Key Points
IL-10 secretion by DLBCL cells prevents the drift of the lymphoma microenvironment toward a highly immune-suppressive state.
Hallmarks of IL-10 deficiency predict R-CHOP response but reflect poor antitumor immunity in preclinical models and patients with DLBCL.
Visual Abstract
The contribution of interleukin-10 (IL-10), secreted by tumoral B cells, to the progression and shaping of the microenvironment in diffuse large B-cell lymphoma (DLBCL) with activated B-cell–like (ABC) phenotype is not yet completely understood. To shed light on this issue, we generated an immunocompetent mouse model of ABC-DLBCL with conditional knockout of IL-10 specifically in malignant B cells. Paradoxically, these mice had significantly worse overall survival when left untreated but experienced increased sensitivity to conventional anti-CD20 immunotherapy or regulatory T-cell depletion. We identified various immunomodulatory mechanisms involved in this behavior. In particular, we show that IL-10–deficient lymphomas acquire a highly immunosuppressed and T-cell–exhausted microenvironment with increased angiogenesis that results in a more aggressive phenotype, which is refractory to PD-1 immune checkpoint blockade. However, the response of IL-10–deficient mice to anti-CD20 immunotherapy was greatly enhanced by upregulation of calcium channels in B cells. In general, IL-10 autocrine signaling promotes the survival of malignant B cells, whereas the paracrine action of B-cell–derived IL-10 maintains an immunoreactive microenvironment that influences the efficacy of emerging immunotherapy strategies targeting the lymphoma microenvironment. Furthermore, IL-10–associated transcriptional signatures derived from our studies may correctly predict clinical outcomes of patients with DLBCL treated with R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone). Thus, our work provides important functional and mechanistic insights into the role of B-cell–derived IL-10 in the biology of ABC-DLBCL.
Introduction
Diffuse large B-cell lymphomas (DLBCLs) are the most common type of non-Hodgkin lymphoma (NHL).1 DLBCLs arise from germinal center (GC) B cells and show marked molecular heterogeneity, with several genetic subgroups2-4 and 2 main transcriptional subgroups: activated B-cell–like (ABC) and GC B-cell–like (GCB).5 The ABC subtype is thought to arise from GC-experienced B cells that avoid terminal plasmacytic differentiation either as plasmablasts or aberrant memory B cells.6,7 The more aggressive ABC-DLBCL accounts for most of the 30% to 40% of patients experiencing relapsed or refractory responses to R-CHOP immunochemotherapy (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone).8 Identification of factors contributing to malignant DLBCL cell survival might predict immunochemotherapy responses and help design treatment alternatives for higher-risk patients.
Interleukin-10 (IL-10) and IL-10 receptor (IL-10R) have been proposed to play an unfavorable role in DLBCL,9-12 creating an autostimulatory loop via STAT3 signaling1,11,13-15 and inducing programmed death-ligand 1 (PD-L1) immune checkpoint expression.16 IL-10 is a potent growth and differentiation factor for activated B cells,17 enhancing the viability and expression of major histocompatibility complex (MHC) class II antigens in resting murine B cells.18 Under inflammatory conditions, frequently found in tumor microenvironments, IL-10 can be upregulated and inhibit the secretion of proinflammatory cytokines, MHC molecules, and costimulatory molecules.19,20 In normal B cells, STAT3 activation is usually transient, but DLBCL shows constitutive activation of STAT3, promoting tumorigenesis.14,21,22 Although multiple lines of evidence support the autocrine contribution of IL-10 to lymphomagenesis, the potentially more complex in vivo role of the B-cell IL-10/STAT3 axis in DLBCL progression remains uncertain. It is unclear whether IL-10 secreted by malignant B cells may elicit antitumor responses in DLBCL via paracrine effects, given its pleiotropic roles with both stimulatory and inhibitory functions.23-25
To explore in vivo the relevance of lymphoma-derived IL-10, we generated a quintuple transgenic mouse model that we call pBICΔ10, after crossing our previous ABC-DLBCL-like pBIC mice,26 a “push-and-block” model driven by NF-κB activation (constitutively active IKK2), genomic instability (p53 loss), and blocked terminal differentiation (Blimp1 deletion), with mice with a floxed Il10 gene,27 for conditional knockout of IL-10 specifically in malignant ABC-DLBCL cells. Analyses of human DLBCL data sets supported the clinical implications of our findings. Collectively, our study provides new functional and mechanistic insights into the role of lymphoma B-cell–derived IL-10 in DLBCL pathogenesis and immunotherapy response.
Methods
Animal studies
Control YC and lymphoma pBIC compound mice, previously described,26 carried conditional loxP alleles (p: p53, B: Blimp1, I: IKK2ca.IRES.GFP, or Y: eYFP) and Cγ1-cre (C). pBICΔ10 mice were generated by crossing pBIC mice with Il10F/F mice.27 Both models were followed-up for tumoral onset without chronic immunization and subjected to in vivo immunotherapy combinations (detailed in the supplemental Methods, available on the Blood website). All procedures were approved by the Ethical Committee of Animal Experimentation of the University of Navarra (089c-17, 085-19), following European Directive 2010/63/EU.
Ex vivo studies
Cell viability in fresh primary murine tumors was evaluated by flow cytometry in response to IL-10/JAK1/STAT3 axis inhibitors (anti-IL-10R, ruxolitinib, and pyrimethamine) and anti-CD20 immunotherapy. Murine serum IL-10 levels were determined by OptEIA mouse IL-10 enzyme-linked immunosorbent assay (ELISA) set. See details in the supplemental Methods.
Immunophenotypic studies
Flow cytometry and chromogenic multiplex immunohistochemistry were performed using population markers and antibodies (supplemental Table 1) and standard protocols as detailed in the supplemental Methods, using CytExpert/FlowJo for cytometry and Leica Aperio CS2/QuPath for image analysis.
Transcriptomic studies
RNA was extracted using TRIzol reagent from the population of interest for reverse transcription quantitative polymerase chain reaction (RT-qPCR) and RNA sequencing (RNA-seq; GSE255140 and GSE11629026). For single-cell RNA sequencing (scRNA-seq) and T-cell receptor sequencing (scTCR-seq), Chromium Single-Cell Instrument and Chromium Next GEM Single Cell 5' Reagent Kit v2 (Dual Index) from 10× Genomics were used (GSE253955). Human DLBCL cohorts with transcriptional and survival data were obtained from indicated public repositories. For additional information, data processing, and analysis, see the supplemental Methods.
Statistical analyses
Statistical analyses were performed using RStudio and GraphPad Prism V.9.0.2 and are described throughout the text and in the supplemental Methods.
Results
IL-10 promotes survival and PD-L1 expression in tumor cells from DLBCL mice
Using data from available human DLBCL transcriptional studies, we confirmed that although IL-10 expression is highly variable in DLBCL, it is upregulated compared with normal GC B cells, particularly in the more aggressive ABC or (MYD88L265P and CD79B mutation-enriched (MCD) subtypes12-15 (Figure 1A). Because our immunocompetent lymphoma mouse model (pBIC) shows key ABC-DLBCL–like hallmarks,26,28 we also detected increased expression of Il10 by RNA-seq and RT-qPCR in fluorescence-activated cell sorter (FACS)-sorted B220+GFP+ lymphoma cells compared with normal GC B cells isolated from sheep red blood cell–immunized control YC mice (Figure 1B). Previous studies in ABC/MCD-DLBCL demonstrated the establishment of a feed-forward prosurvival loop through autocrine IL-10R signaling, relying on JAK1/STAT3 activation (Figure 1C).13,14,29-31 Consistently, we observed significantly higher levels of intracellular IL-10 and phosphorylated STAT3 (pSTAT3) in CD19+GFP+ pBIC lymphoma cells than in normal B cells from control mice (Figure 1D). Ex vivo perturbation of this IL-10R/JAK1/STAT3 axis with monoclonal antibodies blocking IL-10R or inhibiting JAK1 (ruxolitinib) and STAT3 (pyrimethamine)1,32 led to significant killing of pBIC lymphoma cells over adjacent normal B cells (Figure 1E). Furthermore, the known link between IL-10/STAT3 signaling and PD-L1 expression in human DLBCL16 could also be demonstrated in pBIC tumors, with significant reduction of PD-L1 surface levels in B220+GFP+ lymphoma cells exposed to JAK1/STAT3 inhibitors (Figure 1F). Altogether, these data support a pro-oncogenic role of autocrine IL-10/STAT3 signaling inside DLBCL cells, promoting cellular viability and PD-L1 immune checkpoint upregulation.
Tumor survival and PD-L1 expression are promoted by increased levels of IL-10 in DLBCL. (A) Baseline expression of IL10 across normal GC B cells and human DLBCL data sets classified according to COO gene expression or LymphGen genetic subtypes; merged expression arrays (left) from GSE56315 and GSE12195; RNA-seq (right) from Schmitz/Wright et al2,4 (t test). (B) Expression of Il10 by RNA-seq and RT-qPCR between normal GC B cells (B220+CD38lowFAS+YFP+) from control YC mice and lymphoma cells (B220+GFP+) from pBIC tumors (t test and Mann-Whitney, respectively). Values from our previous GSE116290 (triangles) or current GSE255140 (circles) RNA-seq experiments were normalized and analyzed combined here. (C) Model depicting the IL-10/JAK/STAT3 autocrine pathway that promotes survival and upregulation of PD-L1 in DLBCL cells, showing different strategies to inhibit this survival loop. (D) IL-10 expression and pSTAT3 quantification measured as MFI by intracellular flow cytometry of lymphoma (CD19+GFP+) and adjacent normal B cells (CD19+GFP–), respectively (Mann-Whitney). (E) Normalized cell viability assays of lymphoma cells (B220+GFP+) cells compared with adjacent normal B cells (B220+GFP–) studied by flow cytometry after 24-hour ex vivo culture with increasing doses of anti–IL-10R, ruxolitinib, or pyrimethamine inhibitors (n ≥ 3) and 50% inhibitory concentration (IC50) calculations from baseline-corrected data. (F) Relative changes in PD-L1 surface expression between adjacent normal B cells (B220+GFP–) and lymphoma cells (B220+GFP+) after 24 hours untreated vs treated with concentrations above the IC50 of ruxolitinib (100 μM) and pyrimethamine (200 μM; n ≥ 3; analysis of variance [ANOVA]). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. MFI, median fluorescence intensity.
Tumor survival and PD-L1 expression are promoted by increased levels of IL-10 in DLBCL. (A) Baseline expression of IL10 across normal GC B cells and human DLBCL data sets classified according to COO gene expression or LymphGen genetic subtypes; merged expression arrays (left) from GSE56315 and GSE12195; RNA-seq (right) from Schmitz/Wright et al2,4 (t test). (B) Expression of Il10 by RNA-seq and RT-qPCR between normal GC B cells (B220+CD38lowFAS+YFP+) from control YC mice and lymphoma cells (B220+GFP+) from pBIC tumors (t test and Mann-Whitney, respectively). Values from our previous GSE116290 (triangles) or current GSE255140 (circles) RNA-seq experiments were normalized and analyzed combined here. (C) Model depicting the IL-10/JAK/STAT3 autocrine pathway that promotes survival and upregulation of PD-L1 in DLBCL cells, showing different strategies to inhibit this survival loop. (D) IL-10 expression and pSTAT3 quantification measured as MFI by intracellular flow cytometry of lymphoma (CD19+GFP+) and adjacent normal B cells (CD19+GFP–), respectively (Mann-Whitney). (E) Normalized cell viability assays of lymphoma cells (B220+GFP+) cells compared with adjacent normal B cells (B220+GFP–) studied by flow cytometry after 24-hour ex vivo culture with increasing doses of anti–IL-10R, ruxolitinib, or pyrimethamine inhibitors (n ≥ 3) and 50% inhibitory concentration (IC50) calculations from baseline-corrected data. (F) Relative changes in PD-L1 surface expression between adjacent normal B cells (B220+GFP–) and lymphoma cells (B220+GFP+) after 24 hours untreated vs treated with concentrations above the IC50 of ruxolitinib (100 μM) and pyrimethamine (200 μM; n ≥ 3; analysis of variance [ANOVA]). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. MFI, median fluorescence intensity.
Genetic deletion of IL-10 in DLBCL cells accelerates DLBCL progression
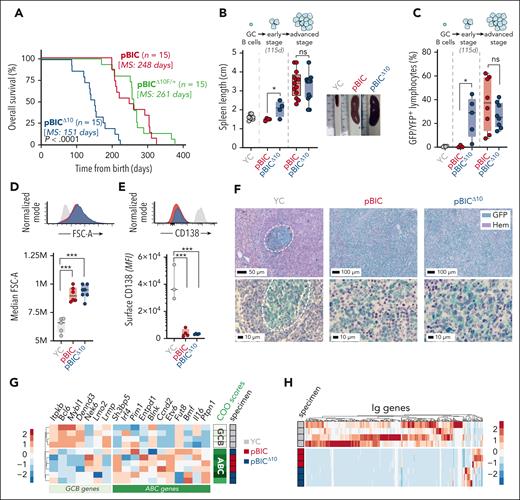
To evaluate in vivo the contribution of autocrine IL-10 to lymphoma progression, we generated a conditional knockout mouse with specific deletion of Il10 in lymphoma cells by crossing Il10F/F mice27 with our original DLBCL-like pBIC mice (hereafter, pBICΔ10). Compared with IL-10–proficient pBIC mice, complete abrogation of IL-10 in GC-triggered lymphomas (supplemental Figure 1) severely impaired overall survival of pBICΔ10 mice, whereas a single wild-type Il10 allele (pBICΔ10F/+) was haplosufficient to restore the original survival rates (Figure 2A). All pBIC and pBICΔ10 mice exhibited splenomegaly at advanced stages (Figure 2B) due to the development of B-cell lymphomas, confirmed by significant expansion of CD19+GFP+ cells (Figure 2C), which were large (Figure 2D) and failed terminal differentiation into CD138+ plasma cells (Figure 2E). Consistent with a more aggressive course, IL-10–deficient pBICΔ10 mice evidenced lymphoma progression at earlier stages (Figure 2B-C), which was independent of sex (supplemental Figure 2A). Histopathologic analysis of the tumors confirmed a diffuse growth pattern of large GFP+ lymphoma cells that disrupted spleen architecture (Figure 2F; supplemental Figure 3). In addition, RNA-seq profiling of fluorescence-activated cell sorter-sorted lymphoma cells from pBIC/pBICΔ10 mice showed downregulation of GCB-linked genes and upregulation of ABC-linked genes,26,33 supporting the classification of pBIC/pBICΔ10 lymphomas as ABC-DLBCL–like (Figure 2G). As expected, these lymphomas were oligoclonal, with downregulation of the normally diverse repertoire of immunoglobulin (Ig) genes expressed in GC B cells (Figure 2H). These results support a host-protective effect of tumor-derived IL-10 in DLBCL progression, likely through direct or indirect paracrine functions, paradoxically overriding the prosurvival effects of the autocrine IL-10/JAK/STAT3 loop.
Genetic deletion of IL-10 in DLBCL cells unexpectedly accelerates DLBCL progression. (A) Overall survival curves (and log-rank test statistic) of pBIC (IL-10 proficient), pBICΔ10 (IL-10 deficient), and pBICΔ10F/+ (IL-10 haplosufficient) mice. Time in the x-axis is represented as the time from the moment of birth in days. (B) Spleen length of pBIC and pBICΔ10 at early (115 days) or advanced (moribund mice) stages compared with control YC mice (t test). Representative images illustrate splenomegaly at advanced stages of disease compared with age-matched control YC mice. (C) Expansion of splenic lymphoma cells (CD19+ GFP+) at different stages of the disease when compared with normal GC B cells (CD19+ YFP+) from splenocytes of SRBC-immunized control YC mice (t test). (D) Representative fluorescence-activated cell sorter (FACS) profiles and cellular sizes as measured by FSC-A parameter of large lymphoma cells (CD19+ GFP+) or control GC B cells (CD19+ YFP+; ANOVA). (E) Representative FACS profiles and comparative levels of plasma cell marker CD138 within lymphoma cells (CD19+ GFP+) or control terminally differentiated PCs (YFP+ CD138+; ANOVA). (F) IHC staining of GFP/YFP (green) and hematoxylin (Hem) in control YC and lymphoma pBIC and pBICΔ10 mice (scale bar in μm). (G) Heat map showing z scores of ABC- and GCB-related genes and RNA-seq–based COO classifier revealing an ABC-DLBCL subtype in both pBIC/pBICΔ10 murine lymphomas, compared with normal GC B cells from control YC mice. (H) Heat map of Ig-related transcripts shows oligoclonality in both pBIC and pBICΔ10 models compared with normal GC B cells from control YC mice. ∗P < .05; ∗∗∗P < .001; d, days; FSC-A, forward scatter area; IHC, immunohistochemistry; MS, median survival; ns, nonsignificant; PCs, plasma cells; SRBC, sheep red blood cells.
Genetic deletion of IL-10 in DLBCL cells unexpectedly accelerates DLBCL progression. (A) Overall survival curves (and log-rank test statistic) of pBIC (IL-10 proficient), pBICΔ10 (IL-10 deficient), and pBICΔ10F/+ (IL-10 haplosufficient) mice. Time in the x-axis is represented as the time from the moment of birth in days. (B) Spleen length of pBIC and pBICΔ10 at early (115 days) or advanced (moribund mice) stages compared with control YC mice (t test). Representative images illustrate splenomegaly at advanced stages of disease compared with age-matched control YC mice. (C) Expansion of splenic lymphoma cells (CD19+ GFP+) at different stages of the disease when compared with normal GC B cells (CD19+ YFP+) from splenocytes of SRBC-immunized control YC mice (t test). (D) Representative fluorescence-activated cell sorter (FACS) profiles and cellular sizes as measured by FSC-A parameter of large lymphoma cells (CD19+ GFP+) or control GC B cells (CD19+ YFP+; ANOVA). (E) Representative FACS profiles and comparative levels of plasma cell marker CD138 within lymphoma cells (CD19+ GFP+) or control terminally differentiated PCs (YFP+ CD138+; ANOVA). (F) IHC staining of GFP/YFP (green) and hematoxylin (Hem) in control YC and lymphoma pBIC and pBICΔ10 mice (scale bar in μm). (G) Heat map showing z scores of ABC- and GCB-related genes and RNA-seq–based COO classifier revealing an ABC-DLBCL subtype in both pBIC/pBICΔ10 murine lymphomas, compared with normal GC B cells from control YC mice. (H) Heat map of Ig-related transcripts shows oligoclonality in both pBIC and pBICΔ10 models compared with normal GC B cells from control YC mice. ∗P < .05; ∗∗∗P < .001; d, days; FSC-A, forward scatter area; IHC, immunohistochemistry; MS, median survival; ns, nonsignificant; PCs, plasma cells; SRBC, sheep red blood cells.
Lymphoma-derived IL-10 regulates the expression of L-type Ca2+ channels and associates with worse response to anti-CD20 immunotherapy
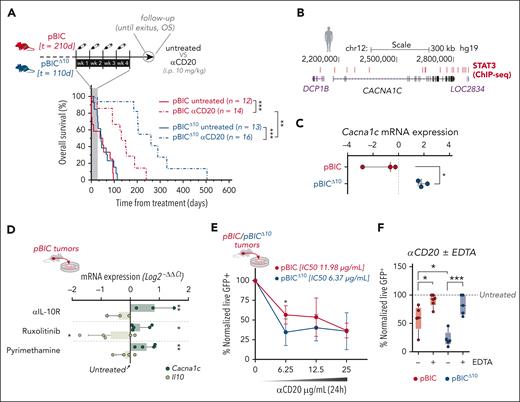
Given the intriguing antitumoral impact of lymphoma-derived IL-10, we explored the response of both DLBCL models to anti-CD20 immunotherapy (as surrogate for rituximab in patients with DLBCL). Randomized pBIC/pBICΔ10 mice at advanced stages of disease were treated intraperitoneally with anti-CD20 (Figure 3A, scheme). Even though IL-10–deficient pBICΔ10 lymphomas are more aggressive in the absence of treatment, they surprisingly responded significantly better to anti-CD20 immunotherapy than IL-10–proficient pBIC mice (Figure 3A). Although this is consistent with the association of higher IL-10 levels and worse clinical outcomes with R-CHOP in patients with DLBCL,9-12 the contradictory aggressiveness of IL-10–deficient murine lymphomas anticipates a complex contribution of tumor-derived IL-10 to DLBCL biology.
Lymphoma-derived IL-10 regulates the expression of L-type Ca2+ channels and associates with worse responses to conventional CD20 immunotherapy. (A) Comparison of survival curves (log-rank test) between pBIC and pBICΔ10 mice, either untreated or treated with anti- (α)CD20, after normalizing the time measurements to the initiation of treatment. (B) Genome Browser representation of STAT3-binding sites identified by ChIP-seq in human ABC-DLBCL cells (GSE10684434). (C) Expression of Cacna1c by RNA-seq (log counts per million (logCPM) in FACS-sorted lymphoma cells (B220+GFP+) from pBIC and pBICΔ10 tumors (t test). (D) Expression of Cacna1c and Il-10 by RT-qPCR in pBIC lymphoma cells after 24-hour ex vivo treatment with 10 μg/mL of αIL-10R and concentrations slightly rounded over IC50 of ruxolitinib (100 μM) or pyrimethamine (200 μM; n = 3), as calculated in Figure 1E. Expression of each gene in untreated cells was used for normalization (ANOVA). (E) Normalized cell viability assays of pBIC/pBICΔ10 lymphoma cells (GFP+ 7AAD–) studied by flow cytometry after 24 hours of ex vivo culture with increasing doses of αCD20 (t test), or (F) after 6 hours in the presence of 12.5 μg/mL of αCD20 with or without 20 mM of EDTA (n = 3; ANOVA). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ChIP-seq, chromatin immunoprecipitation-seq; h, hours; i.p., intraperitoneal; mRNA, messenger RNA; wk, week; OS, overall survival.
Lymphoma-derived IL-10 regulates the expression of L-type Ca2+ channels and associates with worse responses to conventional CD20 immunotherapy. (A) Comparison of survival curves (log-rank test) between pBIC and pBICΔ10 mice, either untreated or treated with anti- (α)CD20, after normalizing the time measurements to the initiation of treatment. (B) Genome Browser representation of STAT3-binding sites identified by ChIP-seq in human ABC-DLBCL cells (GSE10684434). (C) Expression of Cacna1c by RNA-seq (log counts per million (logCPM) in FACS-sorted lymphoma cells (B220+GFP+) from pBIC and pBICΔ10 tumors (t test). (D) Expression of Cacna1c and Il-10 by RT-qPCR in pBIC lymphoma cells after 24-hour ex vivo treatment with 10 μg/mL of αIL-10R and concentrations slightly rounded over IC50 of ruxolitinib (100 μM) or pyrimethamine (200 μM; n = 3), as calculated in Figure 1E. Expression of each gene in untreated cells was used for normalization (ANOVA). (E) Normalized cell viability assays of pBIC/pBICΔ10 lymphoma cells (GFP+ 7AAD–) studied by flow cytometry after 24 hours of ex vivo culture with increasing doses of αCD20 (t test), or (F) after 6 hours in the presence of 12.5 μg/mL of αCD20 with or without 20 mM of EDTA (n = 3; ANOVA). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ChIP-seq, chromatin immunoprecipitation-seq; h, hours; i.p., intraperitoneal; mRNA, messenger RNA; wk, week; OS, overall survival.
Antibody-mediated crosslinking of CD20 molecules induces lymphoma B-cell cytotoxicity via intracellular Ca2+ influx,35,36 requiring the accessory CACNA1C cell surface L-type calcium channels for CD20 stability and rituximab-induced cell death.37 STAT3 directly binds (Figure 3B) and represses CACNA1C expression in pSTAT3high human DLBCL cell lines.34 Consistently, we observed increased Cacna1c expression in pBICΔ10 lymphomas compared with pBIC by RNA-seq (Figure 3C) and after ex vivo treatment with anti-IL-10R or JAK/STAT3 inhibition in primary pBIC lymphoma cells (Figure 3D), supporting the transcriptional repression of L-type CACNA1C calcium channels by the autocrine IL-10/JAK/STAT3 pathway. To further explore the role of this IL-10+/pSTAT3+/CACNA1Clow axis in the response to anti-CD20, ex vivo treatment of fresh primary pBIC/pBICΔ10 tumors with increasing doses of anti-CD20 confirmed increased sensitivity (lower IC50, 50% inhibitory concentration values) of IL-10–deficient pBICΔ10 lymphoma cells (Figure 3E). Notably, this was dependent on extracellular Ca2+ because chelating calcium with EDTA significantly inhibited anti-CD20–induced cell death in both models (Figure 3F). Altogether, these results suggest that the absence of IL-10/JAK/STAT3 autocrine signaling sensitizes DLBCL cells to antibody-mediated cell killing through increased L-type calcium channels that elicit Ca2+ influx after CD20 crosslinking, resulting in better responses to anti-CD20 immunotherapy despite the initially more aggressive behavior of IL-10–/CACNA1Chigh pBICΔ10 lymphomas.
Transcriptomic analyses identify lymphoma-derived IL-10 as a key immunomodulator of DLBCL microenvironment
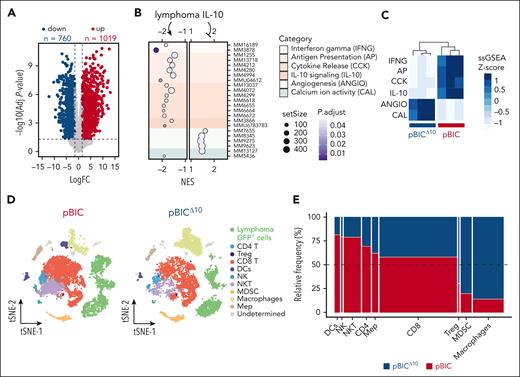
To elucidate the complex role of lymphoma-derived IL-10 in vivo, we compared the transcriptomes of pBIC/pBICΔ10 lymphomas. IL-10 depletion led to broad changes in gene expression (Figure 4A; supplemental Table 2), with 1779 differentially expressed genes involved in functional categories significantly enriched or depleted in pBICΔ10 mice according to gene set enrichment analysis (GSEA) (Figure 4B; supplemental Table 3). Single-sample GSEA (supplemental Table 4) of selected gene signatures revealed a dual role of lymphoma-derived IL-10, enabling immune-associated processes (interferon gamma [IFNG], antigen presentation [AP], cytokine release [CKK], and IL-10 signaling) while repressing others (angiogenesis [ANGIO] and calcium activity [CAL], the later exemplified by the Cacna1c gene; Figure 4C).
Transcriptomic analyses identify lymphoma-derived IL-10 as key immunomodulator of DLBCL microenvironment. (A) Volcano plot of differentially expressed genes between pBICΔ10 vs pBIC FACS-sorted B220+GFP+ lymphoma cells (supplemental Table 2). (B) GSEA comparing pBICΔ10 and pBIC lymphoma cells, highlighting significantly enriched and depleted gene signatures (supplemental Table 3). Functional categories are displayed and grouped by color, based on differential expression analysis of RNA-seq data. (C) Gene signatures summarizing the functional categories found in panel B (supplemental Table 4) were used in single-sample GSEA (ssGSEA) and correctly cluster pBIC and pBICΔ10 mice. (D) t-distributed Stochastic Neighbor Embedding (t-SNE) graph of an integration of 18 343 cells from 1 representative pBIC and pBICΔ10 murine lymphoma. (E) Mosaic plot displaying the distribution of cell types defined by scRNA-seq in pBIC vs pBICΔ10 lymphomas. The width of each cell type bar represents the proportion of that cell type within the total sample population, whereas the height indicates the relative frequency of that cell type between the 2 lymphoma models. NES, normalized enrichment score.
Transcriptomic analyses identify lymphoma-derived IL-10 as key immunomodulator of DLBCL microenvironment. (A) Volcano plot of differentially expressed genes between pBICΔ10 vs pBIC FACS-sorted B220+GFP+ lymphoma cells (supplemental Table 2). (B) GSEA comparing pBICΔ10 and pBIC lymphoma cells, highlighting significantly enriched and depleted gene signatures (supplemental Table 3). Functional categories are displayed and grouped by color, based on differential expression analysis of RNA-seq data. (C) Gene signatures summarizing the functional categories found in panel B (supplemental Table 4) were used in single-sample GSEA (ssGSEA) and correctly cluster pBIC and pBICΔ10 mice. (D) t-distributed Stochastic Neighbor Embedding (t-SNE) graph of an integration of 18 343 cells from 1 representative pBIC and pBICΔ10 murine lymphoma. (E) Mosaic plot displaying the distribution of cell types defined by scRNA-seq in pBIC vs pBICΔ10 lymphomas. The width of each cell type bar represents the proportion of that cell type within the total sample population, whereas the height indicates the relative frequency of that cell type between the 2 lymphoma models. NES, normalized enrichment score.
To delve deeper into the immunomodulatory role of IL-10 on adjacent immune cells, we performed scRNA-seq of splenocytes from representative pBIC and pBICΔ10 tumors (1 from each group), composed of 20% GFP+ lymphoma cells and 80% nontumoral GFP– cells (supplemental Figure 4A). In this exploratory analysis, we identified a total of 11 distinct cell types (Figure 4D) in an integrated data set of 18 343 splenocytes (10 049 from pBIC and 8294 from pBICΔ10), verified by the expression of canonical cell type–specific markers (supplemental Figure 4B). The absence of lymphoma-derived IL-10 promoted differences in infiltrating populations (Figure 4E), with relative increase of immune-suppressor cells (regulatory T cells [Tregs] and macrophages) and relative decrease of immune-effector (CD8 and natural killer [NK]/NKT) or antigen-presenting cells (dendritic cells [DCs]). These results support the existence of autocrine and paracrine interconnected gene networks controlled by IL-10, which may ultimately rewire antitumor immune responses.
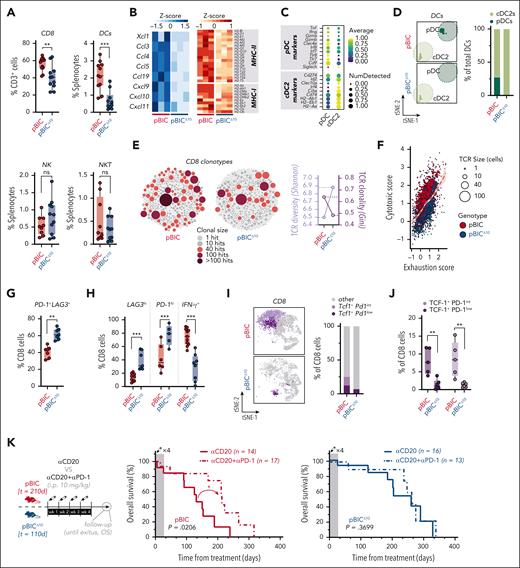
Lymphoma-derived IL-10 prevents the burnout of CD8+ TILs and predicts responses to ICB
Remarkably, the accelerated tumoral onset in IL-10–deficient lymphomas was accompanied by a decrease in CD8+ T cells and DCs without clear changes in NK/NKT cells, both by scRNA-seq (Figure 4E) and flow cytometry (Figure 5A). Consistently, several chemokines involved in the attraction of CD8 cells (eg, CCL5/CXCL9)38 and DCs (eg, CCL4)38-41 appeared particularly downregulated in lymphoma cells from pBICΔ10 mice, together with reduced expression of MHC-I and -II antigen-presenting genes (Figure 5B). Further inspection of scRNA-seq data revealed 2 subclusters inside DCs (Figure 5C). In both pBIC/pBICΔ10 models, DCs were primarily type 2 conventional DCs (cDC2s; Figure 5D) and expressed Cd274/Pd-l1 (Figure 5C), indicating a potential suppressive effect on T cells, in line with previous association of cDC2s with ABC-DLBCL in humans.42 In contrast, plasmacytoid DCs (pDCs), which respond to CXCL19/CXCL10 for recruitment,43,44 are IFN-producing, and may contribute to protective immunity,39,45 were only found in the pBIC microenvironment (Figure 5D). This reveals a role of lymphoma-derived IL-10 in facilitating antigen presentation and pDC-mediated contribution to tumor control. Indirect support for this was the increased TCR clonal diversity (higher Shannon index, based on scTCR-seq data of the same tumors analyzed with scRNA-seq; supplemental Figure 4A) in IL-10–deficient tumors, coinciding with a reduction in the prevalence of larger clones and TCR clonality (lower Gini index; Figure 5E). Altogether, these results support a role for IL-10 in facilitating CD8 priming and antigen-mediated selection of antitumoral T-cell clones.
Lymphoma-derived IL-10 prevents the burnout of CD8+ TILs and predicts responses to immune-checkpoint blockade. (A) Overall percentage of CD8 (from total CD3 lymphocytes), DCs, NK, and NKT (from total splenocytes) cells by flow cytometry in pBIC and pBICΔ10 tumors (t test). (B) Heat map (z scores) of chemokines involved in the attraction of CD8/NK/DCs cells (left) and MHC-I and -II antigen-presenting genes (right), derived from RNA-seq analysis of FACS-sorted lymphoma cells (B220+GFP+) from pBIC and pBICΔ10 tumors. (C) Dot plot based on scRNA-seq data showing scaled expression levels of pDC or cDC2 marker genes in the dendritic cell population infiltrating pBIC and pBICΔ10 tumors. (D) t-SNE distribution (left) and proportion of each DC subtype (from total DCs, right) detected by scRNA-seq in pBIC and pBICΔ10 tumors. (E) Bubble plot of CD8 TCR repertoire identified by scTCR-seq in the same pBIC and pBICΔ10 tumors submitted to scRNA-seq (supplemental Figure 4A). Each bubble represents a unique TCR clonotype, sized and colored according to cell count abundance (hits). Shannon index for evaluation of relative TCR diversity and Gini index for evaluation of TCR diversity are shown. (F) Scatterplot of cytotoxic and exhaustion programs within each CD8 clonotype from scTCR-seq showing a trend toward an exhaustion state in pBICΔ10 TCRα/β-CDR3 clonotypes. (G) Overall percentage of double-positive PD-1+LAG3+ CD8 T cells by flow cytometry (t test). (H) Proportion of CD8 cells with the highest expression of LAG3 or PD-1; and overall percentage of IFN-γ+ secretor cells by flow cytometry of pBIC and pBICΔ10 tumors (t test). (I) Proportion of Tcf1/Pd1 populations detected by scRNA-seq within the CD8+ T-cell compartment. (J) Overall percentage of TCF-1+ (int, intermediate, or high) by flow cytometry within pBIC and pBICΔ10 tumor-infiltrating CD8+ cells (ANOVA). (K) In vivo regime for αCD20 either in monotherapy or in combination with αPD-1; and associated survival curves from the time of treatment in pBIC and pBICΔ10 mice (log-rank test). ∗∗P < .01; ∗∗∗P < .001; d, days; hi, high; i.p., intraperitoneal; int, intermediate; ns, nonsignificant; wk, week.
Lymphoma-derived IL-10 prevents the burnout of CD8+ TILs and predicts responses to immune-checkpoint blockade. (A) Overall percentage of CD8 (from total CD3 lymphocytes), DCs, NK, and NKT (from total splenocytes) cells by flow cytometry in pBIC and pBICΔ10 tumors (t test). (B) Heat map (z scores) of chemokines involved in the attraction of CD8/NK/DCs cells (left) and MHC-I and -II antigen-presenting genes (right), derived from RNA-seq analysis of FACS-sorted lymphoma cells (B220+GFP+) from pBIC and pBICΔ10 tumors. (C) Dot plot based on scRNA-seq data showing scaled expression levels of pDC or cDC2 marker genes in the dendritic cell population infiltrating pBIC and pBICΔ10 tumors. (D) t-SNE distribution (left) and proportion of each DC subtype (from total DCs, right) detected by scRNA-seq in pBIC and pBICΔ10 tumors. (E) Bubble plot of CD8 TCR repertoire identified by scTCR-seq in the same pBIC and pBICΔ10 tumors submitted to scRNA-seq (supplemental Figure 4A). Each bubble represents a unique TCR clonotype, sized and colored according to cell count abundance (hits). Shannon index for evaluation of relative TCR diversity and Gini index for evaluation of TCR diversity are shown. (F) Scatterplot of cytotoxic and exhaustion programs within each CD8 clonotype from scTCR-seq showing a trend toward an exhaustion state in pBICΔ10 TCRα/β-CDR3 clonotypes. (G) Overall percentage of double-positive PD-1+LAG3+ CD8 T cells by flow cytometry (t test). (H) Proportion of CD8 cells with the highest expression of LAG3 or PD-1; and overall percentage of IFN-γ+ secretor cells by flow cytometry of pBIC and pBICΔ10 tumors (t test). (I) Proportion of Tcf1/Pd1 populations detected by scRNA-seq within the CD8+ T-cell compartment. (J) Overall percentage of TCF-1+ (int, intermediate, or high) by flow cytometry within pBIC and pBICΔ10 tumor-infiltrating CD8+ cells (ANOVA). (K) In vivo regime for αCD20 either in monotherapy or in combination with αPD-1; and associated survival curves from the time of treatment in pBIC and pBICΔ10 mice (log-rank test). ∗∗P < .01; ∗∗∗P < .001; d, days; hi, high; i.p., intraperitoneal; int, intermediate; ns, nonsignificant; wk, week.
Next, we explored the functional state of these CD8+ tumor-infiltrating lymphocytes (TILs) in murine DLBCL lymphomas. Combining scRNA-seq and TCR-seq data from pBIC and pBICΔ10 CD8+ T cells, we estimated the exhaustion state by the average expression of previously published exhaustion and cytotoxic programs46 across each T-cell clone (cells with identical TCRα/β-CDR3 clonotypes; supplemental Table 5). CD8+ T-cell clones in pBICΔ10 had higher exhaustion scores relative to cytotoxicity scores than pBIC (Figure 5F), consistent with a more dysfunctional T-cell compartment in the absence of lymphoma-derived IL-10. Confirmed by flow cytometry, IL-10–deficient pBICΔ10 lymphomas exhibited increased proportion of exhausted CD8+ T cells, marked by coexpression of inhibitory receptors such as PD-1 and LAG3 (Figure 5G), with especially high expression of LAG3 and PD-1 but lower IFN-γ production (Figure 5H). The hyper-exhausted phenotype of this population of CD8+ TILs resembled the effector burned-out phenotype previously described for dysfunctional CD8+ TILs, which can predict resistance to immune checkpoint blockade (ICB) in lung cancer.47 Moreover, murine pBIC lymphomas evidenced a population of Tcf1+Pd1int/low CD8+ cells by scRNA-seq (Figure 5I), confirmed by flow cytometry (Figure 5J), which may derive from an intratumoral niche of stem-like CD8+ cells responsive to ICB.48-50 We observed a decrease in the abundance of these precursor CD8+ T cells in pBICΔ10 mice (Figure 5I-J), supporting the notion that IL-10R signaling is necessary for the maintenance of stem-like CD8+ TILs.51 Therefore, we reasoned that the shift toward effector burned-out dysfunctional pBICΔ10 lymphomas resulted from the simultaneous deterioration of progenitor TCF-1+PD-1int/low CD8+ stem-like cells and progressive differentiation of CD8+ TILs to terminally exhausted TCF-1–PD-1hi cells.
To test whether this unbalance of intratumoral CD8+ subsets might contribute to immune escape and resistance to ICB in DLBCL, we evaluated the impact of anti–PD-1 therapy in long-term responses to anti-CD20 immunotherapy in pBICΔ10 mice, as we had previously demonstrated in pBIC mice.26 Randomized pBIC/pBICΔ10 mice at advanced stages of disease were treated with anti-CD20, anti–PD-1, or their combination. Although T-cell–targeted ICB was ineffective as a monotherapy in both pBIC and pBICΔ10 mice (supplemental Figure 5), consistent with our earlier findings,26 a significant response to ICB combination with anti-CD20 was observed only in IL-10–proficient pBIC mice but not in IL-10–deficient pBICΔ10 mice (Figure 5K). This suggests that insufficient IL-10 signaling from DLBCL cells fails to sustain the niche of anti–PD-1 responsive stem-like CD8+ cells and allows for the chronic activation and burnout of CD8+ TILs, ultimately contributing to a less responsive lymphoma microenvironment (LME) and resistance to ICB.
Lymphoma-derived IL-10 prevents macrophage expansion and exhibits antiangiogenic functions
Tumor-associated macrophages (TAMs) have been associated with tumor growth, immune suppression, and resistance to ICB.52 TAMs increased in aggressive pBICΔ10 tumors, as seen by scRNA-seq (Figure 4E) and flow cytometry (Figure 6A), consistent with IL-10 attenuating the activation, proliferation, and recruitment of TAMs.53-55 Furthermore, scRNA-seq revealed coexpression of M1/antitumoral and M2/protumoral markers in both pBIC and pBICΔ10 TAMs (Figure 6B), recapitulating a noncanonical polarization recently uncovered in human DLBCL.56 This suggests that lymphoma-derived IL-10 limits the contribution of macrophages to lymphoma progression.
Lymphoma-derived IL-10 unbalances other immune suppressive subsets, exhibits antiangiogenic functions, and attenuates the response to Treg-depleting immunotherapy. (A) Overall percentage of CD11b+ F4/80+ TAMs identified by flow cytometry in pBIC and pBICΔ10 tumors (t test). (B) Dot plot based on scRNA-seq data showing scaled expression levels of M1 and M2 marker genes in the macrophage population infiltrating pBIC and pBICΔ10 tumors. (C) Representative images of chromogenic multiplex immunohistochemistry (cmIHC; green represents GFP+ lymphoma cells; red, CD31) illustrating distinct CD31+ vascular extensions in pBIC and pBICΔ10 lymphomas. The tissue structure was stained with Hem. (D) Percentage of CD31+ vascular area over the total tissue area (t test). Slides from 3 independent mice were analyzed within each genotype. (E) Representative images of cmIHC illustrating differences in vascular morphology in pBIC or pBICΔ10 lymphomas. Arrowheads mark regions lacking detectable CD31 immunoreactivity and the presence of GFP-expressing lymphoma cells in apparent contact with the lumen of mosaic vessels. Continuous and robust CD31 staining is indicated by a solid line. (F) Overall percentage by flow cytometry of intratumoral MDSCs (left; CD11b+ F4/80– non–double-positive [CD11c+ and IAb+]) and subsets (right) of Gr-MDSCs (Ly6G+Ly6C–) or Mo-MDSCs (Ly6G–Ly6C+) from total splenocytes in pBIC and pBICΔ10 tumoral spleens (t test). (G) Mo-MDSCs studied by flow cytometry in PBMCs from control nontumoral mice (nonimmunized Cγ1-negative littermates of pBIC mice) and tumoral models at advanced disease stages (ANOVA). (H) Heat map representation of IL-10 concentrations measured by ELISA in serum samples of pBIC and pBICΔ10 mice at different time points. (I) Overall percentage of Treg cells (from the total of CD4+ cells) in tumoral spleens from untreated pBIC and pBICΔ10 mice, further compared with mice treated for 4 weeks with αCD25NIB Treg-depleting antibody (t test). (J) In vivo regime for αCD25NIB treatments. (K) Flow cytometry study of PD-1+LAG3+ CD8 cell populations obtained from the spleens of mice either untreated or treated with αCD25NIB, after 4 weeks of treatment (ANOVA). (L) Survival curves from the time of treatment in pBIC and pBICΔ10 mice after αCD25NIB treatment (log-rank test). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; d, days; Gr-MDSC, granulocytic-MDSCs; Mo-MDSC, monocytic-MDSCs; ns, nonsignificant; OS, overall survival; PBMCs, peripheral blood mononuclear cells; wk, week.
Lymphoma-derived IL-10 unbalances other immune suppressive subsets, exhibits antiangiogenic functions, and attenuates the response to Treg-depleting immunotherapy. (A) Overall percentage of CD11b+ F4/80+ TAMs identified by flow cytometry in pBIC and pBICΔ10 tumors (t test). (B) Dot plot based on scRNA-seq data showing scaled expression levels of M1 and M2 marker genes in the macrophage population infiltrating pBIC and pBICΔ10 tumors. (C) Representative images of chromogenic multiplex immunohistochemistry (cmIHC; green represents GFP+ lymphoma cells; red, CD31) illustrating distinct CD31+ vascular extensions in pBIC and pBICΔ10 lymphomas. The tissue structure was stained with Hem. (D) Percentage of CD31+ vascular area over the total tissue area (t test). Slides from 3 independent mice were analyzed within each genotype. (E) Representative images of cmIHC illustrating differences in vascular morphology in pBIC or pBICΔ10 lymphomas. Arrowheads mark regions lacking detectable CD31 immunoreactivity and the presence of GFP-expressing lymphoma cells in apparent contact with the lumen of mosaic vessels. Continuous and robust CD31 staining is indicated by a solid line. (F) Overall percentage by flow cytometry of intratumoral MDSCs (left; CD11b+ F4/80– non–double-positive [CD11c+ and IAb+]) and subsets (right) of Gr-MDSCs (Ly6G+Ly6C–) or Mo-MDSCs (Ly6G–Ly6C+) from total splenocytes in pBIC and pBICΔ10 tumoral spleens (t test). (G) Mo-MDSCs studied by flow cytometry in PBMCs from control nontumoral mice (nonimmunized Cγ1-negative littermates of pBIC mice) and tumoral models at advanced disease stages (ANOVA). (H) Heat map representation of IL-10 concentrations measured by ELISA in serum samples of pBIC and pBICΔ10 mice at different time points. (I) Overall percentage of Treg cells (from the total of CD4+ cells) in tumoral spleens from untreated pBIC and pBICΔ10 mice, further compared with mice treated for 4 weeks with αCD25NIB Treg-depleting antibody (t test). (J) In vivo regime for αCD25NIB treatments. (K) Flow cytometry study of PD-1+LAG3+ CD8 cell populations obtained from the spleens of mice either untreated or treated with αCD25NIB, after 4 weeks of treatment (ANOVA). (L) Survival curves from the time of treatment in pBIC and pBICΔ10 mice after αCD25NIB treatment (log-rank test). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; d, days; Gr-MDSC, granulocytic-MDSCs; Mo-MDSC, monocytic-MDSCs; ns, nonsignificant; OS, overall survival; PBMCs, peripheral blood mononuclear cells; wk, week.
TAMs are also major producers of proangiogenic factors, contributing to the formation of new blood vessels in B-cell NHL.57,58 Indeed, transcriptional analysis of pBICΔ10 lymphoma cells by RNA-seq revealed an enrichment in the expression of genes associated with angiogenesis compared with pBIC (Figure 4B-C), suggesting that the proangiogenic activity of lymphoma cells could synergize with a larger subset of TAMs toward enhanced tumor vascularization. Because an angiogenesis-related signature (named stromal-2)59 has been associated with increased microvessel density in human DLBCL,60,61 we examined the platelet-endothelial adhesion molecule CD31 (PECAM-1) using chromogenic multiplex immunohistochemistry (Figure 6C). CD31+ vascular area was significantly increased in pBICΔ10 lymphomas compared with pBIC (Figure 6D), supporting the antiangiogenic effects of IL-10, consistent with previous observations in Burkitt lymphoma55 and other tumors.62-64 Furthermore, pBIC exhibited a recurrent morphology of mosaic vessels with interspersed GFP+ lymphoma cells, whereas pBICΔ10 tumors showed more continuous CD31 staining and less evidence of focal regions (Figure 6E). Mosaic tumor vessels have been associated with attenuated endothelial markers and abnormal endothelium,65,66 suggesting stabilization and maturation of vessels in the absence of lymphoma-derived IL-10. This supports the antiangiogenic role of lymphoma-derived IL-10 in DLBCL, at least in part via the expansion of noncanonical TAMs.
Lymphoma-derived IL-10 unbalances other immune-suppressive subsets and attenuates the response to Treg-depleting immunotherapy
We next investigated the contribution of other IL-10–producing immune populations to the maintenance of a tolerant LME in IL-10–deficient DLBCLs. Myeloid-derived suppressor cells (MDSCs) are immune-suppressive immature cells, classified as either granulocytic (Gr-MDSCs) or monocytic MDSCs (Mo-MDSCs).67,68 Both pBIC and pBICΔ10 mice exhibited relatively normal proportions of intratumoral granulocytic MDSCs (Figure 6F), consistent with the small proportion of MDSCs observed in normal mouse spleens (2%-4%) or in peripheral blood from healthy individuals (<2%).67,69 Only a slight accumulation of Mo-MDSCs was detected in pBIC lymphomas (Figure 6F) or peripheral blood (Figure 6G). Indeed, previous studies have associated IL-10 and increased serum IL-10 levels with Mo-MDSC promotion in B-cell NHL and other cancers.70-72 However, despite differences in IL-10 production between pBIC and pBICΔ10 lymphomas, serum IL-10 was barely detectable and not significantly different (Figure 6H), suggesting that tumor-derived IL-10 may have a rather localized impact in the LME. Altogether, these results suggest a minor pathogenic role of MDSCs in promoting immune suppression in pBICΔ10 mice.
To explore other populations that could contribute to the accrued tolerogenic LME of IL-10–deficient lymphomas, we examined the presence of intratumoral CD4+FOXP3+ Tregs, which have shown otherwise inconsistent associations with DLBCL clinical outcomes.73 In comparison with pBIC lymphomas, Tregs were significantly enriched in IL-10–deficient pBICΔ10 tumors, as evidenced by scRNA-seq (Figure 4E) and flow cytometry (Figure 6I). To test the functional relevance of this expanded Treg compartment in DLBCL progression, pBIC and pBICΔ10 mice at advanced stages of disease were treated IV with anti-CD25NIB (Figure 6J), a Treg-depleting mouse IgG2a antibody that does not interfere with IL-2 signaling on effector T cells,74 which efficiently reduced intratumoral Tregs in vivo (Figure 6I). Notably, anti-CD25NIB therapy boosted T-cell activation by limiting the proportion of CD8 TILs coexpressing PD-1+LAG3+ (Figure 6K) and significantly delayed tumoral onset only in the initially more aggressive pBICΔ10 tumors (Figure 6L). Altogether, these results suggest that IL-10–deficient lymphomas are particularly dependent on Tregs, acquiring high sensitivity to anti-CD25 immunotherapy.
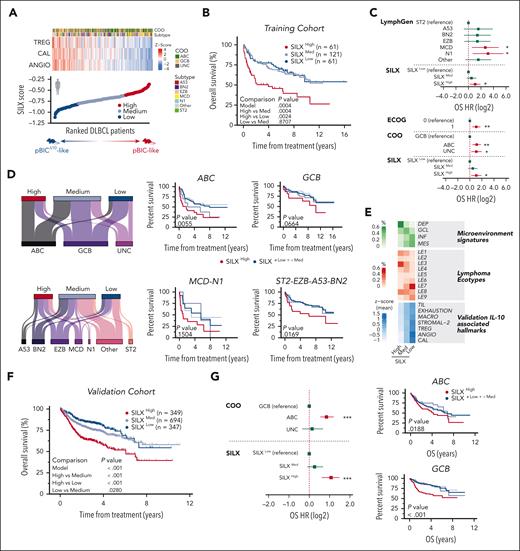
IL-10–associated transcriptional hallmarks stratify patients with DLBCL with distinct responses to R-CHOP
We then investigated whether the IL-10–associated hallmarks identified in pBIC/ pBICΔ10 mice might have some impact on the prognosis and the response to immunochemotherapy in human patients with DLBCL. As a training cohort, we took advantage of the Schmitz et al2 data set, which included bulk RNA-seq and survival data of newly diagnosed patients with DLBCL who received R-CHOP–like therapy (dbGaP phs001444.v2.p1; n = 243). We selected the 3 functional and immunophenotypic categories more significantly enriched in the murine LME after Il10 deletion (Treg [TREG], angiogenesis [ANGIO], and calcium activity [CAL]; supplemental Table 6) and obtained the single-sample GSEA values for each one in every human sample (Figure 7A, top). We then developed a metric called score for IL-10 (SILX) to capture the state of IL-10–associated hallmarks in bulk transcriptomes of human DLBCL samples (supplemental Methods). We ranked all samples according to SILX values (Figure 7A, bottom) and found that the top 25% of the samples, hereafter referred to as SILXhigh (n = 61), showed significantly poorer overall survival after R-CHOP immunochemotherapy than the rest of the patients with medium (SILXmed, n = 121) or low values (SILXlow, n = 61; Figure 7B). Multivariate Cox proportional hazard analysis showed that a SILXhigh score was a risk factor independent of Eastern Cooperative Oncology Group (ECOG) performance status, transcriptional cell-of-origin (COO),5 or mutational LymphGen2,4 assignments (Figure 7C). Sex was not a contributing factor when included in the multivariate Cox model (supplemental Figure 2B). On the contrary, SILXlow/med patients showed more favorable responses to R-CHOP (Figure 7B). In fact, SILX identified a subset with better outcomes inside the initially worse prognosis DLBCL subtypes (COO/ABC5 or mutational/MCD-N12,3), as well as a subset with worse outcomes inside the initially good prognosis DLBCL subtypes (COO/GCB5 or mutational/ST2-EZB-A53-BN22,3; Figure 7D). These findings were highly consistent with our preclinical data, in which IL-10–deficient pBICΔ10 mice showed significantly better in vivo responses to anti-CD20 (Figure 3A). Taken together, this suggests that some of the biological vulnerabilities identified in pBIC/pBICΔ10 mouse models, captured by the SILX score, add a new layer of information on top of the current molecular DLBCL classifications and could help predict R-CHOP responses.
Scoring IL-10–associated transcriptional hallmarks stratifies patients with DLBCL with distinct responses to standard-of-care R-CHOP. (A) Heat map of ssGSEA values (z scores) of 3 genetic signatures (TREG, ANGIO, and CAL) in patients with DLBCL from Schmitz et al2 (n = 243). Samples are ranked by the SILX score calculated for each sample (shown at the bottom). Predicted correspondences with preclinical mouse models are depicted. (B) Survival analysis of this cohort stratified by SILX values. (C) Multivariate cox proportional hazard analysis including ECOG, COO, LymphGen (genetic subtypes), and SILX group. (D) Sankey plots showing the distribution of each SILX group in defined COO and LymphGen subtypes (left). Survival curves for the SILX-stratified patients within the more aggressive (ABC and MCD-N1) or less aggressive (GCB and ST2-EZB-A53-BN2) subtypes (right). Red represents SILXHigh; dark blue represents SILXLow + Med; medium blue, SILXLow; light blue, SILXMed. (E) Percentage of SILXHigh, SILXMed, or SILXLow samples that belong to each microenvironment group defined by Kotlov et al75 (top; green) or to each lymphoma ecotype defined by Steen et al76 (middle; red); and average of normalized ssGSEA values for several IL-10–associated microenvironment hallmarks (bottom; blue). (F) Survival analysis in the validation cohort (n = 1390). (G) Multivariate Cox proportional hazard analysis including COO and SILX annotations in the validation cohort (left). Survival curves of SILX-stratified patients from the validation cohort within ABC and GCB subtypes (right). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. ANGIO, angiogenesis; CAL, calcium activity; DEP, depleted; ECOG, Eastern Cooperative Oncology Group performance status; GCL, germinal center-like; HR, hazard ratio; INF, inflammatory; MACRO, macrophages; MES, mesenchymal; OS, overall survival; TIL, tumor infiltrating lymphocytes; TREG, T regulatory cells; UNC, unclassified.
Scoring IL-10–associated transcriptional hallmarks stratifies patients with DLBCL with distinct responses to standard-of-care R-CHOP. (A) Heat map of ssGSEA values (z scores) of 3 genetic signatures (TREG, ANGIO, and CAL) in patients with DLBCL from Schmitz et al2 (n = 243). Samples are ranked by the SILX score calculated for each sample (shown at the bottom). Predicted correspondences with preclinical mouse models are depicted. (B) Survival analysis of this cohort stratified by SILX values. (C) Multivariate cox proportional hazard analysis including ECOG, COO, LymphGen (genetic subtypes), and SILX group. (D) Sankey plots showing the distribution of each SILX group in defined COO and LymphGen subtypes (left). Survival curves for the SILX-stratified patients within the more aggressive (ABC and MCD-N1) or less aggressive (GCB and ST2-EZB-A53-BN2) subtypes (right). Red represents SILXHigh; dark blue represents SILXLow + Med; medium blue, SILXLow; light blue, SILXMed. (E) Percentage of SILXHigh, SILXMed, or SILXLow samples that belong to each microenvironment group defined by Kotlov et al75 (top; green) or to each lymphoma ecotype defined by Steen et al76 (middle; red); and average of normalized ssGSEA values for several IL-10–associated microenvironment hallmarks (bottom; blue). (F) Survival analysis in the validation cohort (n = 1390). (G) Multivariate Cox proportional hazard analysis including COO and SILX annotations in the validation cohort (left). Survival curves of SILX-stratified patients from the validation cohort within ABC and GCB subtypes (right). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. ANGIO, angiogenesis; CAL, calcium activity; DEP, depleted; ECOG, Eastern Cooperative Oncology Group performance status; GCL, germinal center-like; HR, hazard ratio; INF, inflammatory; MACRO, macrophages; MES, mesenchymal; OS, overall survival; TIL, tumor infiltrating lymphocytes; TREG, T regulatory cells; UNC, unclassified.
To add support to these findings, we explored the association between our SILX groups and other stratification signatures previously established. We observed that SILXlow patients were enriched in inflammatory and mesenchymal signatures75 and also in lymphoma ecotypes LE7 and LE976 (Figure 7E), which are associated with better responses to R-CHOP. On the contrary, SILXhigh samples were overrepresented in the depleted signature75 and in lymphoma ecotypes LE1 and LE376 (Figure 7E), associated with poor survival rates to R-CHOP. Furthermore, SILXlow patients resembled the characteristics of pBICΔ10 murine tumors according to other stablished microenvironment signatures, such as TILs with increased exhaustion markers, Tregs and macrophages, increased proangiogenic features (including the stromal-2 signature), and increased calcium signaling signatures (Figure 7E, bottom; supplemental Table 6). Importantly, the predictive potential of SILX was further validated in additional pretreatment tumor biopsies from patients with DLBCL with available bulk gene expression and survival data: GSE10846 (n = 232),59 GSE98588 (n = 101),3 GSE87371 (n = 116),77,78 GSE31312 (n = 469),79 and GSE117556 (n = 472).80 We calculated the SILX score separately in each cohort to avoid batch effects and then merged their survival data. This showed significantly better overall survival rates in SILXlow than SILXhigh patients after R-CHOP–like treatment (Figure 7F) and confirmed that SILX is independent of transcriptional COO, identifying patients with significantly worse prognosis within the ABC and GCB subtypes (Figure 7G). These results are fully consistent with the biology described here in our murine models upon conditional genetic ablation of lymphoma-derived IL-10 and offer promising potential for the prediction of the efficacy of novel immunotherapies currently under investigation for patients with DLBCL.
Discussion
The data presented here provide important insights into the role played by tumor-produced IL-10 in DLBCL biology. Our pBIC lymphoma model recapitulates the IL-10/JAK1/STAT3 autostimulatory prosurvival loop observed in ABC/MCD-DLBCL,13,14,29-31 in strong agreement with previous observations that anti–IL-10R neutralization or Il10r/Stat3 ablation impairs B-cell proliferation and tumor growth in a cell-intrinsic manner.12,81 Moreover, the direct correlation between IL-10 and PD-L1 expression in pBIC/pBICΔ10 tumors aligns with the recently described immune-suppressive IL-10/STAT3/PD-L1 pathway in DLBCL,16 supporting immune evasion through the PD-1/PD-L1 axis in IL-10–expressor lymphomas.
Transcriptomic and immunophenotypic analyses of lymphoma-infiltrating immune populations in IL-10–proficient and –deficient murine ABC-DLBCL models uncovered multifaceted roles for lymphoma-derived IL-10: promoting immune-associated processes (immune chemotaxis, antigen presentation, and higher percentages of effector CD8 T cells and dendritic cells) while restraining the pro-oncogenic effects of Tregs or noncanonical M1/M2 macrophages, the major subset of macrophages in human DLBCL.56 Therefore, the absence of lymphoma-derived IL-10 results in an immune-suppressed and exhausted microenvironment, prompting aggressive NF-κB–driven pBICΔ10 lymphomas that develop more quickly. These findings align with observations in IL-10/IL-10R–deficient pediatric patients, in whom the constitutive absence of functional IL-10 signaling leads to NF-κB dysregulation and local T-cell immunodeficiency, explaining their Mendelian predisposition to B-cell lymphomagenesis.82 Altogether, these evidences support a role for IL-10 in maintaining immune equilibrium and tumor suppression.
Additionally, lymphoma-derived IL-10 shows antiangiogenic activity in the stroma, because its absence increased CD31+ vascular area and vessels robustness, consistent with the unfavorable angiogenic switch observed in patients with DLBCL.60,83,84 Our results confirmed another important role for lymphoma-derived IL-10 as a repressor of L-type calcium channels, rendering lymphomas refractory to anti-CD20–dependent cell killing.37,85 This, together with the increased angiogenic stroma (probably facilitating access of therapeutic agents to tumors86) and antibody-dependent phagocytic populations such as TAMs,52 could explain the improved survival rates of pBICΔ10 mice after anti-CD20 therapy. This paradoxical situation, in which lymphomas exhibit increased aggressiveness without treatment but greater sensitivity to CD20 immunotherapy, highlights the importance of our preclinical models in elucidating the dual impact of IL-10 in antitumoral immunity.
Another important observation with potential translational implications is that therapies involving T-cell–targeted immune checkpoint therapy (anti–PD-1) or Treg depletion (anti-CD25) were differentially influenced by lymphoma-derived IL-10. Our results show a shift toward burned-out dysfunctional CD8+ T cells and expansion of Tregs in the absence of IL-10, which could explain the resistance to anti–PD-1 immunotherapy in pBICΔ10 mice. This suggests an antitumoral role of IL-10, acting as an immune modulator that maintains the fitness of CD8 effector TILs, in contrast to the notion that it behaves as a broadly immune-suppressive cytokine. Indeed, IL-10 can promote the expansion and activation of CD8+ TILs by enhancing the expression of IFN-γ and MHC-I/II molecules.19,87 Furthermore, prevention of tumor establishment, growth, and metastasis have been related to IL-10 expression for other cancers,19,54,88,89 and patients have recently shown promising responses and CD8+ T-cell invigoration after treatment with pegylated IL-10.90 We show that IL-10 can enhance antigen presentation by improving MHC-I/II gene expression in lymphoma cells and by facilitating chemokine-mediated attraction of cDCs and protective IFN-producing pDCs.38-41,45 Similarly, CD25NIB Treg-depleting therapy74 demonstrated superior tumor control in Treg-infiltrated IL-10–deficient lymphomas, allowing for T-cell activation and antilymphoma immunity.
Given the potential implications of our findings for predicting the response of patients with DLBCL to various therapeutic strategies, we developed a metric that captures characteristic hallmarks of either high or low expression of IL-10 in tumoral B cells (the SILX score). This score stratified several cohorts of human patients with DLBCL (treated with R-CHOP–like regimes) into SILXhigh or SILXmed/low groups, showing significant differences in overall survival. In fact, SILX was an independent risk factor, distinct from COO or genetic classifications. Consistent with previous studies associating poor outcomes with high serum IL-10 levels9,10 and elevated intratumoral IL-10/IL-10R in 30% to 45% of primary DLBCLs,11 SILXhigh patients exhibited significantly worse responses to R-CHOP–like treatment. However, because IL-10 sustains the intratumoral subset of anti–PD-1 responsive TCF1+PD-1+CD8+ T cells with stem-like potential48-50 and IL-10–expressor pBIC mice benefited from combined ICB immunotherapy, our findings suggest that SILXhigh patients with DLBCL might be good candidates for combined CD20 targeting and ICB immunotherapy. On the contrary, Mac/Treg/exhausted TIL signatures, previously defined from transcriptional deconvolution methods in human DLBCL,75,76 are enriched in SILXlow patients with DLBCL. Because PD-1 blockade did not add significant benefit to anti-CD20 treatment in pBICΔ10 mice, we predict that anti–PD-1 or other T-cell–based immunotherapy approaches would show limited efficacy in the dysfunctional LME of SILXlow patients. However, our results suggest that novel combination therapies incorporating Treg-depleting strategies91 or even macrophage immune checkpoint inhibitors92 might hold promising potential to improve clinical outcomes for SILXlow patients with DLBCL. For clinical applicability, future research should focus on refining SILX normalization, incorporating clinical parameters, and enhancing detection of SILX-integrated biomarkers to improve the stratification of patients with DLBCL for emerging immunotherapy options tailored to the unique immune landscape modulated by lymphoma-derived IL-10.
In summary, we have identified lymphoma-derived IL-10 as a key immunomodulator in DLBCL, acting through interconnected autocrine and paracrine pathways. Our study reveals 2 distinct biological settings and vulnerabilities in IL-10–proficient and –deficient DLBCL microenvironments, recapitulated in genetically engineered pBIC and pBICΔ10 mice, respectively. Although IL-10 promotes autocrine lymphoma survival and resistance to anti-CD20 immunotherapy, it also prevents the drift toward a highly immune-suppressive LME, deprived of stem-like reactive CD8 T cells and enriched in Tregs, TAMs, and burned-out exhausted CD8 TILs. Therefore, DLBCL lymphomas low in tumor-derived IL-10 thrive in a T-cell–dysfunctional microenvironment deficient in cytotoxic effector cells but are more sensitive to anti-CD20 immunotherapy because of improved angiogenesis, expanded macrophages, and increased expression of L-type calcium channels. IL-10–associated hallmarks integrated into the SILX score helped in risk stratification and outcome prediction of future immunotherapy interventions in patients with DLBCL. These models help advance our understanding of DLBCL biology and may serve as in vivo platforms for developing and evaluating novel clinical trial strategies.
Acknowledgments
The authors thank Axel Roers from the Carl Gustav Carus Faculty of Medicine, Institute for Immunology at the Technische Universität Dresden, for access to the mouse line Il10F/F; and Genentech for the generous gift of in vivo functional grade anti-mouse CD20 monoclonal antibody (mAb; clone 5D2). The authors also acknowledge the excellent assistance provided for bioinformatics by Victor Segura; for cytometry and cell sorting by Diego Alignani and members of CIMA Lab Diagnostics; for immunohistochemistry by the Morphology unit; and for animal husbandry by members of the Animal Facility, all of them at Cima Universidad de Navarra. They also thank the technical staff of the Department of Pathology, Anatomy and Physiology at the University of Navarra for their assistance and support.
This work was supported by grants to S.R. from Proyecto PID2020-112994RB-I00 financed by MICIU/AEI/10.13039/501100011033, and from Proyectos PID2023-15011OB-I00 and SAF2017-82309-R, cofinanced by MICIU/AEI/10.13039/501100011033 and Fondo Europeo de Desarrollo Regional (FEDER) Una manera de hacer Europa, with the collaboration of the Gilead Scholarship Program for biomedical research (GLD19-00082); and by F. Hoffmann-La Roche through NAV4 imCORE project for the study of anti-mouse CD25NIB mAb. Additional support was provided by Spanish Ministry of Health, Instituto de Salud Carlos III through CIBERONC (CB16/12/00489; S.R. and J.A.M.-C.), CIBEREHD (CB06/04/0006; P.S.), and Fondo de Investigación en Salud (FIS) grant (PI22/00983; J.A.M.-C.), all of them cofinanced by FEDER Una manera de hacer Europa. Training support was received by Ministerio de Universidades (FPU20/03256; S.C.G.) and (FPU22/01581; A.A.-L.), by Plan de Formación y de I+D 2019 from Gobierno de Navarra (34E/2020; J.M.), and by Asociación de Amigos de la Universidad de Navarra (ADA/2023; B.S.B.).
Authorship
Contribution: M.G.-L., S.C.G., F.J.N., and S.R. were involved in conceptualization; M.G.-L., S.C.G., E.G., I.C.-L., O.B., F.J.N., and S.R. performed formal analysis; S.R. was involved in funding acquisition; M.G.-L., S.C.G., J.M., M.P., S.M.-S., M.A.B., A.A.-L., and B.S.B. performed investigation; M.G.-L., S.C.G., J.M., M.P., S.M.-S., M.A.B., and S.R. contributed to methodology; M.A., C.K., P.U., and P.S. contributed to resources; M.C., J.A.M.-C., J.J.L., and S.R. provided critical feedback on experimental design and data interpretation; M.G.-L., S.C.G., F.J.N., and S.R. were involved in visualization and wrote the original draft; and all authors reviewed and edited the draft.
Conflict-of-interest disclosure: J.A.M.-C. obtained research funding from Roche-Genentech, Bristol Myers Squibb-Celgene, Janssen, Priothera, Palleon, AstraZeneca, and K36 Therapeutics; and is a founder of and holds stock options in MIMO Biosciences. M.C. had a consultancy agreement with BeiGene, Bristol Myers Squibb, Incyte, Janssen, Karyopharm, Kite, Kyowa, Lilly, Roche, and Takeda; and was on the speaker’s bureau for Kite, Kyowa, Roche, and Takeda. M.A., C.K., and P.U. are employed by Roche Innovation Center Zurich and declare ownership of stock and patents with Roche. S.R. obtained research funding from Roche-Genentech. The remaining authors declare no competing financial interests.
Correspondence: Sergio Roa, Department of Biochemistry and Genetics, Universidad de Navarra, 1 Irunlarrea, 31008, Pamplona, Spain; email: sroa@unav.es.
References
Author notes
M.G.-L. and S.C.G. contributed equally to this work.
Files have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (GEO) database (GEO series accession numbers GSE255140 [RNA-seq] and GSE253955 [scRNA-seq and scTCR-seq]).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Tumor survival and PD-L1 expression are promoted by increased levels of IL-10 in DLBCL. (A) Baseline expression of IL10 across normal GC B cells and human DLBCL data sets classified according to COO gene expression or LymphGen genetic subtypes; merged expression arrays (left) from GSE56315 and GSE12195; RNA-seq (right) from Schmitz/Wright et al2,4 (t test). (B) Expression of Il10 by RNA-seq and RT-qPCR between normal GC B cells (B220+CD38lowFAS+YFP+) from control YC mice and lymphoma cells (B220+GFP+) from pBIC tumors (t test and Mann-Whitney, respectively). Values from our previous GSE116290 (triangles) or current GSE255140 (circles) RNA-seq experiments were normalized and analyzed combined here. (C) Model depicting the IL-10/JAK/STAT3 autocrine pathway that promotes survival and upregulation of PD-L1 in DLBCL cells, showing different strategies to inhibit this survival loop. (D) IL-10 expression and pSTAT3 quantification measured as MFI by intracellular flow cytometry of lymphoma (CD19+GFP+) and adjacent normal B cells (CD19+GFP–), respectively (Mann-Whitney). (E) Normalized cell viability assays of lymphoma cells (B220+GFP+) cells compared with adjacent normal B cells (B220+GFP–) studied by flow cytometry after 24-hour ex vivo culture with increasing doses of anti–IL-10R, ruxolitinib, or pyrimethamine inhibitors (n ≥ 3) and 50% inhibitory concentration (IC50) calculations from baseline-corrected data. (F) Relative changes in PD-L1 surface expression between adjacent normal B cells (B220+GFP–) and lymphoma cells (B220+GFP+) after 24 hours untreated vs treated with concentrations above the IC50 of ruxolitinib (100 μM) and pyrimethamine (200 μM; n ≥ 3; analysis of variance [ANOVA]). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. MFI, median fluorescence intensity.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/145/23/10.1182_blood.2024025755/2/m_blood_bld-2024-025755-gr1.jpeg?Expires=1769079010&Signature=Jx22JKUH~rIwUyBqPfHJJUtAu3KIjGdkTTJrgm5ELpOs9mwnAA-j2OKeZjQDnFiE9b1xe7XRBld04krotygLgDgVk1sY2uawdRpZbG26DCwYLvARS9oPOcPloIAuIMr8nW94ksJPa6uLeVUDPNnC2exVkwpfqx3kKK9mXW5NLoO7gi5-80UqvnKANc-ivDB3jLAUvgjP7UIkmsBR0XYop1wsopu~WR7BFKHKCWh7dGj-pqTEyjml4ljlIulkSmFBAUeC2Tv7LU6M9ETVH97IkuEZFq1M9r4C9CZxWJrWLnFjNT1I-nY~eOGrw-s9eJPgHi8OTayHEcgQYW7ewNaS4A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Lymphoma-derived IL-10 unbalances other immune suppressive subsets, exhibits antiangiogenic functions, and attenuates the response to Treg-depleting immunotherapy. (A) Overall percentage of CD11b+ F4/80+ TAMs identified by flow cytometry in pBIC and pBICΔ10 tumors (t test). (B) Dot plot based on scRNA-seq data showing scaled expression levels of M1 and M2 marker genes in the macrophage population infiltrating pBIC and pBICΔ10 tumors. (C) Representative images of chromogenic multiplex immunohistochemistry (cmIHC; green represents GFP+ lymphoma cells; red, CD31) illustrating distinct CD31+ vascular extensions in pBIC and pBICΔ10 lymphomas. The tissue structure was stained with Hem. (D) Percentage of CD31+ vascular area over the total tissue area (t test). Slides from 3 independent mice were analyzed within each genotype. (E) Representative images of cmIHC illustrating differences in vascular morphology in pBIC or pBICΔ10 lymphomas. Arrowheads mark regions lacking detectable CD31 immunoreactivity and the presence of GFP-expressing lymphoma cells in apparent contact with the lumen of mosaic vessels. Continuous and robust CD31 staining is indicated by a solid line. (F) Overall percentage by flow cytometry of intratumoral MDSCs (left; CD11b+ F4/80– non–double-positive [CD11c+ and IAb+]) and subsets (right) of Gr-MDSCs (Ly6G+Ly6C–) or Mo-MDSCs (Ly6G–Ly6C+) from total splenocytes in pBIC and pBICΔ10 tumoral spleens (t test). (G) Mo-MDSCs studied by flow cytometry in PBMCs from control nontumoral mice (nonimmunized Cγ1-negative littermates of pBIC mice) and tumoral models at advanced disease stages (ANOVA). (H) Heat map representation of IL-10 concentrations measured by ELISA in serum samples of pBIC and pBICΔ10 mice at different time points. (I) Overall percentage of Treg cells (from the total of CD4+ cells) in tumoral spleens from untreated pBIC and pBICΔ10 mice, further compared with mice treated for 4 weeks with αCD25NIB Treg-depleting antibody (t test). (J) In vivo regime for αCD25NIB treatments. (K) Flow cytometry study of PD-1+LAG3+ CD8 cell populations obtained from the spleens of mice either untreated or treated with αCD25NIB, after 4 weeks of treatment (ANOVA). (L) Survival curves from the time of treatment in pBIC and pBICΔ10 mice after αCD25NIB treatment (log-rank test). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; d, days; Gr-MDSC, granulocytic-MDSCs; Mo-MDSC, monocytic-MDSCs; ns, nonsignificant; OS, overall survival; PBMCs, peripheral blood mononuclear cells; wk, week.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/145/23/10.1182_blood.2024025755/2/m_blood_bld-2024-025755-gr6.jpeg?Expires=1769079010&Signature=SsbSwtJzjHXzj1o6diUHrlIfrktUEpzrV9mDPGxrFI8ideFYGvJzpX9zhLxRxAMO0E9UCDJlBNNeEDE7bcDhwIn3BxI2G7AkOyXeu4~7svWTxyUucm9UOG4c7WS-mUxvPqoka1hREU3FYL4My81mCVngV8yNXfZwjiYpxcV4q~CqLw6ZyKPp8ltLEaezGHWEnS7JQJuegoVlAAJHao-ognPjk3ovchAzjBA6ksk4FhQst0mKQw~7~kYcecVtklFXXgsJO6gvqDy0d2XmfStfHTceu6SQ2qZlre2L3g5qyDYrEVmXsAK5ODcboycc9WrGtuOV6ra8hALZqpxxiYOM3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal