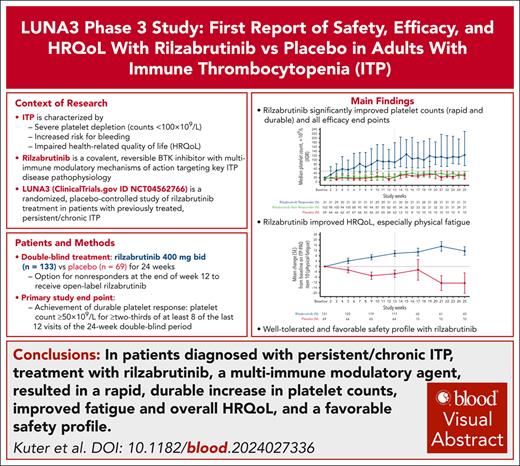
Key Points
Rilzabrutinib acts through multi-immune modulation mechanisms targeting key aspects of ITP disease pathophysiology.
Rilzabrutinib had rapid and durable platelet response, reduced rescue use and bleeding, improved physical fatigue, and favorable safety.
Visual Abstract
Rilzabrutinib is a covalent, reversible Bruton tyrosine kinase inhibitor targeting multiple immune thrombocytopenia (ITP)-related mechanisms. The phase 3 LUNA3 study in previously treated adults with persistent/chronic ITP evaluated oral rilzabrutinib 400 mg twice daily (n = 133) vs placebo (n = 69) for 24 weeks. At baseline overall, median age was 47 years, 63% female, 7.7 year median ITP duration, and 28% prior splenectomy. Overall (N = 202), 85 (64%) rilzabrutinib and 22 (32%) placebo patients achieved platelet response (≥50 × 109/L or 30 × 109/L to <50 × 109/L and doubled from baseline) during the first 12 weeks and were eligible to continue. The primary end point, durable platelet response (platelet count ≥50 × 109/L for ≥two-thirds of ≥8 of the last 12 of 24 weeks without rescue therapy), was observed in 31 (23%) rilzabrutinib vs 0 placebo patients (P < .0001). All secondary efficacy end points were significantly superior for rilzabrutinib (P < .05). Median time to first platelet response was 15 days in rilzabrutinib responders. Rilzabrutinib significantly reduced rescue therapy use by 52% (P = .0007) and improved week 25 bleeding scores (P = .0006). Improved physical fatigue was sustained from week 13 (P = .01) through 25 (P = .0003). Treatment-related adverse events were mainly grade 1/2. One rilzabrutinib patient with multiple risk factors had serious treatment-related grade 3 peripheral embolism (lower left leg), and another died from unrelated pneumonia. Rilzabrutinib in patients who failed multiple previous ITP therapies showed rapid and durable platelet response, reduced rescue medication and bleeding, improved physical fatigue, and favorable safety. Trial registration: www.clinicaltrials.gov (#NCT04562766) and www.clinicaltrialsregister.eu (#2020-002063-60).
Introduction
The acquired autoimmune hematologic disease immune thrombocytopenia (ITP) is estimated to have a worldwide prevalence of 10 to 23 per 100 000 people and incidence of ∼2 to 4 per 100 000 person-years in the adult and pediatric general population.1-7 As a disease, ITP is characterized mainly by autoantibody-mediated platelet destruction with impaired platelet production, resulting in thrombocytopenia (ie, platelet count of <100 × 109/L), increased bleeding risk, and impaired health-related quality of life (HRQoL).8-10
Treatment for adults with ITP focuses on overt disease characteristics aiming at increasing platelet counts while lowering the bleeding risks.11-13 Current ITP therapy, although effective in raising platelet counts in many patients, fails in others, and is also hampered by high relapse rates among initial responders, side effects, and long-term tolerability, and often fails to address HRQoL issues, especially fatigue.11,14,15 For patients with severe or chronic ITP, the disease burden is more significant, especially in those unresponsive to current therapy, contributing to elevated mortality rates and reinforcing the high unmet therapeutic needs for these individuals.16,17
Rilzabrutinib is an oral, reversible, covalent Bruton tyrosine kinase (BTK) inhibitor optimized for safety and efficacy in autoimmune and/or inflammatory diseases.18 Tailored covalency and high selectivity and specificity of rilzabrutinib is thought to contribute to long BTK target engagement while decreasing the risk for off-target effects (eg, atrial fibrillation, bleeding) relative to other BTK inhibitors.18,19 Biochemically, rilzabrutinib is characterized by a rapid on-rate and slow off-rate for BTK, achieving >80% BTK occupancy within the first hour that is maintained over 24 hours; in vivo studies confirm high and durable BTK occupancy despite rilzabrutinib plasma levels dropping to <3 ng/mL at 14 hours after treatment.19 Rilzabrutinib mediates its therapeutic effect through multi-immune modulation mechanisms identified through preclinical studies, targeting key aspects of ITP disease pathophysiology, such as inhibiting B-cell activation, reducing pathogenic autoantibody production, interrupting platelet phagocytosis by Fcγ receptor in the spleen and liver, and inhibiting inflammatory pathways.19 Results from the open-label phase 1/2 study of rilzabrutinib for patients with difficult-to-treat ITP showed rapid and durable platelet response,20 with robust efficacy and safety profiles in a long-term extension study.21 This pivotal phase 3 is, to our knowledge, the first, randomized, placebo-controlled study evaluating the efficacy and safety of rilzabrutinib in adult and pediatric patients with persistent/chronic ITP.22 This report focuses on the final results in adults for the double-blind period.
Methods
Trial design and oversight
LUNA3 was a phase 3, multicenter, placebo-controlled, parallel group study with open-label and long-term extensions (ClinicalTrials.gov identifier: NCT04562766; EudraCT 2020-002063-60).22 Adults were randomized from multiple countries in a 2:1 ratio to oral rilzabrutinib 400 mg twice daily or placebo for the 24-week double-blind period. Randomization was stratified by prior splenectomy and thrombocytopenia severity (platelet count of <15 × 109/L or ≥15 × 109/L). At the end of week 12, patients were evaluated for “platelet response,” defined as at least 1 platelet count of ≥50 × 109/L or 30 × 109/L to <50 × 109/L and at least a doubling from baseline in the absence of rescue therapy during the first 12 weeks. Responders could continue double-blinded treatment through week 24; nonresponders could either discontinue from the study or enter the 28-week open-label period receiving rilzabrutinib 400 mg twice daily (initial treatment remained blinded) and were classified as nonresponders for the primary end point analysis.
The study adhered to International Council for Harmonisation Tripartite Guidelines on Good Clinical Practice, applicable US Food and Drug Administration Codes of Federal Regulations, and the Declaration of Helsinki. The protocol/informed consent documents were reviewed/approved by each institutional review board/independent ethics committee. All patients provided written informed consent.
Participants
Key inclusion criteria were as follows. Adult patients were aged ≥18 years with primary persistent/chronic23 ITP. Patients had to have 2 platelet counts of <30 × 109/L at least 5 days apart during the screening period, with no single platelet count of >35 × 109/L within 14 days of treatment. Included patients had to have had a prior response (ie, platelet count of ≥50 × 109/L) to IV immunoglobulin (IVIg)/anti-Rh0(D) immunoglobulin infusion (anti-D) or corticosteroids (CS) that was not sustained. In addition, patients had to have documented intolerance, insufficient response, or any contraindication to any other ITP therapy. Additional eligibility criteria details are provided in the supplemental Methods, available on the Blood website.
Only stable concomitant CS and/or thrombopoietin receptor agonist (TPO-RA) was allowed. Adjustments in the doses of concomitant ITP medications were permitted for associated safety concerns only. Rescue medication (IVIg, high-dose CS, platelet infusion, or anti-D) to raise platelet counts was allowed for platelets of <20 × 109/L or bleeding/wet purpura.
End points
The primary efficacy end point was durable platelet response: platelet counts of ≥50 × 109/L for two-thirds or more of at least 8 nonmissing weekly scheduled platelet measurements during the last 12 weeks in the absence of rescue therapy. Additionally, at least 2 of these platelet counts of ≥50 × 109/L had to be during the last 6 weeks of the 24-week blinded treatment period. Only in the European Union and United Kingdom, to be consistent with the European Medicines Agency’s regulatory recommendation, durable platelet response was defined as platelet counts of ≥50 × 109/L for ≥8 of the last 12 weeks of the 24-week blinded treatment period in the absence of rescue therapy. Patients were considered nonresponders if receiving rescue medication after 8 treatment weeks, discontinuing before week 12 of treatment, or discontinuing after week 12 and before week 25 because of lack of response or related AEs.
Key secondary efficacy end points during the double-blind period were number of weeks with platelet count of ≥50 × 109/L or ≥30 × 109/L to <50 × 109/L and at least doubled from baseline in the absence of rescue therapy, number of weeks with platelet counts of ≥30 × 109/L and at least doubled from baseline in the absence of rescue therapy, time to first platelet count of ≥50 × 109/L or ≥30 × 109/L to <50 × 109/L and at least doubled from baseline, proportion of patients requiring rescue therapy, change from baseline at week 13 on item 10 of the ITP patient assessment questionnaire24,25 (ITQ-PAQ; ie, physical fatigue; scores 0 [worst] to 100 [best] with higher score indicating better HRQoL), and change from baseline at week 25 in the idiopathic thrombocytopenic purpura bleeding scale26 (IBLS; ie, bleeding score). Overall IBLS score was derived from an assessment system grading bleeding scores from 0 (none) to 2 (marked bleeding) based on 11 site-specific assessments.26
Other secondary/exploratory end points during the double-blind period included change from baseline at week 25 on ITP-PAQ HRQoL domains,24,25 safety (per Common Terminology Criteria for AEs, version 5.0), platelet counts of ≥50 × 109/L for 4 of the last 8 weeks of the 24-week treatment period, overall platelet response (ie, platelet count of ≥50 × 109/L at 2 consecutive visits), complete platelet response (ie, platelet count of ≥100 × 109/L with no bleeding/rescue therapy at 2 consecutive visits), and platelet count of >250 × 109/L and >450 × 109/L in patients receiving concomitant TPO-RA.
Secondary efficacy end points corrected for multiplicity were number of weeks with platelet response, time to platelet response, percent of patients requiring rescue therapy, and change from baseline in physical fatigue (ITP-PAQ, item 10) and IBLS.
Statistical analyses
Efficacy was evaluated in the intent-to-treat (randomized) population, and safety in the randomized and exposed population. A sample size of 194 (129 rilzabrutinib, 65 placebo) was estimated to provide 95% power at an α level of .05 to detect a 20% difference in durable response assuming the true response rate of 25% for rilzabrutinib and 5% for placebo.
The primary end point, durable platelet response, was analyzed using the Cochran-Mantel-Haenszel test adjusted by randomization and stratification factors, and including asymptotic confidence interval (CI). Missing data were not imputed. The number of weeks with platelet response were analyzed using a mixed-effect model with repeated measures approach. The log-rank test compared time to platelet response and time to rescue therapy. Change from baseline in ITP-PAQ and IBLS were analyzed using an analysis of covariance model. A fixed-sequence procedure was applied to control the multiplicity. Anchor-based psychometric analyses determined between-group meaningful score difference thresholds for ITP-PAQ item 10 (physical fatigue) using change from baseline data to weeks 13 and 25. Between-group mean change scores and mixed-models for repeated measures were used to estimate a between-group meaningful score difference threshold range of 8 to 18.
Results
Patients
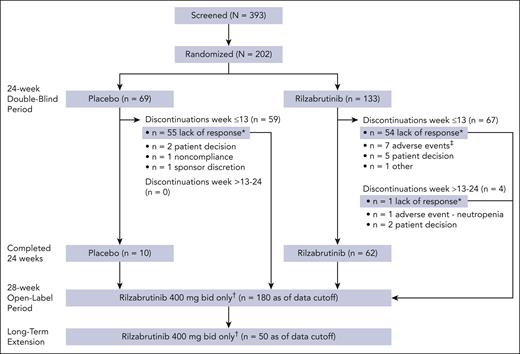
A total of 202 adult patients were randomized to rilzabrutinib (n = 133) or placebo (n = 69) from December 2020 to September 2023 in 26 countries (Figure 1). At baseline, median ages for patients in the rilzabrutinib and placebo arms, respectively, were 47 and 46 years, and 59% and 71% were female (Table 1). Median duration of ITP since diagnosis was longer for rilzabrutinib vs placebo at 8.1 years (range, 0.3-52.2) vs 6.2 years (range, 0.3-35.8). Median baseline platelet counts were 15 × 109/L (range, 1× 109/L to 54 × 109/L) for both arms. Approximately half of all patients had received ≥5 prior unique ITP therapies (43% rilzabrutinib, 52% placebo); 28% overall had prior splenectomy. For all patients, the most common prior treatments were CS (96%) and TPO-RA (69%). At baseline, the number of patients with no recorded response (defined as at least 1 platelet count of ≥50 × 109/L) to specific prior ITP therapies were 53 of 193 (27%) patients who had received CS, 52 of 139 (37%) patients who had received TPO-RAs, 32 of 118 (27%) patients who had received IVIg or anti-D Ig, 48 of 71 (68%) who received rituximab, and 17 of 24 (71%) who received fostamatinib.
CONSORT flow diagram. ∗After 12 weeks, responders with platelet count of ≥50 × 109/L or 30 × 109/L to <50 × 109/L and at least doubled from baseline (referred to as “platelet response”) could continue blinded treatment; nonresponders could discontinue or enter the 28-week open-label period receiving rilzabrutinib 400 mg twice daily (initial treatment remained blinded). Patients who discontinued the 24-week double-blind period because of lack of response were given the option to enter the 28-week open-label period. †Includes patients who have entered as of the data cutoff. All patients in the 28-week open-label and long-term extension periods received rilzabrutinib 400 mg twice daily despite their initial treatment arm assignment. ‡Included 1 patient who discontinued because of an AE of pneumonia that was deemed unrelated to treatment per investigator judgment and lasted 16 days before rilzabrutinib was discontinued and the patient died (see the supplemental Safety Results for details). bid, twice daily.
CONSORT flow diagram. ∗After 12 weeks, responders with platelet count of ≥50 × 109/L or 30 × 109/L to <50 × 109/L and at least doubled from baseline (referred to as “platelet response”) could continue blinded treatment; nonresponders could discontinue or enter the 28-week open-label period receiving rilzabrutinib 400 mg twice daily (initial treatment remained blinded). Patients who discontinued the 24-week double-blind period because of lack of response were given the option to enter the 28-week open-label period. †Includes patients who have entered as of the data cutoff. All patients in the 28-week open-label and long-term extension periods received rilzabrutinib 400 mg twice daily despite their initial treatment arm assignment. ‡Included 1 patient who discontinued because of an AE of pneumonia that was deemed unrelated to treatment per investigator judgment and lasted 16 days before rilzabrutinib was discontinued and the patient died (see the supplemental Safety Results for details). bid, twice daily.
Characteristics of patients at baseline (safety population)
| Characteristic . | Rilzabrutinib (n = 133) . | Placebo (n = 69) . | All patients (N = 202) . |
|---|---|---|---|
| Age, median (range), y | 47 (18-80) | 46 (19-79) | 47 (18-80) |
| Sex, n (%) | |||
| Male | 55 (41) | 20 (29) | 75 (37) |
| Female | 78 (59) | 49 (71) | 127 (63) |
| Duration of ITP, median (range), y | 8.1 (0.3-52.2) | 6.2 (0.3-35.8) | 7.7 (0.3-52.2) |
| Baseline platelet count, median (range),∗ ×109/L | 15 (1-32) | 15 (1-54) | 15 (1-54) |
| Baseline platelet count by category, n (%) | |||
| <15 × 109/L | 65 (49) | 32 (46) | 97 (48) |
| ≥15 × 109/L | 68 (51) | 37 (54) | 105 (52) |
| Prior unique therapies,†n (%) | |||
| 1-2 | 43 (32) | 15 (22) | 58 (29) |
| 3-4 | 33 (25) | 18 (26) | 51 (25) |
| ≥5 | 57 (43) | 36 (52) | 93 (46) |
| No. of unique prior ITP therapies,† median (range) | 4 (1-15) | 5 (1-12) | 4 (1-15) |
| Prior splenectomy,† n (%) | 37 (28) | 19 (28) | 56 (28) |
| Most common prior ITP therapies,†n (%) | |||
| CS | 127 (95) | 66 (96) | 193 (96) |
| TPO-RA | 88 (66) | 51 (74) | 139 (69) |
| IVIg or anti-D immunoglobulin | 72 (54) | 40 (58) | 118 (58) |
| Immunosuppressants | 67 (50) | 42 (61) | 109 (54) |
| Rituximab | 42 (32) | 29 (42) | 71 (35) |
| Fostamatinib | 17 (13) | 7 (10) | 24 (12) |
| Prior response to most common prior ITP therapies,†response (n)/received (n), (% response) | |||
| CS | 93/127 (73) | 47/66 (71) | 140/193 (73) |
| TPO-RA | 55 /88 (63) | 32/51 (63) | 87/139 (63) |
| IVIg or anti-D immunoglobulin | 55/72 (76) | 31/40 (78) | 86 /118 (73) |
| Immunosuppressants | NR/67 (NR) | NR/42 (NR) | NR/109 (NR) |
| Rituximab | 11/42 (26) | 12/29 (41) | 23/71 (32) |
| Fostamatinib | 4/17 (24) | 3/7 (43) | 7/24 (29) |
| Concomitant ITP therapy, n (%) | |||
| None | 53 (40) | 23 (33) | 76 (38) |
| CS | 34 (26) | 20 (29) | 54 (27) |
| TPO-RA | 25 (19) | 12 (17) | 37 (18) |
| CS + TPO-RA | 21 (16) | 14 (20) | 35 (17) |
| Geographic region, n (%) | |||
| Asia/Pacific | 44 (33) | 26 (38) | 70 (35) |
| Western Europe | 33 (25) | 16 (23) | 49 (24) |
| Eastern Europe | 19 (14) | 13 (4) | 32 (16) |
| South America | 24 (18) | 11 (16) | 35 (17) |
| North America | 13 (10) | 3 (4) | 16 (8) |
| Characteristic . | Rilzabrutinib (n = 133) . | Placebo (n = 69) . | All patients (N = 202) . |
|---|---|---|---|
| Age, median (range), y | 47 (18-80) | 46 (19-79) | 47 (18-80) |
| Sex, n (%) | |||
| Male | 55 (41) | 20 (29) | 75 (37) |
| Female | 78 (59) | 49 (71) | 127 (63) |
| Duration of ITP, median (range), y | 8.1 (0.3-52.2) | 6.2 (0.3-35.8) | 7.7 (0.3-52.2) |
| Baseline platelet count, median (range),∗ ×109/L | 15 (1-32) | 15 (1-54) | 15 (1-54) |
| Baseline platelet count by category, n (%) | |||
| <15 × 109/L | 65 (49) | 32 (46) | 97 (48) |
| ≥15 × 109/L | 68 (51) | 37 (54) | 105 (52) |
| Prior unique therapies,†n (%) | |||
| 1-2 | 43 (32) | 15 (22) | 58 (29) |
| 3-4 | 33 (25) | 18 (26) | 51 (25) |
| ≥5 | 57 (43) | 36 (52) | 93 (46) |
| No. of unique prior ITP therapies,† median (range) | 4 (1-15) | 5 (1-12) | 4 (1-15) |
| Prior splenectomy,† n (%) | 37 (28) | 19 (28) | 56 (28) |
| Most common prior ITP therapies,†n (%) | |||
| CS | 127 (95) | 66 (96) | 193 (96) |
| TPO-RA | 88 (66) | 51 (74) | 139 (69) |
| IVIg or anti-D immunoglobulin | 72 (54) | 40 (58) | 118 (58) |
| Immunosuppressants | 67 (50) | 42 (61) | 109 (54) |
| Rituximab | 42 (32) | 29 (42) | 71 (35) |
| Fostamatinib | 17 (13) | 7 (10) | 24 (12) |
| Prior response to most common prior ITP therapies,†response (n)/received (n), (% response) | |||
| CS | 93/127 (73) | 47/66 (71) | 140/193 (73) |
| TPO-RA | 55 /88 (63) | 32/51 (63) | 87/139 (63) |
| IVIg or anti-D immunoglobulin | 55/72 (76) | 31/40 (78) | 86 /118 (73) |
| Immunosuppressants | NR/67 (NR) | NR/42 (NR) | NR/109 (NR) |
| Rituximab | 11/42 (26) | 12/29 (41) | 23/71 (32) |
| Fostamatinib | 4/17 (24) | 3/7 (43) | 7/24 (29) |
| Concomitant ITP therapy, n (%) | |||
| None | 53 (40) | 23 (33) | 76 (38) |
| CS | 34 (26) | 20 (29) | 54 (27) |
| TPO-RA | 25 (19) | 12 (17) | 37 (18) |
| CS + TPO-RA | 21 (16) | 14 (20) | 35 (17) |
| Geographic region, n (%) | |||
| Asia/Pacific | 44 (33) | 26 (38) | 70 (35) |
| Western Europe | 33 (25) | 16 (23) | 49 (24) |
| Eastern Europe | 19 (14) | 13 (4) | 32 (16) |
| South America | 24 (18) | 11 (16) | 35 (17) |
| North America | 13 (10) | 3 (4) | 16 (8) |
NR, not reported.
Defined as average of first and second qualifying screening platelet counts and study day 1 platelet count.
Unique ITP therapies were identified using standard medication term, different CS were counted as 1 therapy, and splenectomy could be counted as 1 prior ITP therapy. Patients could receive >1 therapy. Prior response was recorded on the electronic case report form as platelet count ≥50 × 109/L at any time. The Immunosuppressants category included immunosuppressant and other immunomodulatory agents (including cyclophosphamide).
Most participants completed the initial 12 weeks of the double-blind period in both groups (121 [91%] for rilzabrutinib, and 66 [96%] for placebo). A higher percentage of participants completed the 24-week double-blind period in the rilzabrutinib group (62 [47%]) than in the placebo group (10 [14%]). The main reason for discontinuation during the overall 24-week double-blind period was because of lack of platelet response per predefined levels (55 [41%] for rilzabrutinib vs 55 [80%] for placebo). Other common reasons through the 24 weeks for discontinuation (≥5% in either group) were patient decision (7 [5%] for rilzabrutinib vs 2 [3%] for placebo) and AEs (8 [6.0%] for rilzabrutinib vs none for placebo). All patients were evaluable for efficacy and safety.
Patients in the rilzabrutinib and placebo arms received double-blind treatment for a median of 98 (range, 22-182) and 84 (range, 17-173) days, respectively. At study entry, 80 (60%) and 46 (67%) of patients in the rilzabrutinib and placebo arms, respectively, received concomitant ITP medication (Table 1).
Efficacy
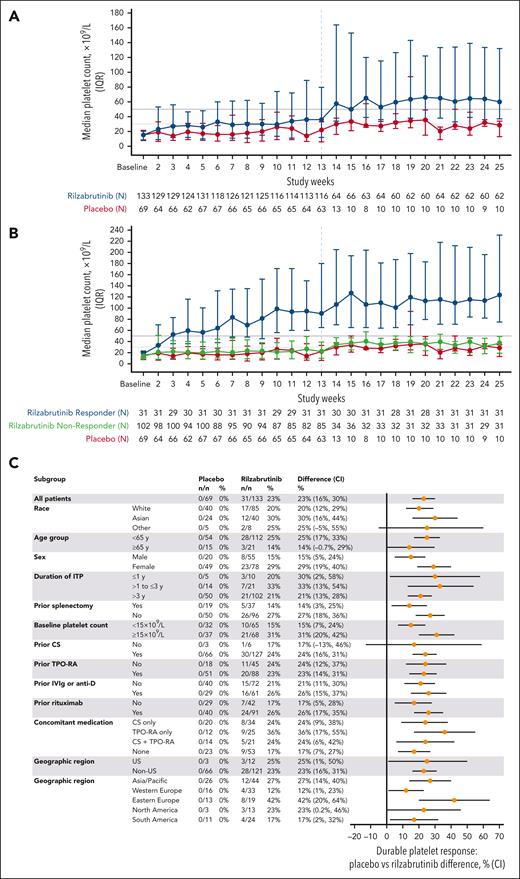
During the first 12 weeks, 85 (64%) vs 22 (32%) patients who received rilzabrutinib vs placebo, respectively, achieved a platelet response and were eligible to continue the double-blind period. The primary end point of durable response was met in 31 (23%; 95% CI, 16-30) vs 0 (95% CI, 0-0) patients who received rilzabrutinib vs placebo, respectively (P < .0001), identical for both durable response definitions. Results were confirmed by sensitivity analyses (supplemental Table 1). Platelet counts improved over time with rilzabrutinib (Figure 2A), with median platelet counts in rilzabrutinib durable responders exceeding 30 × 109/L, 50 × 109/L, and 100 × 109/L at week 2, 3, and 14, respectively, and maintaining >100 × 109/L thereafter (Figure 2B). For rilzabrutinib nondurable responders, median platelet counts were >30 × 109/L from week 14 to 25. No patients receiving placebo had a durable response; these patients had median platelet counts of <30 × 109/L from baseline through week 13. After week 13, patients receiving placebo who were eligible to continue the double-blind period had median platelet counts of <30. ×109/L for 50% of weeks 14 through 25. Durable response was consistent across prespecified subgroups (Figure 2C).
Platelet counts and subgroup analyses. Median platelet counts (IQR) over time for rilzabrutinib vs placebo arms (A) and by responder status (B), and subgroup analysis of durable responses (C). Durable platelet response was defined as the proportion of patients achieving platelet counts of ≥50 × 109/L for two-thirds or more of at least 8 nonmissing weekly scheduled platelet measurements during the last 12 weeks of the 24-week blinded treatment period in the absence of rescue therapy, provided that at least 2 nonmissing weekly platelet measurements were ≥50 × 109/L during the last 6 weeks of the 24-week blinded treatment period. Vertical dashed line at week 13 represents measurement of patient response and continuation of responders to treatment through 24 weeks. IQR, interquartile range; y, year.
Platelet counts and subgroup analyses. Median platelet counts (IQR) over time for rilzabrutinib vs placebo arms (A) and by responder status (B), and subgroup analysis of durable responses (C). Durable platelet response was defined as the proportion of patients achieving platelet counts of ≥50 × 109/L for two-thirds or more of at least 8 nonmissing weekly scheduled platelet measurements during the last 12 weeks of the 24-week blinded treatment period in the absence of rescue therapy, provided that at least 2 nonmissing weekly platelet measurements were ≥50 × 109/L during the last 6 weeks of the 24-week blinded treatment period. Vertical dashed line at week 13 represents measurement of patient response and continuation of responders to treatment through 24 weeks. IQR, interquartile range; y, year.
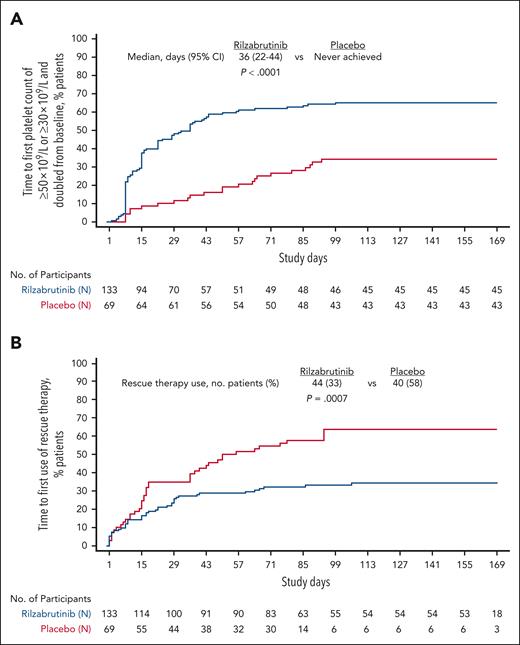
All prespecified secondary efficacy end points were statistically significantly superior for rilzabrutinib over placebo. The improved durability of achieving multiple platelet count thresholds was consistently longer for rilzabrutinib vs placebo (Table 2). The least squares (LS) mean number of weeks with platelet response was longer with rilzabrutinib vs placebo by 6.46 weeks for all patients (P < .0001) and 8.83 weeks in responders (ie, achieved platelet response during the double-blind period; P < .0001). Median time to platelet response was 36 days in all patients with rilzabrutinib (vs never achieved with placebo [P < .0001; Figure 3A]), and was 15 days in rilzabrutinib responders.
Durability of platelet counts during 24-week double-blind period
| No. of weeks with platelet count (in the absence of rescue therapy) . | All patients . | Responders∗ . | ||||
|---|---|---|---|---|---|---|
| Rilzabrutinib . | Placebo . | P value . | Rilzabrutinib . | Placebo . | P value . | |
| 50 × 109/L or between ≥30 × 109/L and <50 × 109/L and at least doubled from baseline | n = 133 | n = 69 | n = 85 | n = 23 | ||
| LS mean (SE) | 7.18 (0.75) | 0.72 (0.35) | <.0001 | 11.40 (0.99) | 2.57 (0.86) | <.0001 |
| LS mean difference (SE) vs placebo 95% CI | 6.46 (0.78) 4.92-7.99 | 8.83 (1.08) 6.72-10.93 | ||||
| ≥30 × 109/L and at least doubled from baseline | n = 133 | n = 69 | n = 84 | n = 23 | ||
| LS mean (SE) | 6.95 (0.75) | 0.64 (0.34) | <.0001 | 11.20 (1.01) | 2.38 (0.86) | <.0001 |
| LS mean difference (SE) vs placebo 95% CI | 6.31 (0.78) 4.79-7.83 | 8.81 (1.08) 6.71-10.92 | ||||
| No. of weeks with platelet count (in the absence of rescue therapy) . | All patients . | Responders∗ . | ||||
|---|---|---|---|---|---|---|
| Rilzabrutinib . | Placebo . | P value . | Rilzabrutinib . | Placebo . | P value . | |
| 50 × 109/L or between ≥30 × 109/L and <50 × 109/L and at least doubled from baseline | n = 133 | n = 69 | n = 85 | n = 23 | ||
| LS mean (SE) | 7.18 (0.75) | 0.72 (0.35) | <.0001 | 11.40 (0.99) | 2.57 (0.86) | <.0001 |
| LS mean difference (SE) vs placebo 95% CI | 6.46 (0.78) 4.92-7.99 | 8.83 (1.08) 6.72-10.93 | ||||
| ≥30 × 109/L and at least doubled from baseline | n = 133 | n = 69 | n = 84 | n = 23 | ||
| LS mean (SE) | 6.95 (0.75) | 0.64 (0.34) | <.0001 | 11.20 (1.01) | 2.38 (0.86) | <.0001 |
| LS mean difference (SE) vs placebo 95% CI | 6.31 (0.78) 4.79-7.83 | 8.81 (1.08) 6.71-10.92 | ||||
Responders included patients who achieved the platelet count threshold being measured.
Time to event analyses. Kaplan-Meier plot of time to first platelet count of ≥50 × 109/L or ≥30 × 109/L and doubled from baseline (A) and time to first use of rescue therapy (B) during the 24-week double-blind period. Cox regression model was used with treatment group and stratification factors as covariates. Log-rank test was adjusted by stratification factors.
Time to event analyses. Kaplan-Meier plot of time to first platelet count of ≥50 × 109/L or ≥30 × 109/L and doubled from baseline (A) and time to first use of rescue therapy (B) during the 24-week double-blind period. Cox regression model was used with treatment group and stratification factors as covariates. Log-rank test was adjusted by stratification factors.
A smaller proportion of patients (33%) on rilzabrutinib required rescue therapy compared with placebo (58%). Rilzabrutinib significantly reduced the need for rescue therapy by 52% (P = .0007). Median time to rescue therapy was never reached for rilzabrutinib vs 56 days for placebo (Figure 3B).
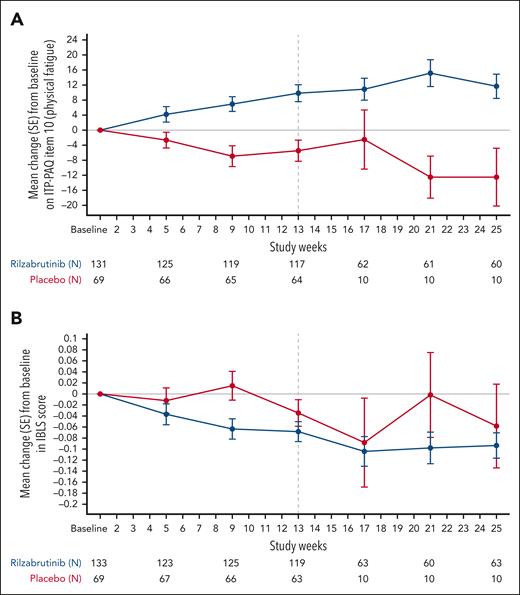
Improvements in both physical fatigue (Figure 4A) and IBLS bleeding score (Figure 4B) with rilzabrutinib compared with placebo were observed as early as week 5. The secondary end point of change from baseline on physical fatigue at week 13 was significantly improved with rilzabrutinib vs placebo (LS mean, 7.95; standard error [SE], 2.13 vs −0.13; SE, 2.86; P = .0114). LS mean differences in physical fatigue for rilzabrutinib vs placebo were 8.08 (SE, 3.19 [95% CI, 1.82-14.34]; P = .01) at week 13 and 12.0 (P = .0003) at week 25. Among rilzabrutinib durable responders, physical fatigue score improved with mean changes from baseline of 17.5 and 15.8 at weeks 13 and 25, respectively (supplemental Table 2). Additionally, nondurable responders receiving rilzabrutinib showed improvement in physical fatigue, with mean change from baseline of 7.2 and 7.5 at weeks 13 and 25, respectively. Patients receiving placebo exhibited no improvement in physical fatigue.
Physical fatigue and bleeding scores. Mean change (SE) from baseline on physical fatigue (item 10 of the ITP-PAQ) (A), and mean change (SE) from baseline in IBLS (B) during the 24-week double-blind period. Vertical dashed line at week 13 represents measurement of patient response and continuation of responders to treatment through 24 weeks.
Physical fatigue and bleeding scores. Mean change (SE) from baseline on physical fatigue (item 10 of the ITP-PAQ) (A), and mean change (SE) from baseline in IBLS (B) during the 24-week double-blind period. Vertical dashed line at week 13 represents measurement of patient response and continuation of responders to treatment through 24 weeks.
The secondary end point of change from baseline in IBLS score at week 25 showed significant improvement (ie, decreased score) with rilzabrutinib vs placebo (LS mean [SE]: −0.04 [0.02] vs 0.05 [0.02]), with an LS mean difference of −0.09 (SE, 0.03 [95% CI, −0.14 to −0.04]; P = .0006).
For patients completing 24 weeks of treatment, 34 of 62 (55%) on rilzabrutinib and 0 of 10 on placebo had ≥4 of the last 8 platelet counts of ≥50 × 109/L. Overall, 53 (40%) patients on rilzabrutinib vs 6 (9%) on placebo achieved a platelet count of ≥50 × 109/L on 2 consecutive visits (ie, overall response), and 29 (22%) vs 2 (3%) patients on rilzabrutinib vs placebo, respectively, had platelet counts of ≥100 × 109/L (ie, complete response). Among patients receiving concomitant TPO-RA, platelet counts of >250 × 109/L and >450 × 109/L on 2 consecutive visits were observed in 10 of 46 (22%) and 2 of 46 (4%) patients on rilzabrutinib, respectively, and in 1 of 26 (4%) patients on placebo for both measurements.
At week 25, rilzabrutinib showed clinically meaningful differences (ie, clinically minimal important differences25; defined range, 8-12) compared with baseline and improvements over placebo in ITP-PAQ domains for psychological health (11.9 vs −1.0), overall HRQoL (10.6 vs 2.3), symptoms (10.3 vs 2.1), bother-physical health (10.0 vs −4.7), and social activity (9.4 vs 1.3); and improvements over placebo from baseline for activity (9.96 vs −1.3), fatigue/sleep (9.3 vs −5.0), fear (7.8 vs −5.0), women’s reproductive health (5.8 vs −16.7), and work (4.9 vs 3.8).
Safety
Cumulative treatment exposure duration (participant-years) was 44.3 for rilzabrutinib vs 17.9 for placebo. Similar percentages of respective patients on rilzabrutinib and placebo had all-cause, any-grade AEs (83% vs 75%), serious AEs (9% vs 12%), and grade ≥3 AEs (11% vs 14%; supplemental Table 3). Eight (6%) rilzabrutinib vs 0 placebo patients discontinued because of AEs (supplemental Table 4).
Most AEs were assessed as grade 1 or 2 for both treatments. Any cause, grade ≥2 gastrointestinal AEs were comparable between rilzabrutinib and placebo arms (6% vs 4%; supplemental Table 3).
Any-grade, treatment-related AEs for 51% rilzabrutinib vs 17% placebo patients were reported (Table 3). The most common treatment-related AEs for rilzabrutinib vs placebo were diarrhea (23% vs 4%), nausea (17% vs 6%), headache (8% vs 1%), and abdominal pain (6% vs 1%). All other related AEs were ≤5%. One patient on placebo had treatment-related grade 3 purpura lasting for 8 days that resolved with no treatment changes. Two rilzabrutinib-related AEs per investigator judgment were grade 4 neutropenia lasting 14 days (no associated infections, no change to treatment) and grade 3 serious AE of peripheral embolism (lower left leg) in a patient with multiple risk factors that led to treatment discontinuation (supplemental Safety Results). In the rilzabrutinib arm, 1 patient died from pneumonia lasting 16 days that was deemed unrelated to treatment per investigator judgment (supplemental Safety Results).
Summary of any cause and treatment-related AEs by treatment arm during the 24-week double-blind period (safety population)
| Event, no. of patients (%) . | Rilzabrutinib (n = 133) . | Placebo (n = 69) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EA rate∗ . | Any . | Related . | Any grade ≥3† . | Related grade ≥3 . | EA rate∗ . | Any . | Related . | Any grade ≥3‡ . | Related grade ≥3 . | |
| Any AE | 858 | 111 (83) | 68 (51) | 15 (11) | 2 (2) | 676 | 52 (75) | 12 (17) | 10 (14) | 1 (1) |
| Diarrhea | 131 | 43 (32) | 30 (23) | 0 | 0 | 43 | 7 (10) | 3 (4) | 0 | 0 |
| Nausea | 74 | 27 (20) | 23 (17) | 0 | 0 | 23 | 4 (6) | 4 (6) | 0 | 0 |
| Headache | 64 | 24 (18) | 10 (8) | 0 | 0 | 30 | 5 (7) | 1 (1) | 0 | 0 |
| COVID-19 | 44 | 18 (14) | 0 | 1 (1) | 0 | 17 | 3 (4) | 0 | 0 | 0 |
| Arthralgia | 29 | 12 (9) | 6 (5) | 0 | 0 | 17 | 3 (4) | 1 (1) | 0 | 0 |
| Dizziness | 26 | 11 (8) | 3 (2) | 0 | 0 | 6 | 1 (1) | 1 (1) | 0 | 0 |
| Abdominal pain | 24 | 10 (8) | 8 (6) | 0 | 0 | 6 | 1 (1) | 1 (1) | 0 | 0 |
| Nasopharyngitis | 21 | 9 (7) | 0 | 0 | 0 | 12 | 2 (3) | 0 | 0 | 0 |
| Vomiting | 22 | 9 (7) | 7 (5) | 0 | 0 | 6 | 1 (1) | 1 (1) | 0 | 0 |
| Upper abdominal pain | 17 | 7 (5) | 5 (4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dyspepsia | 16 | 7 (5) | 4 (3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anemia | 12 | 5 (4) | 0 | 1 (1) | 0 | 23 | 4 (6) | 0 | 0 | 0 |
| Upper respiratory tract infection | 12 | 5 (4) | 2 (2)§ | 0 | 0 | 17 | 3 (4) | 0 | 0 | 0 |
| Rash | 12 | 5 (4) | 2 (2) | 0 | 0 | 6 | 1 (1) | 1 (1) | 0 | 0 |
| Abdominal discomfort | 9 | 4 (3) | 4 (3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fatigue | 9 | 4 (3) | 2 (2) | 0 | 0 | 23 | 4 (6) | 1 (1) | 0 | 0 |
| Abdominal distension | 7 | 3 (2) | 2 (2) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Increased alanine aminotransferase | 7 | 3 (2) | 1 (1) | 0 | 0 | 11 | 2 (3) | 2 (3) | 0 | 0 |
| Increased aspartate aminotransferase | 7 | 3 (2) | 1 (1) | 0 | 0 | 11 | 2 (3) | 2 (3) | 0 | 0 |
| Neutropenia | 2 | 1 (1) | 1 (1) | 1 (1) | 1 (1) | 0 | 0 | 0 | 0 | 0 |
| Peripheral embolism | 2 | 1 (1) | 1 (1) | 1 (1) | 1 (1) | 0 | 0 | 0 | 0 | 0 |
| Purpura§ | 0 | 0 | 0 | 0 | 0 | 11 | 2 (3) | 1 (1) | 1 (1) | 1 (1) |
| Chronic sinusitis | 2 | 0 | 1 (1)|| | 0 | 0 | 0 | 0 | 0 | 0 | |
| Event, no. of patients (%) . | Rilzabrutinib (n = 133) . | Placebo (n = 69) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EA rate∗ . | Any . | Related . | Any grade ≥3† . | Related grade ≥3 . | EA rate∗ . | Any . | Related . | Any grade ≥3‡ . | Related grade ≥3 . | |
| Any AE | 858 | 111 (83) | 68 (51) | 15 (11) | 2 (2) | 676 | 52 (75) | 12 (17) | 10 (14) | 1 (1) |
| Diarrhea | 131 | 43 (32) | 30 (23) | 0 | 0 | 43 | 7 (10) | 3 (4) | 0 | 0 |
| Nausea | 74 | 27 (20) | 23 (17) | 0 | 0 | 23 | 4 (6) | 4 (6) | 0 | 0 |
| Headache | 64 | 24 (18) | 10 (8) | 0 | 0 | 30 | 5 (7) | 1 (1) | 0 | 0 |
| COVID-19 | 44 | 18 (14) | 0 | 1 (1) | 0 | 17 | 3 (4) | 0 | 0 | 0 |
| Arthralgia | 29 | 12 (9) | 6 (5) | 0 | 0 | 17 | 3 (4) | 1 (1) | 0 | 0 |
| Dizziness | 26 | 11 (8) | 3 (2) | 0 | 0 | 6 | 1 (1) | 1 (1) | 0 | 0 |
| Abdominal pain | 24 | 10 (8) | 8 (6) | 0 | 0 | 6 | 1 (1) | 1 (1) | 0 | 0 |
| Nasopharyngitis | 21 | 9 (7) | 0 | 0 | 0 | 12 | 2 (3) | 0 | 0 | 0 |
| Vomiting | 22 | 9 (7) | 7 (5) | 0 | 0 | 6 | 1 (1) | 1 (1) | 0 | 0 |
| Upper abdominal pain | 17 | 7 (5) | 5 (4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dyspepsia | 16 | 7 (5) | 4 (3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anemia | 12 | 5 (4) | 0 | 1 (1) | 0 | 23 | 4 (6) | 0 | 0 | 0 |
| Upper respiratory tract infection | 12 | 5 (4) | 2 (2)§ | 0 | 0 | 17 | 3 (4) | 0 | 0 | 0 |
| Rash | 12 | 5 (4) | 2 (2) | 0 | 0 | 6 | 1 (1) | 1 (1) | 0 | 0 |
| Abdominal discomfort | 9 | 4 (3) | 4 (3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fatigue | 9 | 4 (3) | 2 (2) | 0 | 0 | 23 | 4 (6) | 1 (1) | 0 | 0 |
| Abdominal distension | 7 | 3 (2) | 2 (2) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Increased alanine aminotransferase | 7 | 3 (2) | 1 (1) | 0 | 0 | 11 | 2 (3) | 2 (3) | 0 | 0 |
| Increased aspartate aminotransferase | 7 | 3 (2) | 1 (1) | 0 | 0 | 11 | 2 (3) | 2 (3) | 0 | 0 |
| Neutropenia | 2 | 1 (1) | 1 (1) | 1 (1) | 1 (1) | 0 | 0 | 0 | 0 | 0 |
| Peripheral embolism | 2 | 1 (1) | 1 (1) | 1 (1) | 1 (1) | 0 | 0 | 0 | 0 | 0 |
| Purpura§ | 0 | 0 | 0 | 0 | 0 | 11 | 2 (3) | 1 (1) | 1 (1) | 1 (1) |
| Chronic sinusitis | 2 | 0 | 1 (1)|| | 0 | 0 | 0 | 0 | 0 | 0 | |
Includes treatment-related AEs occurring in ≥2 patients, AEs due to any cause occurring in ≥5% patients, and AEs of special interest. Relatedness of the AE was determined by the investigators. Multiple AEs may have occurred in a single patient.
The exposure-adjusted (EA) rate is defined as the number of patients with at least 1 AE per 100 patient-years.
In patients on rilzabrutinib who had an AE of grade ≥3 that was due to any cause and occurred in a single patient each, these AEs were asthenia, epistaxis, esophageal varices hemorrhage, facial paralysis, hepatic cirrhosis, hepatic enzyme increased, hyperglycemia, ITP, intermenstrual bleeding, otitis media acute, pelvic inflammatory disease, periorbital hematoma, platelet count decreased, pneumonia, renal abscess, sepsis, swelling of eyelid, thrombocytopenia, urinary tract infection, and vaginal hemorrhage.
In patients on placebo who had an AE of grade ≥3 that was due to any cause and occurred in a single patient each, these AE swere conjunctival hemorrhage, heavy menstrual bleeding, hematemesis, hypertension, ITP, mouth hemorrhage, and syncope.
Grade 3 purpura was the only treatment-related bleeding event in the placebo arm; there were no grade ≥2 bleeding-related AEs in the rilzabrutinib arm.
Grade 2 upper respiratory tract infection in 2 patients and grade 2 chronic sinusitis in 1 patient were the only rilzabrutinib-related infectious events; there were no grade ≥2 infectious events in the placebo arm.
Grade ≥2 bleeding events due to any cause were reported in 4% of patients on rilzabrutinib and 12% of patients on placebo (supplemental Table 3). The only treatment-related grade ≥2 bleeding event was grade 3 purpura with placebo. There were no treatment-related grade ≥2 infections with placebo, and 3 (2%) in those treated with rilzabrutinib, 2 of whom had grade 2 upper respiratory tract infection (resolved, dose unchanged) and 1 with grade 2 chronic sinusitis (not resolved, dose unchanged).
Pharmacokinetics/markers
Median rilzabrutinib plasma concentrations 2 hours post-dose on first dosing days of weeks 1 and 25 were 144 and 193 ng/mL, respectively. Respective predose (ie, trough) rilzabrutinib plasma concentrations on first dosing days of weeks 1, 13, and 25 were 0, 5.6, and 4.2 ng/mL, respectively. No notable changes over time were observed in haptoglobin, immunoglobulins, T- or B-lymphocytes, natural killer cells, hemoglobin, leukocytes, or neutrophils for either arm (supplemental Table 5).
Discussion
This is, to our knowledge, the first phase 3 placebo-controlled trial providing substantial evidence of a beneficial therapeutic effect of rilzabrutinib for adults with ITP who received multiple prior therapies. Enrolled patients had difficult-to-treat and overwhelmingly chronic ITP based on their duration of ITP for a median of 7.7 years, 4 prior unique ITP therapies (46% of patients receiving ≥5 prior therapies), and low baseline platelet counts (48% of patients with counts of <15 × 109/L). The many observed beneficial therapeutic effects of rilzabrutinib, including rapid and durable platelet increase and reduced fatigue and bleeding, provide robust evidence supporting the efficacy and safety of BTK inhibition in addressing complex ITP pathophysiology.11,27
In the LUNA3 study, the definition of durable response used weekly platelet counts during the last 12 weeks of the double-blind period, representing longer, more frequent platelet response evaluations relative to other phase 3 ITP studies and guidelines.23,28,29 Two durable response definitions were examined: first, based on the proportion (ie, two-thirds or more) of available (≥8 nonmissing weekly) platelet counts of ≥50 × 109/L over the last 12 weeks to account for potential missing platelet counts; and the second, based on a fixed number (ie, at least 8) of platelet counts of ≥50 × 109/L for 8 of the last 12 visits. Interestingly, both definitions resulted in the same durable response rate of 23% for rilzabrutinib vs 0% placebo, reflecting marginal effects of missing platelet counts, with the observed 0 placebo response reinforcing the degree of disease refractoriness and very low likelihood of spontaneous remission in these patients.
In this study with rilzabrutinib, durable platelet response, overall response, and median time to first platelet count of ≥50 × 109/L among responders were 23%, 40%, and 15 days, respectively. Although the phase 2, part A study used slightly different response definitions, similar platelet response and time to response results for LUNA3 were observed.20 Phase 2 part B reported a 35% (9/26) durable response (per the second LUNA3 primary end point definition) and median time to first platelet count of ≥50 × 109/L (in patients reaching platelet count of ≥50 × 109/L) of 15 days.21,30
Although current second-line options, TPO-RAs, rituximab, fostamatinib, or splenectomy show robust evidence in ITP, challenges may include incomplete targeting of disease-related pathophysiology, elevated long-term toxicity, risks for bleeding/thromboembolic/surgery-related events, and/or impaired HRQoL effects.12,13,23,31 Varying study designs, patient populations, and durable response definitions make cross-trial comparisons difficult, and later lines of therapy are often based on individual needs, treatment availability, and patient/physician preferences.23,28,29 In patients with a shorter ITP duration and fewer prior therapies, durable response defined as platelet count of ≥50 × 109/L during ≥6 of the last 8 weeks without rescue therapy was 49% with romiplostim (combined patients with/without splenectomy) vs 2% with patients on placebo.29 Fostamatinib- vs placebo-treated patients achieved 18% vs 2% stable response, defined as platelet count of ≥50 × 109/L at 4 of 6 biweekly visits in weeks 14 through 24 without rescue therapy.28
Notably, rilzabrutinib (vs placebo) significantly reduced the need for ITP rescue therapy by 52% and improved multiple HRQoL (including physical fatigue) and bleeding scores. Improved fatigue is unparalleled and particularly significant, given the unmet need highlighted by patient surveys and clinical guidelines.14,15,23 Changes from baseline in fatigue score with rilzabrutinib were improved in durable responders and nonresponders, the latter suggesting positive therapeutic effects mediated through pathways independent of increased platelet count, potentially through amelioration of ITP-associated chronic inflammatory states.32,33
Oral rilzabrutinib demonstrated predominantly low-grade AEs, consistent with phase 1/2 studies.20,21,34 Despite the higher diarrhea rate for rilzabrutinib vs placebo, all events were grade 1 or 2, most patients recovered without treatment interruption, and only 3 patients discontinued treatment because of gastrointestinal-related AEs. Relative to other BTK inhibitors,35-37 rilzabrutinib’s safety profile showed fewer severe infections and cardiac, liver-, cytopenia-, or bleeding-related AEs. Unlike other ITP studies,28,38,39 patients with a medical history of thromboembolic events (a relatively common complication of the disease with an annualized incidence rate of 4% to 7% with TPO-RA) were allowed in this study.40,41 Despite the potential risk of thrombosis, only 1 patient on rilzabrutinib (who had multiple factors elevating their risk for thromboembolism) experienced peripheral embolism of the lower left leg. Recent preclinical studies have shown that rilzabrutinib could have an antithrombotic effect without increasing bleeding risk through human platelet C-type lectin-like type II (CLEC-2) inhibition, together with the lack of off-target effects.42 As an inhibitor of BTK, rilzabrutinib may directly regulate the NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome, with the potential for downstream innate immune and anti-inflammatory effects beyond platelet count increases (eg, improving fatigue, decreasing thromboembolism risk). These potential roles for rilzabrutinib in preventing ITP-associated effects warrant further study.
Results for this large phase 3 study were strengthened by the placebo-controlled study design in a large population of previously treated patients with ITP from multiple global study centers. Consistent with previous phase 3 ITP trials, LUNA3 allowed concomitant ITP medication (CS and/or TPO-RA) at stable doses during the double-blind period that were balanced between treatment groups. Subgroup analyses of durable response confirmed that rilzabrutinib’s efficacy was comparable between those receiving rilzabrutinib as monotherapy or with concomitant ITP medication, highlighting its clinical benefit irrespective of patient treatment status. Limitations were exclusion of newly diagnosed patients and involvement of a small number of patients with persistent ITP (ie, ITP duration of 3-12 months23). Bleeding is a very important symptom for patients with ITP.43-45 Similar to previous clinical development programs (eg, fostamatinib46), IBLS was used to evaluate the effect of treatment with rilzabrutinib on bleeding.26 Psychometric properties of IBLS are being validated using LUNA3 study data as part of their validation in the ITP population.47
In conclusion, this pivotal phase 3 trial provides robust evidence of the efficacy and safety of BTK inhibition with rilzabrutinib in patients with persistent/chronic ITP who failed multiple prior therapies. Treatment with rilzabrutinib resulted in statistically and clinically meaningful improvements in all aspects of the disease, including rapid and durable platelet response, improved physical fatigue, decreased bleeding, and an acceptable safety profile. The ongoing open-label and long-term extension parts of the study will help characterize the long-term efficacy and safety of rilzabrutinib in adults with ITP. In addition, the ongoing pediatric part of the LUNA3 study may help with evaluating safety and efficacy in children aged ≥10 years.
Acknowledgments
The authors thank the patients who participated in the trial, their caregivers, trial investigators, and safety monitoring board. Publication support and critical feedback during manuscript development were provided by Erin Reineke of Sanofi. The authors received editorial support in the preparation of the manuscript from Julie Kern of Second City Science LLC, funded by Sanofi.
This study was supported by Sanofi.
The sponsor (Sanofi) and lead investigators designed the trial in collaboration. Trial conduct and analyses were overseen by the sponsor; data were collected by investigators. An independent data safety monitoring board reviewed unblinded data. First-draft development was led by the first author with sponsor-supported medical writing assistance. The authors directed development of the manuscript and are fully responsible for all content and editorial decisions for this manuscript.
Authorship
Contribution: D.J.K., W.G., N.C., H.A.L., M.L., and A.D. contributed to the design of the study; D.J.K., W.G., N.C., H.A.L., L.Z., Y.M., W.H., L.E.M.G., A.L.B., C.W.T., G.S., M.L.H.-K., C.C.-A., D.G.-A., H.T., H.-J.S., A.D.d.C.J., Z.L., C.P.I., I.K., E.L., G.K., M.F., S.A., and M.T. participated in patient enrollment and treatment, data collection, and assembly of data, and performed the research; M.C., R.D., M.Y., I.G., and A.D. analyzed data; D.J.K., M.C., R.D., M.Y., I.G., and A.D. wrote the initial manuscript draft; and all authors provided their reviews and feedback during the development of the manuscript, vouch for data accuracy/completeness, trial fidelity, and complete adverse event reporting, provided final approval for the manuscript prior to submission, and approved the final submitted manuscript.
Conflict-of-interest disclosure: D.J.K. reports support for self and institution for present manuscript from Principia Biopharma (Sanofi) and Sanofi; grants or contracts for institution from BioCryst, Hutchmed, Novartis, and Takeda; royalties or licenses from UpToDate; consulting fees from Alexion, Alpine, Amgen, argenx, BioCryst, Bristol Myers Squibb, Caremark, Chugai, Hengrui, Hutchmed, Immunovant, Medscape, Merck Sharp & Dohme, New York Blood Center, Novartis, PeerView, Physicians' Education Resource (PER), Pfizer, Regeneron, Rigel, Sanofi, Seismic, Sobi, Takeda, UCB Pharma, UpToDate, and Verve; and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Alexion, Alpine, Amgen, argenx, BioCryst, Bristol Myers Squibb, Caremark, Chugai, Hengrui, Hutchmed, Immunovant, Medscape, Merck Sharp & Dohme, New York Blood Center, Novartis, PeerView, PER, Pfizer, Principia, Regeneron, Rigel, Sanofi, Seismic, Sobi, Takeda, UCB Pharma, UpToDate, and Verve. W.G. reports grants or contracts from Janssen, Sanofi, Sobi, and UCB Pharma; consulting fees from Alpine, Amgen, argenx, Cellphire, HiBio, Hutchmed, Kedrion, Novartis, Pfizer, Principia Biopharma (Sanofi), Sanofi, Sobi, Grifols, UCB Pharma, and Takeda; and lecture honoraria from Amgen, Bayer, Bristol Myers Squibb, Grifols, Novartis, Pfizer, Sanofi, and Sobi. N.C. reports support for this manuscript from Sanofi; consulting fees from argenx, Novartis, Sanofi, and Sobi; honoraria for lectures at educational meetings from Novartis, Sanofi, and Sobi; travel and hotel accommodations for the American Society of Hematology 2023 and the European Hematology Association 2024 annual meetings from Novartis; and research support/grant funding for institution for separate studies from Novartis (ITP) and Rigel (COVID-19). H.A.L. reports consulting fees from Alpine Immune Sciences and noncompensated consulting with Sanofi. Y.M. reports grants/contracts from Alexion, BioMarin, CSL Behring, Chugai, Janssen, Novartis, Novo Nordisk, Pfizer, Sanofi, and UCB Pharma; consulting fees from argenx, Kyowa Kirin, Novartis, and Zenyaku Kogyo; honoraria from Alexion, Kissei, Sanofi, and Takeda; support for attending meetings and/or travel from argenx, BioMarin, and Sanofi; and participation on a data safety monitoring board or an advisory board with argenx, Novartis, Pfizer, Sanofi, and UCB Pharma. M.L.H.-K. reports support for attending meetings and/or travel from Jazz and Sobi; and participation in data safety monitoring board or advisory board with Amgen, Grifols, and Sanofi. D.G.-A. served as a speaker for AbbVie, Amgen, Asofarma, Astra, Bristol Myers Squibb, Janssen, Novartis, Roche, Sanofi, Takeda, and Teva and reports participation on an advisory board for Amgen, Janssen, Novartis, Roche, and Teva. C.P.I. reports consulting fees from Amgen, Bayer, Boehringer Ingelheim, Novartis, Sanofi, Sobi, and Takeda and honoraria from Amgen, Boehringer Ingelheim, Grifols, Novartis, Sanofi, Takeda, and Werfen. S.A. reports a contract as a member of the grant committee for Grifols; reports honoraria for lectures from Amgen, argenx, Grifols, and Novartis; support for attending meetings and/or travel from Amgen, Grifols, and Novartis; participation on advisory board with argenx, Grifols, Novartis, and Sanofi; and is a member of a French referral center for adult autoimmune cytopenia. M.C., R.D., M.Y., I.G., and M.L. are employees of, and receive support for attending meetings and/or travel and stock/stock options from, Sanofi. A.D. is an employee of, receives support for attending meetings and/or travel from, issued patent for use of rilzabrutinib in patients with ITP, and receives stock/stock options from Sanofi and reports participation on a data safety monitoring board or an advisory board member of the Open Session. The remaining authors declare no competing financial interests.
A complete list of the members of the LUNA3 Trial Group appears in the supplemental Data.
Correspondence: David J. Kuter, Hematology Division, Massachusetts General Hospital, Harvard Medical School, Bartlett Hall 150, 55 Fruit St, Boston, MA 02114-2621; email: kuter.david@mgh.harvard.edu.
References
Author notes
Preliminary baseline demographic study results were presented as a publication-only abstract (abstract PB3371) for the European Hematology Association meeting, Madrid, Spain June 13-16, 2024; this study was also presented in abstract form (abstract 5) at the 66th annual meeting of the American Society of Hematology, San Diego, CA, 7-10 December 2024.
Qualified researchers may request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and data set specifications. Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.vivli.org/.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal