (Lin−Sca-1+c-Kit+CD48+), common myeloid progenitors (Lin−Sca-1−c-Kit+CD34+CD16/32−), common lymphoid progenitors (Lin−Sca-1loc-KitloCD127+), and Lin− HSPCs (Figure 1A). We also found that, consistently, human DCAF8 is highly expressed in HSCs compared with downstream progenitor cells analyzed using the BloodSpot database19 (supplemental Figure 1A).
Aging-related decreased expression of DCAF8 leads to increased number of HSCs. (A) Reverse transcription qPCR (RT-qPCR) of Dcaf8 in various murine HSPCs, including LT-HSC (Lin−Sca-1+c-Kit+CD150+CD48−), ST-HSC (Lin−Sca-1+c-Kit+CD150−CD48−), MPP (Lin−Sca-1+c-Kit+CD48+), CMP (Lin−Sca-1−c-Kit+CD34+CD16/32−), CLP (Lin−Sca-1loc-KitloCD127+), and Lin− cells (n = 3 technical replicates, pooled from 3 mice). (B) Immunoblot of DCAF8 in LK cells at different mouse ages. (C) The relative intensity of DCAF8 immunoblot in LK cells, quantified and normalized to β-actin (n = 3 independent experiments). (D) Immunoblot of DOCK11 in LT-HSCs at different mouse ages. (E) The relative intensity of DOCK11 immunoblot in LT-HSCs, quantified and normalized to β-actin (n = 3 independent experiments). (F) Representative flow cytometric plots for HSPCs in the BM of 8-week-old WT and Dcaf8−/− mice. (G-H) Frequency of LT-HSC, ST-HSC, MPP2 (Lin−Sca-1+c-Kit+CD150+CD48+), and MPP3 (Lin−Sca-1+c-Kit+CD150−CD48+) represented as percentages of (G) total BM cells (n = 6 mice) and (H) LSK (Lin−Sca-1+c-Kit+) cells (n = 6 mice) in 8-week-old WT and Dcaf8−/− mice. (I) Surface expression of CD150 of LT-HSCs in WT and Dcaf8−/− mice (n = 6 mice), expressed as mean fluorescence intensity (MFI) analyzed by flow cytometry. (J) Frequency of LT-HSC divided into CD150high and CD150low subgroups represented as percentages of total BM cells in 8-week-old WT and Dcaf8−/− mice (n = 6 mice). (K) Frequency of LT-HSC represented as percentages of LSK cells at different ages in WT and Dcaf8−/− groups (n = 4, 4, 5, 3, and 4 mice for WT; and 5, 4, 4, 3, and 4 mice for Dcaf8−/−). Statistical analysis used 2-way analysis of variance (ANOVA) followed by the Tukey multiple comparisons test. (L) Number of LT-HSC in BM at different ages in WT and Dcaf8−/− groups (n = 4 mice). (M-N) Frequency of (M) myeloid (CD11b+) and (N) B cells (CD19+) in the PB at different ages in WT and Dcaf8−/− mice (n = 12, 14, 9, 6, and 5 mice for WT; and 11, 10, 7, 5, and 4 mice for Dcaf8−/−). Statistical analysis used 2-way ANOVA followed by the Tukey multiple comparisons test. Data are presented as mean ± standard deviation (SD). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001 by unpaired 2-tailed t tests unless otherwise specified. CLP, common lymphoid progenitors; CMP, common myeloid progenitors; LK, Lin−c-Kit+; MPP, multipotent progenitors; ns, no statistical significance; ST-HSC, short-term HSC.
Aging-related decreased expression of DCAF8 leads to increased number of HSCs. (A) Reverse transcription qPCR (RT-qPCR) of Dcaf8 in various murine HSPCs, including LT-HSC (Lin−Sca-1+c-Kit+CD150+CD48−), ST-HSC (Lin−Sca-1+c-Kit+CD150−CD48−), MPP (Lin−Sca-1+c-Kit+CD48+), CMP (Lin−Sca-1−c-Kit+CD34+CD16/32−), CLP (Lin−Sca-1loc-KitloCD127+), and Lin− cells (n = 3 technical replicates, pooled from 3 mice). (B) Immunoblot of DCAF8 in LK cells at different mouse ages. (C) The relative intensity of DCAF8 immunoblot in LK cells, quantified and normalized to β-actin (n = 3 independent experiments). (D) Immunoblot of DOCK11 in LT-HSCs at different mouse ages. (E) The relative intensity of DOCK11 immunoblot in LT-HSCs, quantified and normalized to β-actin (n = 3 independent experiments). (F) Representative flow cytometric plots for HSPCs in the BM of 8-week-old WT and Dcaf8−/− mice. (G-H) Frequency of LT-HSC, ST-HSC, MPP2 (Lin−Sca-1+c-Kit+CD150+CD48+), and MPP3 (Lin−Sca-1+c-Kit+CD150−CD48+) represented as percentages of (G) total BM cells (n = 6 mice) and (H) LSK (Lin−Sca-1+c-Kit+) cells (n = 6 mice) in 8-week-old WT and Dcaf8−/− mice. (I) Surface expression of CD150 of LT-HSCs in WT and Dcaf8−/− mice (n = 6 mice), expressed as mean fluorescence intensity (MFI) analyzed by flow cytometry. (J) Frequency of LT-HSC divided into CD150high and CD150low subgroups represented as percentages of total BM cells in 8-week-old WT and Dcaf8−/− mice (n = 6 mice). (K) Frequency of LT-HSC represented as percentages of LSK cells at different ages in WT and Dcaf8−/− groups (n = 4, 4, 5, 3, and 4 mice for WT; and 5, 4, 4, 3, and 4 mice for Dcaf8−/−). Statistical analysis used 2-way analysis of variance (ANOVA) followed by the Tukey multiple comparisons test. (L) Number of LT-HSC in BM at different ages in WT and Dcaf8−/− groups (n = 4 mice). (M-N) Frequency of (M) myeloid (CD11b+) and (N) B cells (CD19+) in the PB at different ages in WT and Dcaf8−/− mice (n = 12, 14, 9, 6, and 5 mice for WT; and 11, 10, 7, 5, and 4 mice for Dcaf8−/−). Statistical analysis used 2-way ANOVA followed by the Tukey multiple comparisons test. Data are presented as mean ± standard deviation (SD). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001 by unpaired 2-tailed t tests unless otherwise specified. CLP, common lymphoid progenitors; CMP, common myeloid progenitors; LK, Lin−c-Kit+; MPP, multipotent progenitors; ns, no statistical significance; ST-HSC, short-term HSC.
We subsequently investigated the potential for aging-related differential expression of DCAF8. Immunoblotting revealed a progressive decline in DCAF8 protein levels in aged Lin− cells (supplemental Figure 1B), aged Lin−c-Kit+ HSPCs (Figure 1B-C), and aged LT-HSCs (Figure 1D-E) compared with their corresponding young cell groups. Consistently, qPCR showed a significant decrease in Dcaf8 expression in LT-HSCs from 9-month-old mice compared with those from 5-week-old mice (supplemental Figure 1C). These findings demonstrate that DCAF8 is highly expressed in HSCs compared with downstream progenitor cells and, moreover, that its expression in HSCs declines with age.
Dcaf8 knockout leads to increased number of HSCs at a young age
Given that DCAF8 is highly expressed in HSCs and shows an age-related decline, we sought to determine whether its loss affects the HSC compartment. Using a previously described Dcaf8 knockout mouse model,18 we analyzed the HSC population defined with signaling lymphocyte activation molecule family markers.20 We found significantly higher frequencies of LT-HSCs in young Dcaf8−/− mice than WT controls, both within the BM compartment and the Lin−Sca-1+c-Kit+ (LSK) subset (Figure 1F-H; supplemental Figure 1D). These findings were further validated using CD34 and fetal liver kinase-2 (Flk2) markers to define LT-HSCs (LSK CD34−Flk2−),21 showing consistent increase in LT-HSC frequencies in young Dcaf8−/− mice (supplemental Figure 1E-F). The absolute number of LT-HSCs was also increased significantly in Dcaf8−/− mice (Figure 1L). These results indicate a substantial increase in LT-HSC frequency and number in young Dcaf8−/− mice compared with age-matched WT controls.
To further investigate the dynamic of HSC changes over time, we analyzed LT-HSC frequencies across different age groups. At young ages (3 and 6 months), Dcaf8−/− mice showed a significant increase in LT-HSC frequencies. However, in aged mice (16 and 20 months), LT-HSC frequencies decreased significantly in the Dcaf8−/− group compared with WT controls (Figure 1K). This finding was corroborated using CD34 and Flk2 markers (supplemental Figure 1E,G), and the absolute number of LT-HSCs followed a similar trend (Figure 1L), indicating that Dcaf8−/− HSCs do not increase with aging to the same extent as WT HSCs. Of note, the frequency of multipotent progenitor 4 cells, which typically declines with age,22 remains unchanged in Dcaf8−/− mice (supplemental Figure 1H).
CD150 (Slamf1), a marker associated with aged HSCs,23,24 was significantly upregulated in Dcaf8−/− LT-HSCs compared with WT controls (Figure 1I). Moreover, an expansion of the CD150high subsets within the LT-HSC population, which is related to age-associated myeloid-biased differentiation,23 was observed in Dcaf8−/− mice (Figure 1J; supplemental Figure 1I-J). Consistent with this, PB analysis revealed a myeloid skewing in aged Dcaf8−/− mice (Figure 1M), accompanied by a significant decrease in B cells (Figure 1N), whereas T cell numbers remained unaffected (supplemental Figure 1K). The lineage distribution in young Dcaf8−/− mice did not show any significant abnormalities.
Despite these observed changes in HSCs, hematopoietic homeostasis was only mildly affected in Dcaf8−/− mice under steady-state conditions. Complete blood count assays showed no discernible differences between WT and Dcaf8−/− mice across different age groups (supplemental Figure 2A-C). Similarly, BM and spleen cellularity in Dcaf8−/− mice was comparable with that of WT controls (supplemental Figure 2D-E). Within the BM compartment, the frequencies of Lin− cells, LSK cells, and progenitor cells, and lineage distribution were also similar between the 2 groups at both young and old ages (supplemental Figure 2F-K). We also analyzed the cell cycle status of LT-HSCs (supplemental Figure 3A,B,D) and LSKs (supplemental Figure 3C) at steady state, and found no significant differences between WT and Dcaf8−/− mice.
Dcaf8 knockout impairs HSC self-renewal and regeneration capacity
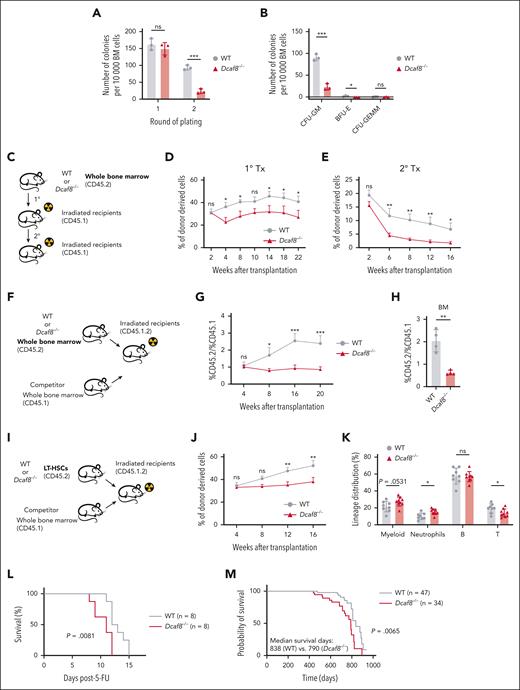
Self-renewal is essential for HSCs to maintain LT blood production.4 To investigate the impact of Dcaf8 knockout on HSC self-renewal, we first performed in vitro serial colony-forming unit assays with whole BM cells from young mice. In the second round of plating, total colonies (Figure 2A), colony-forming unit–granulocyte, macrophage; and burst-forming unit-erythroid colonies (Figure 2B) exhibited significant decreases in BM cells obtained from Dcaf8−/− mice compared with WT controls. These results indicate that Dcaf8 knockout impairs the colony-forming capacity of BM cells.
Dcaf8 knockout leads to impaired self-renewal capacity of HSCs. (A) Total number of colonies after 1 × 104 BM cells were plated for 2 rounds to determine the colony-forming capacity (n = 3 mice). (B) Number of colonies divided by type after the second-round plating as shown in panel A (n = 3 mice). (C) Schematic of the transplantation assay as illustrated in panels D-E. (D-E) Donor chimerism analyses in the PB of (D) primary recipients (n = 6 recipients) and (E) secondary recipients (n = 7 recipients for WT group, n = 10 recipients for Dcaf8−/− group) at the indicated time points. Statistical analysis used 2-way ANOVA followed by the Fisher least significant difference test. (F) Schematic of the competitive transplantation assay as illustrated in panels G-H. (G) Ratio of donor chimerism to competitive mice in the PB of the recipients at the indicated time points (n = 6 recipients for WT group; n = 8 recipients for Dcaf8−/− group). Statistical analysis used 2-way ANOVA followed by the Fisher least significant difference test. (H) Ratio of donor chimerism to competitive mice in the BM of the recipients at the end point (n = 4 recipients). (I) Schematic of the LT-HSC competitive transplantation assay as illustrated in panels J-K. (J) Donor chimerism analyses in the PB of the recipients at the indicated time points (n = 8 recipients for WT group; n = 9 recipients for Dcaf8−/− group). Statistical analysis used 2-way ANOVA followed by the Fisher least significant difference test. (K) Frequencies of myeloid (CD11b+), neutrophils (CD11b+Ly6G+), B cells (CD19+), and T cells (CD3+) in donor-derived cells in the PB of the recipients at the end point (n = 8 recipients for WT group; n = 9 recipients for Dcaf8−/− group). (L) The Kaplan-Meier survival curve of WT and Dcaf8−/− mice after 2 injections of 5-FU (150 mg/kg) administered 7 days apart (n = 8 mice). P value was calculated by log-rank test. (M) The Kaplan-Meier survival curve of WT (n = 47 mice) and Dcaf8−/− (n = 34 mice) mice. P value was calculated by log-rank test. For panels D, E, G, and J, data are presented as mean ± standard error of the mean (SEM); other data are presented as mean ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001 by unpaired 2-tailed t tests unless otherwise specified. B, B cells; BFU-E, burst-forming unit-erythroid; CFU-GEMM, colony-forming unit-granulocyte, erythroid, macrophage, megakaryocyte; CFU-GM, colony-forming unit-granulocyte, macrophage; ns, no statistical significance; T, T cells; Tx, transplantation.
Dcaf8 knockout leads to impaired self-renewal capacity of HSCs. (A) Total number of colonies after 1 × 104 BM cells were plated for 2 rounds to determine the colony-forming capacity (n = 3 mice). (B) Number of colonies divided by type after the second-round plating as shown in panel A (n = 3 mice). (C) Schematic of the transplantation assay as illustrated in panels D-E. (D-E) Donor chimerism analyses in the PB of (D) primary recipients (n = 6 recipients) and (E) secondary recipients (n = 7 recipients for WT group, n = 10 recipients for Dcaf8−/− group) at the indicated time points. Statistical analysis used 2-way ANOVA followed by the Fisher least significant difference test. (F) Schematic of the competitive transplantation assay as illustrated in panels G-H. (G) Ratio of donor chimerism to competitive mice in the PB of the recipients at the indicated time points (n = 6 recipients for WT group; n = 8 recipients for Dcaf8−/− group). Statistical analysis used 2-way ANOVA followed by the Fisher least significant difference test. (H) Ratio of donor chimerism to competitive mice in the BM of the recipients at the end point (n = 4 recipients). (I) Schematic of the LT-HSC competitive transplantation assay as illustrated in panels J-K. (J) Donor chimerism analyses in the PB of the recipients at the indicated time points (n = 8 recipients for WT group; n = 9 recipients for Dcaf8−/− group). Statistical analysis used 2-way ANOVA followed by the Fisher least significant difference test. (K) Frequencies of myeloid (CD11b+), neutrophils (CD11b+Ly6G+), B cells (CD19+), and T cells (CD3+) in donor-derived cells in the PB of the recipients at the end point (n = 8 recipients for WT group; n = 9 recipients for Dcaf8−/− group). (L) The Kaplan-Meier survival curve of WT and Dcaf8−/− mice after 2 injections of 5-FU (150 mg/kg) administered 7 days apart (n = 8 mice). P value was calculated by log-rank test. (M) The Kaplan-Meier survival curve of WT (n = 47 mice) and Dcaf8−/− (n = 34 mice) mice. P value was calculated by log-rank test. For panels D, E, G, and J, data are presented as mean ± standard error of the mean (SEM); other data are presented as mean ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001 by unpaired 2-tailed t tests unless otherwise specified. B, B cells; BFU-E, burst-forming unit-erythroid; CFU-GEMM, colony-forming unit-granulocyte, erythroid, macrophage, megakaryocyte; CFU-GM, colony-forming unit-granulocyte, macrophage; ns, no statistical significance; T, T cells; Tx, transplantation.
Next, we assessed the self-renewal capacity of HSCs in vivo through serial BMT assays (Figure 2C). The Dcaf8−/− group exhibited a significant reduction in donor chimerism levels in the PB compared with the WT group (Figure 2D). Upon secondary transplantation, donor chimerism in Dcaf8−/− PB diminished significantly even further (Figure 2E). We also performed competitive transplantation assays (Figure 2F), in which, the Dcaf8−/− group showed significantly decreased donor chimerism in both the PB and BM compared with WT (Figure 2G-H). To directly assess the function of HSCs, we performed competitive transplantation using purified LT-HSCs (Figure 2I). Consistent with previous results, donor chimerism in the PB was significantly reduced in recipients of Dcaf8−/− HSCs compared with WT controls (Figure 2J). Lineage analysis of the PB in recipients revealed a trend toward myeloid-biased differentiation in the Dcaf8−/− group, with a significantly higher proportion of neutrophils and significantly reduced T cells, despite the overall myeloid increase not reaching significance (P = .0531; Figure 2K). These results indicate the diminished self-renewal capacity of Dcaf8−/− HSCs. Notably, a short-term homing assay ruled out the possibility that this reduction in self-renewal was due to impaired homing capacity (supplemental Figure 3F-H). Further test on mobilization ability showed no significant differences between Dcaf8−/− and WT HSCs (supplemental Figure 3I-K).
To evaluate whether Dcaf8 knockout affects the ability of HSCs to response to stress, we treated Dcaf8−/− mice and WT controls with 2 doses of 5-fluorouracil (5-FU), a myeloablative agent that kills cycling cells and triggers HSC regeneration.25 The Dcaf8−/− group showed significantly impaired survival compared with WT controls (Figure 2L). Although cell cycle analysis under steady-state conditions showed no significant differences, BrdU (bromodeoxyuridine) incorporation assays performed 30 days after a single 5-FU dose revealed significantly reduced proliferation of Dcaf8−/− LT-HSCs (supplemental Figure 3E). These findings suggest that DCAF8 deficiency compromises the regenerative response of HSCs after myeloablative stress. Moreover, a lifelong follow-up revealed that Dcaf8−/− mice had significantly shorter life span than WT controls (Figure 2M). Although we did not observe overt signs of hematopoietic failure under steady-state conditions, the reduced life span may reflect a cumulative effect of hematopoietic dysfunction under stress as well as potential defects in nonhematopoietic tissues caused by DCAF8 loss.14,15
Loss of DCAF8 induces transcriptional changes associated with impaired HSC function
To explore the mechanism by which Dcaf8 knockout leads to functional defects in HSCs, we performed RNA sequencing on LT-HSCs sorted from young Dcaf8−/− mice and WT controls (supplemental Figure 4A; supplemental Table 1). The most differentially expressed genes (adjusted P < .05 and fold change of >1.5 or <0.67) in Dcaf8−/−HSCs were associated with HSC regulation. These include the upregulation of Prtn3, which predicts HSC aging26; Ccl3, which promotes myeloid differentiation27; along with the downregulation of Irf1, whose loss impairs HSC self-renewal28 (supplemental Figure 4B-D).
Gene set enrichment analysis revealed that 2 independent transcriptional signatures of aged HSCs, identified in previous studies,26,29 were significantly enriched in the Dcaf8−/− group (supplemental Figure 4E-F). Despite the enrichment results, canonical aging markers of HSCs such as Itga2b (CD41),30Selp,26 and Clu31 were not upregulated in Dcaf8−/− HSCs (supplemental Table 1). This suggests that the observed transcriptional alterations do not reflect a typical aging program. Collectively, these findings indicate that the absence of Dcaf8 induces changes in HSC transcriptional programs associated with impaired function.
Loss of DCAF8 confers cellular senescence and DNA damage to HSCs
Previous studies have implicated the BM microenvironment in HSC maintenance.1,32,33 However, cytokine levels, including tumor necrosis factor α, interferon gamma, granulocyte-macrophage colony-stimulating factor, interleukin-1β (IL-1β), IL-6, IL-10, and chemokine (C-C motif) ligand 5, were comparable between Dcaf8−/− and WT BM (supplemental Figure 3L). We further conducted BMT from WT donors to irradiated recipients of Dcaf8−/− mice or WT mice (supplemental Figure 3M). Comparable donor chimerism in the PB were observed (supplemental Figure 3N), and the frequency of donor-derived LT-HSCs showed no significant differences between the 2 recipient groups (supplemental Figure 3O), suggesting that the BM microenvironment in Dcaf8−/− mice does not affect hematopoiesis.
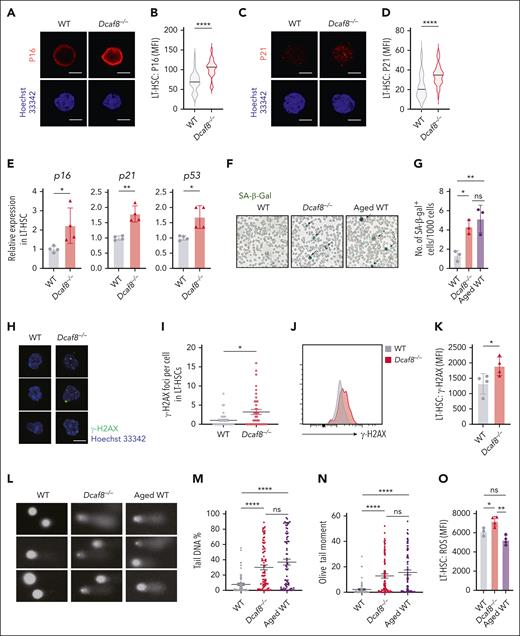
To investigate intrinsic defects in Dcaf8−/− HSCs, we examined established molecular features of cellular senescence. Senescent cells are often featured by the upregulation of tumor suppressor gene p53, cyclin-dependent kinase inhibitors Cdkn1a/p21, and more prominently Cdkn2a/p16.34,35 Notably, p16 expression is increased in HSCs during aging36 or in response to cellular stress such as reactive oxygen species,37 and affects HSC self-renewal.36 Immunofluorescence staining of LT-HSCs from Dcaf8−/− mice revealed significant upregulation of both P16 and P21 (Figure 3A-D). qPCR assays confirmed this upregulation, showing elevated levels of transcription of p16, p21, and p53 in LT-HSCs from Dcaf8−/− mice (Figure 3E). Additionally, senescence-associated β-galactosidase activity38 was significantly higher in the BM cells of Dcaf8−/− mice compared with WT controls, reaching levels comparable with those observed in aged mice (Figure 3F-G). These findings highlight the senescent state of LT-HSCs in Dcaf8−/− mice.
Dcaf8 knockout leads to cellular senescence and DNA damage in HSCs. (A-D) Representative images of (A) P16 and (C) P21 indicated by immunofluorescence, with quantification of (B) P16 (n = 64 cells for WT; n = 67 cells for Dcaf8−/−, pooled from 3 mice) and (D) P21 (n = 39 cells for WT; n = 36 cells for Dcaf8−/−, pooled from 3 mice) by MFI in purified LT-HSCs from young WT and Dcaf8−/− mice. Scale bar, 5 μm. (E) RT-qPCR of p16, p21, and p53 in purified LT-HSCs from young WT and Dcaf8−/− mice (n = 4 technical replicates, pooled from 3 mice). (F-G) Representative images of (F) senescence-associated β-galactosidase (SA-β-gal) staining and (G) quantification in BM cells from young WT, Dcaf8−/− mice, and aged (20 months old) WT controls (n = 3 independent experiments, each pooled from 3 mice). Statistical analysis used 1-way ANOVA followed by the Tukey multiple comparisons test. (H-I) Representative images of (H) phosphorylated H2A histone family member X (γ-H2AX) indicated by immunofluorescence and (I) quantification of γ-H2AX foci in purified LT-HSCs from young WT and Dcaf8−/− mice (n = 34 cells for WT; n = 40 cells for Dcaf8−/−, pooled from 3 mice). Scale bar, 5 μm. Statistical analysis used 2-tailed Mann-Whitney U test. (J) Representative flow cytometric plots and (K) analysis of γ-H2AX expression of LT-HSCs from young WT and Dcaf8−/− mice (n = 4 mice). (L) Representative alkaline comets and quantification by (M) percent of DNA in tail and (N) olive tail moment of purified LT-HSCs from young WT (n = 65 cells, pooled from 3 mice), Dcaf8−/− (n = 79 cells, pooled from 3 mice) mice, and aged (20 months old) WT controls (n = 76 cells, pooled from 3 mice). Statistical analysis used Kruskal-Wallis test followed by the Dunn multiple comparisons test. (O) Analysis of reactive oxygen species levels stained with DCFH-DA by flow cytometry of LT-HSCs from young WT (n = 3 mice), Dcaf8−/− (n = 4 mice) mice, and aged (20 months old) WT controls (n = 4 mice). Statistical analysis used 1-way ANOVA followed by the Tukey multiple comparisons test. For panels I, M, and N, data are presented as mean ± SEM; other data are presented as mean ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001 by unpaired 2-tailed t tests unless otherwise specified. ns, no statistical significance; ROS, reactive oxygen species.
Dcaf8 knockout leads to cellular senescence and DNA damage in HSCs. (A-D) Representative images of (A) P16 and (C) P21 indicated by immunofluorescence, with quantification of (B) P16 (n = 64 cells for WT; n = 67 cells for Dcaf8−/−, pooled from 3 mice) and (D) P21 (n = 39 cells for WT; n = 36 cells for Dcaf8−/−, pooled from 3 mice) by MFI in purified LT-HSCs from young WT and Dcaf8−/− mice. Scale bar, 5 μm. (E) RT-qPCR of p16, p21, and p53 in purified LT-HSCs from young WT and Dcaf8−/− mice (n = 4 technical replicates, pooled from 3 mice). (F-G) Representative images of (F) senescence-associated β-galactosidase (SA-β-gal) staining and (G) quantification in BM cells from young WT, Dcaf8−/− mice, and aged (20 months old) WT controls (n = 3 independent experiments, each pooled from 3 mice). Statistical analysis used 1-way ANOVA followed by the Tukey multiple comparisons test. (H-I) Representative images of (H) phosphorylated H2A histone family member X (γ-H2AX) indicated by immunofluorescence and (I) quantification of γ-H2AX foci in purified LT-HSCs from young WT and Dcaf8−/− mice (n = 34 cells for WT; n = 40 cells for Dcaf8−/−, pooled from 3 mice). Scale bar, 5 μm. Statistical analysis used 2-tailed Mann-Whitney U test. (J) Representative flow cytometric plots and (K) analysis of γ-H2AX expression of LT-HSCs from young WT and Dcaf8−/− mice (n = 4 mice). (L) Representative alkaline comets and quantification by (M) percent of DNA in tail and (N) olive tail moment of purified LT-HSCs from young WT (n = 65 cells, pooled from 3 mice), Dcaf8−/− (n = 79 cells, pooled from 3 mice) mice, and aged (20 months old) WT controls (n = 76 cells, pooled from 3 mice). Statistical analysis used Kruskal-Wallis test followed by the Dunn multiple comparisons test. (O) Analysis of reactive oxygen species levels stained with DCFH-DA by flow cytometry of LT-HSCs from young WT (n = 3 mice), Dcaf8−/− (n = 4 mice) mice, and aged (20 months old) WT controls (n = 4 mice). Statistical analysis used 1-way ANOVA followed by the Tukey multiple comparisons test. For panels I, M, and N, data are presented as mean ± SEM; other data are presented as mean ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001 by unpaired 2-tailed t tests unless otherwise specified. ns, no statistical significance; ROS, reactive oxygen species.
Cellular senescence can be triggered by factors such as telomere shortening and DNA damage.35 We observed a significant increase in phosphorylated H2A histone family member X, a marker of DNA damage, in LT-HSCs from Dcaf8−/− mice, as shown by increased foci number by immunostaining (Figure 3H-I), and elevated expression by flow cytometry (Figure 3J-K). To directly assess DNA damage, we performed an alkaline comet assay, which measures single- and double-strand breaks.39 LT-HSCs from Dcaf8−/− mice exhibited significantly elevated percentages of tail DNA and olive tail moments compared with WT, reaching levels comparable with those in aged controls (Figure 3L-N). Additionally, reactive oxygen species levels in LT-HSCs were significantly increased in Dcaf8−/− mice compared with both WT and aged controls, suggesting that oxidative stress is distinctly elevated in the absence of DCAF8 (Figure 3O).
Together, these results indicate that the loss of DCAF8 induces cellular senescence and DNA damage in HSCs, contributing to their functional decline.
Loss of DCAF8 leads to DOCK11 accumulation via impaired ubiquitination
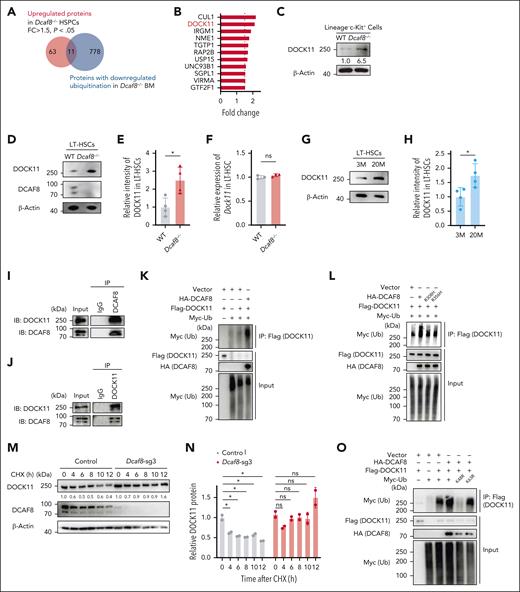
Given DCAF8’s role as a substrate receptor in the E3 ubiquitin ligase complex,13 we expanded the scope of our analysis to include the possibility of posttranslational regulation in DCAF8-deficient cells. Using quantitative proteomics on Lin− HSPCs from Dcaf8−/− mice and WT controls, we identified 74 significantly upregulated proteins and 8 significantly downregulated proteins (P < .05; fold change of >1.5 or <0.67; supplemental Table 2). To pinpoint direct substrates of DCAF8 in hematopoietic cells, we performed a ubiquitin proteomics analysis of BM cells from Dcaf8−/− and WT mice (supplemental Table 3). By intersecting the proteins significantly upregulated in HSPCs of Dcaf8−/− mice with those exhibiting significantly downregulated levels of ubiquitination in the BM of Dcaf8−/− mice (ubiquitin sites uniquely detected in WT group), we examined potential functional substrates (Figure 4A-B).
DOCK11 is upregulated in Dcaf8 knockout HSPCs and is polyubiquitinated and destabilized by DCAF8. (A) Venn diagram for proteins that were upregulated in Dcaf8−/− HSPCs or proteins with downregulated ubiquitination in Dcaf8−/− BM cells. (B) Proteins identified in an overlap analysis as shown in panel A. Fold changes are represented as Dcaf8−/− vs WT HSPCs. (C) Immunoblot of DOCK11 in LK cells from WT and Dcaf8−/− mice. (D) Representative immunoblot of DOCK11 and DCAF8 in LT-HSCs from WT and Dcaf8−/− mice; and (E) the relative intensity of DOCK11 immunoblot, quantified and normalized to β-actin (n = 4 independent experiments). (F) RT-qPCR of Dock11 in LT-HSCs from WT and Dcaf8−/− mice (n = 3 technical replicates, pooled from 3 mice). (G) Representative immunoblot of DOCK11 in LT-HSCs at different mouse ages, and (H) the relative intensity of DOCK11 immunoblot, quantified and normalized to β-actin (n = 3 independent experiments). (I-J) Coimmunoprecipitation of DCAF8 and DOCK11. DCAF8 (I) and DOCK11 (J) were immunoprecipitated in cell lysates of Ba/F3 cells, and the associated DCAF8 and DOCK11 were analyzed. (K) HEK293T cells were transiently transfected with the plasmids indicated. Flag-tagged DOCK11 was immunoprecipitated and the ubiquitination was detected with Myc-tagged ubiquitin. (L) HEK293T cells were transiently transfected with WT HA-tagged DCAF8, or DCAF8 mutants R308H or R356H. Flag-tagged DOCK11 was immunoprecipitated and the ubiquitination was detected with Myc-tagged ubiquitin (Ub). (M) Immunoblot of DCAF8 and DOCK11 in Ba/F3 cells stably expressing vectors or Dcaf8 single-guide RNA (sgRNA). Cells were treated with CHX (50 μg/mL) for the indicated durations and lysed. (N) The relative intensity of DOCK11 in panel M was quantified and normalized to β-actin (n = 2 independent experiments). (O) HEK293T cells were transiently transfected with WT Ub or Ub mutants K48R or K63R. Flag-tagged DOCK11 was immunoprecipitated and the ubiquitination was detected with Myc-tagged Ub. Data are presented as mean ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001 by unpaired 2-tailed t tests unless otherwise specified. CHX, cycloheximide; FC, fold change; Flag, FLAG tag; HA, hemagglutinin tag; IB, immunoblot; IgG, immunoglobulin G; IP, immunoprecipitation; LK, Lin−c-Kit+; M, months; ns, no statistical significance.
DOCK11 is upregulated in Dcaf8 knockout HSPCs and is polyubiquitinated and destabilized by DCAF8. (A) Venn diagram for proteins that were upregulated in Dcaf8−/− HSPCs or proteins with downregulated ubiquitination in Dcaf8−/− BM cells. (B) Proteins identified in an overlap analysis as shown in panel A. Fold changes are represented as Dcaf8−/− vs WT HSPCs. (C) Immunoblot of DOCK11 in LK cells from WT and Dcaf8−/− mice. (D) Representative immunoblot of DOCK11 and DCAF8 in LT-HSCs from WT and Dcaf8−/− mice; and (E) the relative intensity of DOCK11 immunoblot, quantified and normalized to β-actin (n = 4 independent experiments). (F) RT-qPCR of Dock11 in LT-HSCs from WT and Dcaf8−/− mice (n = 3 technical replicates, pooled from 3 mice). (G) Representative immunoblot of DOCK11 in LT-HSCs at different mouse ages, and (H) the relative intensity of DOCK11 immunoblot, quantified and normalized to β-actin (n = 3 independent experiments). (I-J) Coimmunoprecipitation of DCAF8 and DOCK11. DCAF8 (I) and DOCK11 (J) were immunoprecipitated in cell lysates of Ba/F3 cells, and the associated DCAF8 and DOCK11 were analyzed. (K) HEK293T cells were transiently transfected with the plasmids indicated. Flag-tagged DOCK11 was immunoprecipitated and the ubiquitination was detected with Myc-tagged ubiquitin. (L) HEK293T cells were transiently transfected with WT HA-tagged DCAF8, or DCAF8 mutants R308H or R356H. Flag-tagged DOCK11 was immunoprecipitated and the ubiquitination was detected with Myc-tagged ubiquitin (Ub). (M) Immunoblot of DCAF8 and DOCK11 in Ba/F3 cells stably expressing vectors or Dcaf8 single-guide RNA (sgRNA). Cells were treated with CHX (50 μg/mL) for the indicated durations and lysed. (N) The relative intensity of DOCK11 in panel M was quantified and normalized to β-actin (n = 2 independent experiments). (O) HEK293T cells were transiently transfected with WT Ub or Ub mutants K48R or K63R. Flag-tagged DOCK11 was immunoprecipitated and the ubiquitination was detected with Myc-tagged Ub. Data are presented as mean ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001 by unpaired 2-tailed t tests unless otherwise specified. CHX, cycloheximide; FC, fold change; Flag, FLAG tag; HA, hemagglutinin tag; IB, immunoblot; IgG, immunoglobulin G; IP, immunoprecipitation; LK, Lin−c-Kit+; M, months; ns, no statistical significance.
Among these candidates, DOCK11 emerged as one of the most upregulated proteins with reduced ubiquitination levels in Dcaf8−/− mice (Figure 4B). DOCK11, a guanine nucleotide exchange factor for Rho guanosine triphosphatase that specifically activates CDC42, is predominantly expressed in hematopoietic cells.40-42 Elevated CDC42 activity has been previously linked to HSC aging, contributing to cellular senescence and genomic instability.43 We confirmed the upregulation of DOCK11 protein in Lin−c-Kit+ HSPCs in Dcaf8−/− mice (Figure 4C). We further performed analysis on sorted LT-HSCs directly, which confirmed a significant increase in DOCK11 protein expression in Dcaf8−/− HSCs (Figure 4D-E), without a corresponding increase in Dock11 transcript levels (Figure 4F), suggesting posttranslational regulation. Notably, DOCK11 protein was also elevated in LT-HSCs from aged mice compared with young mice (Figure 4G-H), paralleling the age-related decline in DCAF8 expression.
To investigate whether DCAF8 mediates the polyubiquitination of DOCK11, we first validated the interaction between DCAF8 and DOCK11 using coimmunoprecipitation assays in Ba/F3 cells, a murine hematopoietic progenitor cell line (Figure 4I-J). In vitro ubiquitination assays confirmed that the presence of DCAF8 increased the ubiquitination of DOCK11 (Figure 4K; supplemental Figure 5A). Previous studies identified specific mutations in DCAF8 (R314H and R362H) that disrupt its binding to the CRL4 complex.14 We introduced the analogous mutations (R308H and R356H) in the murine Dcaf8 gene, and either mutation abolished DOCK11 ubiquitination by DCAF8 (Figure 4L), indicating that the CRL4DCAF8 E3 ubiquitin ligase complex is essential for DOCK11 ubiquitination and degradation.
As expected, DCAF8 destabilized DOCK11 by promoting its ubiquitination. Knock down of Dcaf8 in Ba/F3 cells significantly prolonged the half-life of DOCK11 (Figure 4M-N), whereas overexpression of Dcaf8 significantly shortened it (supplemental Figure 5B-C). In addition, the K48R ubiquitin mutant, but not the K63R mutant, abolished the ubiquitination of DOCK11 in the presence of DCAF8 (Figure 4O), indicating that DCAF8 facilitates the polyubiquitination of DOCK11 via K48 linkage. Overall, these findings reveal that DCAF8 promotes the polyubiquitination and degradation of DOCK11, and its loss results in DOCK11 accumulation in HSPCs.
DCAF8 deficiency leads to elevated CDC42 activity and loss of cell polarity in HSCs
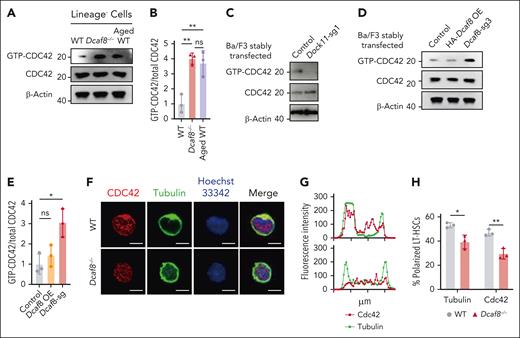
Next, we investigated whether loss of DCAF8 and subsequent DOCK11 accumulation led to elevated activity of CDC42. Our results showed an elevation of CDC42 activity in Lin− cells from Dcaf8−/− mice compared with WT controls, and this elevation was comparable with that observed in aged controls (Figure 5A-B). To directly assess the molecular regulation of CDC42 activity by DCAF8 and DOCK11 in vitro, we detected CDC42 activation in Ba/F3 cells after knockdown of either Dcaf8 or Dock11. CDC42 activity decreased upon Dock11 knockdown (Figure 5C), whereas Dcaf8 knockdown led to a marked increase in CDC42 activation (Figure 5D-E), consistent with the pattern observed in vivo.
Dcaf8 knockout leads to elevated activity of CDC42. (A) Immunoblot of guanosine triphosphate (GTP)-bound (active) CDC42 and total CDC42 in Lin− cells from WT, Dcaf8−/− mice, and aged (20 months old) WT controls, and (B) the ratio of GTP-bound CDC42 to total CDC42 (n = 3 independent experiments). Statistical analysis used 1-way ANOVA followed by the Tukey multiple comparisons test. (C) Immunoblot of GTP-bound (active) CDC42 and total CDC42 in Ba/F3 cells stably expressing vectors or Dock11 sgRNA. (D) Immunoblot of GTP-bound (active) CDC42 and total CDC42 in Ba/F3 cells stably expressing vectors, overexpressing HA-tagged DCAF8, or expressing Dcaf8 sgRNA, and (E) the ratio of GTP-bound CDC42 to total CDC42 (n = 3 independent experiments). Statistical analysis used 1-way ANOVA followed by the Tukey multiple comparisons test. (F) Representative immunofluorescence images showing the distribution of CDC42 and tubulin in purified LT-HSCs from WT and Dcaf8−/− mice. Scale bar, 5 μm. (G) Representative intensity plots along sections through locations of distributed CDC42 and tubulin across a cell, as seen in panel F. (H) Percentages of LT-HSCs with polarized distribution of CDC42 and tubulin in WT and Dcaf8−/− mice (n = 3 independent experiments, in each experiment at least 40 cells pooled from 3 mice were analyzed). Data are presented as mean ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001 by unpaired 2-tailed t tests unless otherwise specified. HA, hemagglutinin tag; ns, no statistical significance.
Dcaf8 knockout leads to elevated activity of CDC42. (A) Immunoblot of guanosine triphosphate (GTP)-bound (active) CDC42 and total CDC42 in Lin− cells from WT, Dcaf8−/− mice, and aged (20 months old) WT controls, and (B) the ratio of GTP-bound CDC42 to total CDC42 (n = 3 independent experiments). Statistical analysis used 1-way ANOVA followed by the Tukey multiple comparisons test. (C) Immunoblot of GTP-bound (active) CDC42 and total CDC42 in Ba/F3 cells stably expressing vectors or Dock11 sgRNA. (D) Immunoblot of GTP-bound (active) CDC42 and total CDC42 in Ba/F3 cells stably expressing vectors, overexpressing HA-tagged DCAF8, or expressing Dcaf8 sgRNA, and (E) the ratio of GTP-bound CDC42 to total CDC42 (n = 3 independent experiments). Statistical analysis used 1-way ANOVA followed by the Tukey multiple comparisons test. (F) Representative immunofluorescence images showing the distribution of CDC42 and tubulin in purified LT-HSCs from WT and Dcaf8−/− mice. Scale bar, 5 μm. (G) Representative intensity plots along sections through locations of distributed CDC42 and tubulin across a cell, as seen in panel F. (H) Percentages of LT-HSCs with polarized distribution of CDC42 and tubulin in WT and Dcaf8−/− mice (n = 3 independent experiments, in each experiment at least 40 cells pooled from 3 mice were analyzed). Data are presented as mean ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001 by unpaired 2-tailed t tests unless otherwise specified. HA, hemagglutinin tag; ns, no statistical significance.
Elevated CDC42 activity has been associated with a loss of cell polarity in HSCs, a hallmark of HSC aging.44-46 In Dcaf8−/− LT-HSCs, we observed a uniform distribution of both proteins throughout the cell body, indicating a loss of polarity. In contrast, LT-HSCs from age-matched WT controls displayed the typical polarized distribution (Figure 5F-H). Importantly, CDC42 protein levels in LT-HSCs were comparable between Dcaf8−/− and WT mice (supplemental Figure 5D), indicating that the altered distribution pattern was not due to differences in CDC42 expression. These findings underscore that Dcaf8 knockout leads to a disruption in HSC polarity as a result of elevated CDC42 activity.
Dock11 knockout alleviates cellular defects and partially restores self-renewal capacity of DCAF8-deficient HSCs
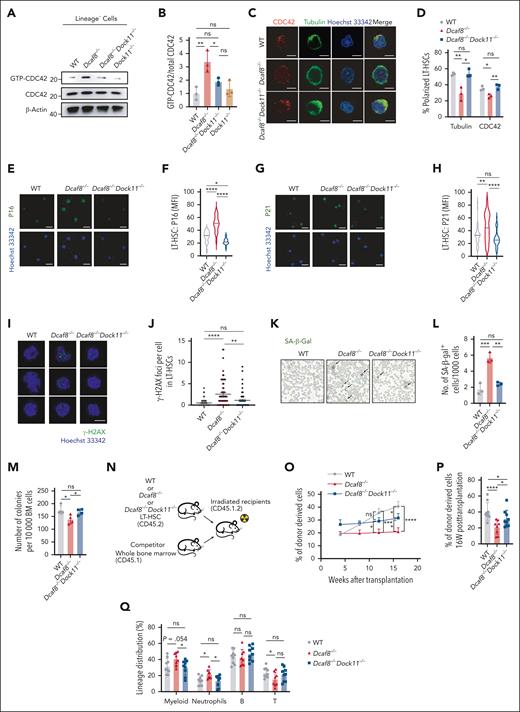
To investigate the functional role of DOCK11 in HSCs in the context of DCAF8 deficiency, we generated Dock11 knockout mice using CRISPR-CRISPR–associated protein 9 and bred them with Dcaf8 knockout mice to create Dcaf8/Dock11 double-knockout mice (supplemental Figure 6A-B). Indeed, CDC42 activity, which elevated in Dcaf8−/− Lin− cells, was restored to WT levels in Dcaf8−/−Dock11−/− cells (Figure 6A-B). Consistently, the loss of polarity observed in Dcaf8−/− LT-HSCs was rescued in the double-knockout group (Figure 6C-D), supporting the DOCK11-CDC42 axis as the mediator of these defects. Remarkably, immunofluorescence staining of purified LT-HSCs showed significant lower expression of P16 (Figure 6E-F) and P21 (Figure 6G-H) in Dcaf8−/−Dock11−/− mice compared with Dcaf8−/− mice. Quantification of phosphorylated H2A histone family member X foci in LT-HSCs revealed a significant reduction in the double-knockout group (Figure 6I-J). Furthermore, senescence-associated β-galactosidase was significantly reduced in the BM cells of Dcaf8−/−Dock11−/− mice compared with Dcaf8−/− mice, comparable with the levels in WT controls (Figure 6K-L). These results revealed that loss of DOCK11 mitigates cellular senescence and DNA damage in Dcaf8−/− LT-HSCs.
Dock11 knockout ameliorates cellular and functional defects in HSCs caused by DCAF8 deficiency. (A) Immunoblot of GTP-bound (active) CDC42 and total CDC42 in Lin− cells from WT, Dcaf8−/−, Dcaf8−/−Dock11−/−, and Dock11−/− mice, and (B) the ratio of GTP-bound CDC42 to total CDC42 (n = 3 independent experiments). Statistical analysis used 1-way ANOVA followed by the Tukey multiple comparisons test. (C) Representative immunofluorescence images showing the distribution of CDC42 and tubulin in purified LT-HSCs from WT, Dcaf8−/−, and Dcaf8−/−Dock11−/− mice. Scale bar, 5 μm. (D) Percentages of LT-HSCs with polarized distribution of CDC42 and tubulin in WT, Dcaf8−/−, and Dcaf8−/−Dock11−/− mice (n = 3 independent experiments, in each experiment at least 40 cells pooled from 3 mice were analyzed). Statistical analysis used 1-way ANOVA followed by the Tukey multiple comparisons test. (E-H) Representative images of (E) P16 and (G) P21 indicated by immunofluorescence, with quantification of (F) P16 (n = 20 cells, pooled from 3 mice) and (H) P21 (n = 40, pooled from 3 mice) by MFI in purified LT-HSCs from WT, Dcaf8−/−, and Dcaf8−/−Dock11−/− mice. Scale bar, 5 μm. Statistical analysis used 1-way ANOVA followed by the Tukey multiple comparisons test. (I) Representative images of γ-H2AX indicated by immunofluorescence, with (J) quantification of γ-H2AX foci in purified LT-HSCs from WT (n = 53 cells, pooled from 3 mice), Dcaf8−/− (n = 67 cells, pooled from 3 mice), and Dcaf8−/−Dock11−/− mice (n = 63 cells, pooled from 3 mice). Scale bar, 5 μm. Statistical analysis used Kruskal-Wallis test followed by the Dunn multiple comparisons test. (K) Representative images of SA-β-gal staining and (L) quantification in the BM cells from WT, Dcaf8−/−, and Dcaf8−/−Dock11−/− mice (n = 3 independent experiments, each pooled from 3 mice). Statistical analysis used 1-way ANOVA followed by the Tukey multiple comparisons test. (M) Total number of colonies after 1 × 104 BM cells were plated to determine the colony-forming capacity of WT, Dcaf8−/−, and Dcaf8−/−Dock11−/− mice (n = 4 mice). Statistical analysis used 1-way ANOVA followed by the Fisher least significant difference test. (N) Schematic of the transplantation assay as illustrated in panels O-Q. (O) Donor chimerism analyses in the PB of recipients at the indicated time points (n = 9 recipients for WT and Dcaf8−/−Dock11−/−, n = 8 recipients for Dcaf8−/−). Statistical analysis used 2-way ANOVA followed by the Fisher least significant difference test. (P) Donor chimerism analyses in the PB of recipients at the end point (n = 9 recipients for WT and Dcaf8−/−Dock11−/−, n = 8 recipients for Dcaf8−/−). Statistical analysis used 2-way ANOVA followed by the Fisher least significant difference test. (Q) Frequencies of myeloid (CD11b+), neutrophils (CD11b+Ly6G+), B cells (CD19+), and T cells (CD3+) in donor-derived cells in the PB of the recipients at the end point (n = 9 recipients for WT and Dcaf8−/−Dock11−/−, n = 8 recipients for Dcaf8−/−). Statistical analysis used 1-way ANOVA followed by the Fisher least significant difference test. For panels J and O, data are presented as mean ± SEM; other data are presented as mean ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001 by unpaired 2-tailed t tests unless otherwise specified. B, B cells; ns, no statistical significance; T, T cells.
Dock11 knockout ameliorates cellular and functional defects in HSCs caused by DCAF8 deficiency. (A) Immunoblot of GTP-bound (active) CDC42 and total CDC42 in Lin− cells from WT, Dcaf8−/−, Dcaf8−/−Dock11−/−, and Dock11−/− mice, and (B) the ratio of GTP-bound CDC42 to total CDC42 (n = 3 independent experiments). Statistical analysis used 1-way ANOVA followed by the Tukey multiple comparisons test. (C) Representative immunofluorescence images showing the distribution of CDC42 and tubulin in purified LT-HSCs from WT, Dcaf8−/−, and Dcaf8−/−Dock11−/− mice. Scale bar, 5 μm. (D) Percentages of LT-HSCs with polarized distribution of CDC42 and tubulin in WT, Dcaf8−/−, and Dcaf8−/−Dock11−/− mice (n = 3 independent experiments, in each experiment at least 40 cells pooled from 3 mice were analyzed). Statistical analysis used 1-way ANOVA followed by the Tukey multiple comparisons test. (E-H) Representative images of (E) P16 and (G) P21 indicated by immunofluorescence, with quantification of (F) P16 (n = 20 cells, pooled from 3 mice) and (H) P21 (n = 40, pooled from 3 mice) by MFI in purified LT-HSCs from WT, Dcaf8−/−, and Dcaf8−/−Dock11−/− mice. Scale bar, 5 μm. Statistical analysis used 1-way ANOVA followed by the Tukey multiple comparisons test. (I) Representative images of γ-H2AX indicated by immunofluorescence, with (J) quantification of γ-H2AX foci in purified LT-HSCs from WT (n = 53 cells, pooled from 3 mice), Dcaf8−/− (n = 67 cells, pooled from 3 mice), and Dcaf8−/−Dock11−/− mice (n = 63 cells, pooled from 3 mice). Scale bar, 5 μm. Statistical analysis used Kruskal-Wallis test followed by the Dunn multiple comparisons test. (K) Representative images of SA-β-gal staining and (L) quantification in the BM cells from WT, Dcaf8−/−, and Dcaf8−/−Dock11−/− mice (n = 3 independent experiments, each pooled from 3 mice). Statistical analysis used 1-way ANOVA followed by the Tukey multiple comparisons test. (M) Total number of colonies after 1 × 104 BM cells were plated to determine the colony-forming capacity of WT, Dcaf8−/−, and Dcaf8−/−Dock11−/− mice (n = 4 mice). Statistical analysis used 1-way ANOVA followed by the Fisher least significant difference test. (N) Schematic of the transplantation assay as illustrated in panels O-Q. (O) Donor chimerism analyses in the PB of recipients at the indicated time points (n = 9 recipients for WT and Dcaf8−/−Dock11−/−, n = 8 recipients for Dcaf8−/−). Statistical analysis used 2-way ANOVA followed by the Fisher least significant difference test. (P) Donor chimerism analyses in the PB of recipients at the end point (n = 9 recipients for WT and Dcaf8−/−Dock11−/−, n = 8 recipients for Dcaf8−/−). Statistical analysis used 2-way ANOVA followed by the Fisher least significant difference test. (Q) Frequencies of myeloid (CD11b+), neutrophils (CD11b+Ly6G+), B cells (CD19+), and T cells (CD3+) in donor-derived cells in the PB of the recipients at the end point (n = 9 recipients for WT and Dcaf8−/−Dock11−/−, n = 8 recipients for Dcaf8−/−). Statistical analysis used 1-way ANOVA followed by the Fisher least significant difference test. For panels J and O, data are presented as mean ± SEM; other data are presented as mean ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001 by unpaired 2-tailed t tests unless otherwise specified. B, B cells; ns, no statistical significance; T, T cells.
Next. we evaluated whether this reduction in cellular senescence and DNA damage resulted in improved self-renewal capacity. In vitro colony-forming unit assays showed a significant increase in colony formation from BM cells of Dcaf8−/−Dock11−/− mice compared with Dcaf8−/− mice (Figure 6M). To assess in vivo self-renewal capacity, we transplanted purified LT-HSCs from each group into irradiated recipient mice (Figure 6N). After stable engraftment, the donor chimerism in the Dcaf8−/−Dock11−/− group showed significant improvement compared with the Dcaf8−/− group (Figure 6O-P). Lineage analysis of donor-derived cells in the PB revealed a significant reduction in overall myeloid and neutrophil output in the Dcaf8−/−Dock11−/− group compared with Dcaf8−/−, reaching levels similar to WT (Figure 6Q). These results revealed that the loss of DOCK11 mitigates the self-renewal defects in Dcaf8−/− HSCs.
To clarify the role of DOCK11 in steady-state hematopoiesis, we comprehensively analyzed Dock11−/− mice. Across multiple time points, Dock11−/− mice exhibited largely normal PB counts, lineage distribution in the PB and BM, and unaffected BM and spleen cellularity (supplemental Figures 6C-H and 7A-B,G,H). Frequencies of Lin−, LSK, and progenitor cells remained comparable with WT (supplemental Figure 7C-F). Notably, a consistent increase in frequencies of short-term HSCs was observed in Dock11−/− mice at both 3 and 6 months (supplemental Figure 7I-L). Despite these changes, Dock11−/− mice exhibited normal colony-forming ability, HSC self-renewal capacity in competitive transplantation (supplemental Figure 8A-E), and stress response to 5-FU (supplemental Figure 8F). These findings support that DOCK11 deficiency alone does not impair steady-state hematopoiesis.
In summary, our findings suggest that the accumulation of DOCK11, due to an age-related decline in DCAF8-mediated degradation, impairs HSC function and contributes to cellular senescence.
Discussion
In this study, we highlight the critical role of DCAF8, a protein highly expressed in HSCs, in maintaining HSC function and preventing cellular senescence. We demonstrate that the age-related decline in DCAF8 expression, coupled with accumulation of DOCK11 and subsequent increase in CDC42 activity, underlies a previously unrecognized mechanism of HSC functional decline.
One of the key observations in this study is the significant age-related decline of DCAF8 expression in HSCs, suggesting a critical role for DCAF8 in the LT regulation of these cells. Loss of DCAF8, as demonstrated in our experiments, correlates with impaired HSC self-renewal capacity and an upregulation of key senescence markers P16 and P21. Although it is well established that HSC function diminishes with age, our findings indicate that reduced DCAF8 expression may contribute to this process through specific cellular mechanisms, particularly the induction of cellular senescence. These findings are consistent with other studies on regulated protein homeostasis in HSC maintenance.7 Similar age-related declines in other ubiquitin ligase components, such as CUL4A (Cullin-4A) and DDB1 (DNA damage-binding protein 1) have been reported,47 suggesting a shared mechanism in HSC aging.
This study also highlights DOCK11 as a guanine nucleotide exchange factor responsible for activating CDC42, leading to disrupted polarity and contributing to HSC aging.44,46,48 We demonstrate that the age-related decline in DCAF8 leads to DOCK11 accumulation and elevated CDC42 activity, revealing a new regulatory pathway in which DCAF8 modulates CDC42 activity via DOCK11 degradation to maintain HSC function.
The disruption of cell polarity is a critical factor in the functional decline of HSCs with age. We propose that the elevated CDC42 activity and loss of polarity of HSCs, which increase mobilization during aging,49 ultimately leads to HSC exhaustion. This loss of polarity not only affects asymmetric division,45 but also impairs the ability of HSCs to interact with BM niche cells.50,51 As a result, aged HSCs are more likely to divide symmetrically, leading to an increase in HSC numbers but a decline in their regenerative potential.45 The Dcaf8−/− mouse model mimics this age-related phenotype, demonstrating reduced self-renewal capacity.
Proper regulation of CDC42 activity is essential for maintaining HSC polarity and function.52 Both deficiency and overactivation of CDC42 have been shown to impair HSC adhesion, homing, and engraftment abilities, as well as disrupt cell polarity.44,53-55 Although the CDC42 inhibitor CASIN has shown promise in restoring HSC function in aged mice,44,56 it also poses potential risks by inhibiting CDC42 in cells in which its activity is normal.57,58 Targeting DOCK11 offers an alternative approach to reducing CDC42 activity in aged HSCs.
Moreover, this study establishes a link between DCAF8 deficiency and increased DNA damage in LT-HSCs, reinforcing the role of ubiquitin proteasome system dysregulation in HSC maintenance. Previous studies reported that elevated CDC42 activity predisposes cells to genomic instability by compromising DNA damage repair mechanisms.43,59,60 This study reinforces its involvement in HSC genomic instability. Notably, Dock11 knockout mitigates the genomic instability observed in Dcaf8−/− HSCs, highlighting the regulatory role of the DCAF8-DOCK11-CDC42 axis.
Although DOCK11 is primarily expressed in the hematopoietic system, previous studies have mainly focused on its role in immune cells and immune-related diseases.40,41,61-63 This study uniquely identifies DOCK11 as a detrimental factor in HSC functional decline. Interestingly, DOCK11 expression is downregulated in immune tissues in aged mice,64,65 suggesting distinct regulatory patterns across different cell types.
In summary, this study reveals that age-related DCAF8 decline in HSCs causes DOCK11 accumulation and elevated CDC42 activity, promoting senescence and dysfunction, thereby identifying potential therapeutic targets for preserving HSC function.
Acknowledgments
The authors thank Yi Jin for her assistance with cell sorting, Yan Shen for her assistance with mouse treatment, and Zheng Ruan for her assistance with confocal fluorescence microscopy.
This work was supported by the National Natural Science Foundation of China (numbers 82170111 and 81970134 [P.L.], numbers 82170147 and 81870112 [R.R.], number 82200120 [X.Z.], and number 81770171 [B.J.]), the Key Project of National Natural Science Foundation of China (number 82230088 [R.R.]), Shanghai Science and Technology Development Funds (number 20Z11900200 [R.R.]), Shanghai Collaborative Innovation Program on Regenerative Medicine and Stem Cell Research (number 2019CXJQ01 [R.R.]), the Samuel Waxman Cancer Research Foundation (R.R.), Shanghai Pujiang Program (number 2021PJD043 [B.J.]), and the Innovative Research Team of High-level Local Universities in Shanghai (R.R. and B.J.).
Authorship
Contribution: P.L. and R.R. conceptualized the study; P.L., R.R., and B.J. developed the methodology; P.X., X.Z., D.L., J.N., and Y.H. performed the investigations; P.X., X.Z., and D.L. performed formal analysis and data curation; Z.X., J.L., Y.D., X.H., L.Y., R.Z., W.H., X.W., S.J., and Y.C. provided resources; P.X. and X.Z. wrote the original manuscript draft; P.X., X.Z., D.L., P.L., and R.R. reviewed and edited the manuscript; and P.L., R.R., and B.J. supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ping Liu, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, No. 197 Ruijin Second Road, Shanghai 200025, China; email: liuping@shsmu.edu.cn; and Ruibao Ren, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, No. 197 Ruijin Second Road, Shanghai 200025, China; International Center for Aging and Cancer, Hainan Medical University, Haikou 571199, China; email: rbren@sjtu.edu.cn.
References
Author notes
P.X., X.Z., and D.L. contributed equally to this study.
RNA sequencing (RNA-seq) data and proteomics and ubiquitin proteomics data have been deposited at the Gene Expression Omnibus (GEO) and ProteomeXchange database and are publicly available as of the date of publication. RNA-seq data are accessible through the National Center for Biotechnology Information GEO (accession number GSE278044). Proteomics of lineage-negative cells are accessible through ProteomeXchange (accession number PXD056107). Ubiquitin proteomics of bone marrow cells are accessible through ProteomeXchange (accession numbers PXD056221and PXD056263).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal