Key Points
This study is the first large-scale meta-analysis to assess the response rate to DDAVP in different bleeding disorders.
The differences in DDAVP response emphasize the need for a standardized response definition and further research into response mechanisms.
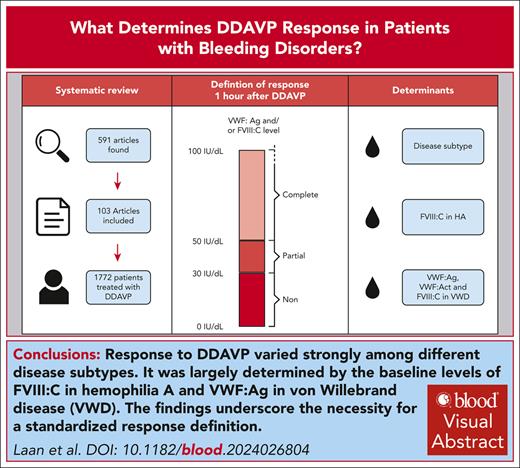
Visual Abstract
Desmopressin (1-desamino-8-d-arginine vasopressin [DDAVP]) can be used to prevent or stop bleeding. However, large interindividual variability is observed in DDAVP response and determinants are largely unknown. In this systematic review and meta-analysis, we aimed to identify the response to DDAVP and the factors that determine DDAVP response in patients. We included studies with patients with any bleeding disorder receiving DDAVP. First and second screening round and risk of bias assessment were performed by independent reviewers. The main outcome was proportion of patients with complete (factor level >50 U/dL) or partial (30-50 U/dL) response to DDAVP. Determinants of response including disease type, age, sex, von Willebrand factor (VWF) and factor VIII (FVIII) mutations, and baseline factor levels were investigated. In total, 591 articles were found and 103 were included. Of these, 71 articles (1772 patients) were suitable for the study’s definition of response. Meta-analysis showed a pooled response proportion of 0.71 (0.64; 0.78) and a significant difference in response between disease subtypes. For hemophilia A, baseline FVIII activity (FVIII:C) was a borderline significant determinant of response. In patients with von Willebrand disease (VWD) type 1, VWF antigen (VWF:Ag), VWF activity, and FVIII:C were significant determinants. A large variation in response was observed for specific mutations in VWF and FVIII. Response to DDAVP varied between disease subtypes and was largely determined by the baseline levels of FVIII:C for hemophilia A and VWF:Ag for VWD. Our findings highlight the significant differences in response and emphasize the need for a standardized response definition and further research into response mechanisms.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited with commendation by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at https://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 1828.
Disclosures
CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declares no competing financial interests.
Learning objectives
Upon completion of this activity, participants will:
Describe the response to desmopressin (DDAVP) in patients with various bleeding disorders, based on a systematic review and meta-analysis
Determine clinical, genetic, and laboratory factors associated with DDAVP response in patients with bleeding disorders, based on a systematic review and meta-analysis
Identify clinical implications of the response to DDAVP and the factors associated with DDAVP response in patients with bleeding disorders, based on a systematic review and meta-analysis
Release date: April 17, 2025; Expiration date: April 17, 2026
Introduction
Patients with bleeding disorders experience frequent bleeding from mucocutaneous tissue of nose, uterus, and bleeding in joints and muscles, causing discomfort and pain. Von Willebrand disease (VWD) and hemophilia A (HA) are 2 of the most common bleeding disorders worldwide with a prevalence of 1:10 000 individuals and 1:5000 males, respectively.1 VWD is characterized by quantitative or qualitative defects of von Willebrand factor (VWF), a large multimeric glycoprotein.2 VWF is produced by endothelial cells and megakaryocytes, can bind to collagen at sites of injury, and mediates the formation of a platelet plug. Furthermore, VWF protects coagulation factor VIII (FVIII) from degradation.3 FVIII is the protein that is (partly) deficient in patients with HA and a cofactor in the FIX mediated activation of FX, which is crucial for thrombin generation.4
The main goal of treatment in patients with bleeding disorders is to prevent or treat bleeding. Treatment options aim to increase plasma FVIII levels in HA or VWF and/or FVIII in VWD.2,5 The most common treatment options are replacement therapy that supplements the (partly) deficient coagulation factor and 1-desamino-8-d-arginine vasopressin (DDAVP), also known as desmopressin.6 DDAVP is a synthetic vasopressin analog that increases endogenous VWF and FVIII levels by an average of three- to fivefold. The ability to increase plasma FVIII and VWF levels in response to DDAVP depends largely on the availability of stored FVIII/VWF, the efficacy of FVIII/VWF secretion, and rate of clearance.7 Therefore, DDAVP is mostly prescribed in patients in which VWF and FVIII are not completely deficient, such as patients with VWD type 1, patients with moderate and mild HA,8 and patients with platelet function disorders (PFDs).9
DDAVP is one of the cheapest and readily available treatments for patients with bleeding disorders. Furthermore, the intranasal formulation of the drug, which can be administered by patients themselves, greatly improves convenience of use. Large interindividual differences exist in the response to DDAVP; therefore, each individual patient receives a DDAVP test dose with multiple blood drawings to investigate how well they respond to the drug. When patients do not respond, they are fully dependent on the alternative treatments, which are costlier and may require hospital visits. Several studies have reported that DDAVP response is partly influenced by certain factors such as age, blood group type, disease severity, or mutation type.7,10-23 However, most studies were conducted in smaller patient cohorts with a large heterogeneity in patient characteristics. Therefore, it is still largely unknown which factors determine a DDAVP response. For that reason, in this systematic review, we aimed to identify the response rate in different diseases and to identify possible determinants that influence DDAVP response to gain a better understanding of the reason behind a nonresponse.
Methods
Search strategy and selection criteria
We performed a systematic review and meta-analysis to explore the factors that determine DDAVP response in patients with a bleeding disorder. Our PROSPERO study protocol is available at https://www.crd.york.ac.uk/prospero/ (CRD42021259033). We performed a comprehensive search in PubMed, Embase, Web of Science, Cochrane Library, and Emcare on 16 October 2020. The search was rerun once before the final analysis on 1 September 2022 (supplemental File 1, available on the Blood website). All studies were entered in Covidence,24 where duplicate records were removed. Title and abstract screening and full text screening were performed by at least 2 reviewers independently (S.L., I.v.M., J.D.C.A.). Published studies were included that were performed in patients with any bleeding disorder treated with DDAVP in any dose or form of administration for the indication to improve hemostasis. Papers written in languages other than English, animal studies, reviews, and studies on patients without a bleeding disorder were excluded. Any disagreements were resolved by consensus.
Data extraction
The primary outcome was the DDAVP response classified according to the following definition: complete response is defined as VWF antigen (VWF:Ag) and/or FVIII activity (FVIII:C) >50 U/dL, for VWD and HA respectively after 1 hour. Partial response is VWF:Ag and/or FVIII:C between 30 U/dL and 50 U/dL, and nonresponse is VWF:Ag and/or FVIII:C levels <30 U/dL. This definition is a slight adjustment on the American Society of Hematology, the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation, and the World Federation of Hemophilia 2021 guidelines.25 In these guidelines, a complete response is defined as VWF or FVIII level increasing at least twofold over baseline, and levels reach >50 U/dL. We will refer to the adjusted definition as the “study definition.” This response definition was used to compare complete responders with partial and nonresponders between studies. DDAVP response was also collected based on the response definitions applied in the respective papers, which usually comprised the categories complete, partial, and nonresponse, and will be referred to as the “article definition.” Data from articles where no definition was given were also collected, referred to as “undefined definition.” Data were extracted by 3 reviewers (S.L., I.v.M., J.D.C.A.) independently, using a template custom made within Microsoft Excel (supplemental File 2, data extraction template). For all articles, summary data were collected and individual patient-level data were obtained if possible. Raw data were requested from the authors of papers if summary data could not be extracted directly from the article. We collected potential determinants of a DDAVP response in patients with bleeding disorders. Expected determinants were diagnosis, blood group, mutations in VWF or FVIII, weight, age, sex, baseline factor levels of VWF or FVIII, multimer pattern of VWF, and dose and administration route of DDAVP. Note that VWF activity was measured with different platelet binding assays, the most of them using VWF ristocetin cofactor activity. In this article, all VWF activity levels will be indicated as VWF:Act.
Study grouping and data analysis
Analyses were performed separately per disease but also in 1 of 5 main disease types, namely VWD type 1; VWD type 2; VWD type 3; HA; and, if not fitting in 1 of these 4 disease types, “other.” We categorized VWD subtypes as a result of their completely different pathophysiology. Most of the studies reported DDAVP as categorical outcome (eg, no, partial, or complete response); other studies reported continuous variables (VWF:Ag, VWF:Act, FVIII:C). Meta-analysis per disease subtype was performed when data from at least 3 studies were available. Because we were interested in the determinants of the DDAVP response with regard to the actual physiological mechanisms of secretion of factors from endothelial cells, we based the “study definition” on the increase in VWF:Ag or FVIII:C. However, this definition does not necessarily reflect an increase of functional VWF:Act as is usually considered in the context of clinical responsiveness and applicability of DDAVP in VWD. Responses in VWF levels in the quantitative sense (on antigen level) or in a qualitative sense (on activity level) both offer different insights into the mechanisms of DDAVP response. Therefore, we also compared VWF:Act as a factor next to VWF:Ag over time in patients with type 1 VWD with patients with type 2 VWD, which are known to have qualitative defects of VWF. In patients with PFD and some HA carriers, baseline factor levels before DDAVP administration were >50 U/dL. These patients were not included in the meta-analysis because they would always have a complete response according to our definition. Meta-regression (supplemental Methods) was performed when the association between a prognostic factor and DDAVP response was evaluated in at least 3 studies. Finally, the effect of VWF/FVIII mutations on response was analyzed per patient.
Results
Study selection and data extraction
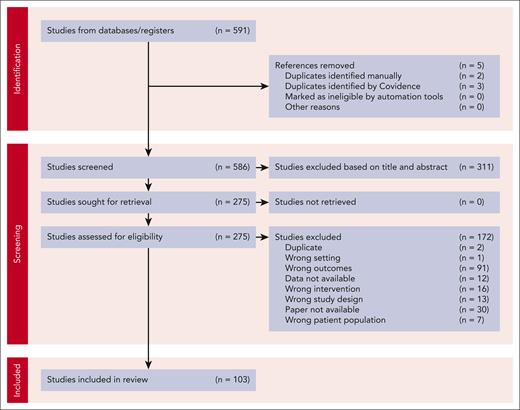
The search strategy identified 570 studies and another 21 original articles were added after the rerun of the search. After first and second round of screening, 103 studies (ranging from publication date 1980 to 2022) were included as shown in the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flow diagram (Figure 1).7,10-23,26-113 The characteristics of all studies are presented in supplemental Table 1. Most of the included studies are prospective case reports/series.
PRISMA flow diagram of inclusion and exclusion of articles. PRISMA, Preferred Reporting Items for Systematic reviews and Meta-Analyses.
PRISMA flow diagram of inclusion and exclusion of articles. PRISMA, Preferred Reporting Items for Systematic reviews and Meta-Analyses.
Data on DDAVP response could be extracted and classified according to the “study definition” in 71 articles and according to the “article definition” in 36 articles (supplemental Table 1). The definitions that were used for the “article definitions” are listed in supplemental Table 2. Data from studies with an “undefined definition” of DDAVP response were also extracted from 52 articles. Unfortunately, due to heterogeneity between article definitions and small patient numbers in the studies only reporting the “article definition” and “undefined definition,” these could not be used for further meta-analysis and meta-regression. Risk of bias (RoB) was assessed, with most articles being low (40/103) or moderate RoB (52/103) with some high RoB studies (11/103). Almost all studies reported only IV (71/103) or IV or subcutaneous administration of DDAVP (13/103). A few reported subcutaneous administration (14/103), intranasal (1/103), or not reported administration (5/103). All extracted data, for example, the RoB score, study design, bleeding disorder, and administration route, per study can be found in supplemental File 3.
Meta-analysis on response to DDAVP per disease subtype
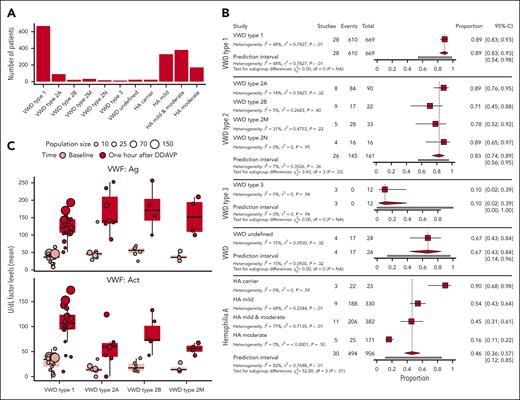
For the meta-analysis, 71 articles were used where the “study definition” of response could be applied. In supplemental Table 1, patient number reflects the patients from which data could be extracted based on the “study definition” (total of 1772 patients). Of these patients, the average age was 34.2 ± 12.2 and 24.9% were female. A large part of the patients were male patients with HA. Patient numbers per disease subtype are shown in Figure 2A. Data from 3 or more articles were obtained for the following subtypes of disease: VWD type 1 (28 studies), VWD type 2A (8 studies), VWD type 2B (9 studies), VWD type 2M (5 studies), VWD type 2N (4 studies), VWD type 3 (3 studies), VWD undefined (4 studies), HA carriers (3 studies), HA mild (9 studies), HA mild and moderate (11 studies), and HA moderate (5 studies). As seen in Figure 2A, most patients had either HA (n = 906) or VWD type 1 (n = 669).
Complete response rate varies per disease subtype according to the study definition. (A) Number of patients per disease type included in the meta-analysis. Disease types with <3 articles are excluded from this analysis. (B) Meta-analysis on response to DDAVP at 1 hour in patients with different bleeding disorders per subtype according to the study definition. Using a random effect model, the proportions of complete response per disease type are shown. The proportion of response is not 0 in zero-event studies due to the continuity correction that was performed to prevent mathematical errors. (C) Response to DDAVP of patients with VWD type 1 and VWD types 2A, 2B, and 2M. In the top panel, VWF:Ag (U/dL) before (light red) and after 1 hour of DDAVP (dark red) are shown. In the bottom panel, VWF:Act (U/dL) is shown. VWD type 2N is not displayed in this figure because only 2 studies had data on both VWF:Ag and VWF:Act.
Complete response rate varies per disease subtype according to the study definition. (A) Number of patients per disease type included in the meta-analysis. Disease types with <3 articles are excluded from this analysis. (B) Meta-analysis on response to DDAVP at 1 hour in patients with different bleeding disorders per subtype according to the study definition. Using a random effect model, the proportions of complete response per disease type are shown. The proportion of response is not 0 in zero-event studies due to the continuity correction that was performed to prevent mathematical errors. (C) Response to DDAVP of patients with VWD type 1 and VWD types 2A, 2B, and 2M. In the top panel, VWF:Ag (U/dL) before (light red) and after 1 hour of DDAVP (dark red) are shown. In the bottom panel, VWF:Act (U/dL) is shown. VWD type 2N is not displayed in this figure because only 2 studies had data on both VWF:Ag and VWF:Act.
The results of the meta-analyses for the complete response rate are presented in Figure 2B. Forest plots presenting data points per study instead of subtype summaries are presented in supplemental Figure 1. Meta-analysis showed a pooled response proportion of 0.71 (0.64; 0.78) for all patients and a significant difference in DDAVP response between disease subtypes. The overall proportion of complete response was 0.89 (confidence interval [CI], 0.83-0.93) in patients with VWD type 1, 0.83 (CI, 0.75-0.89) in type 2, and 0.10 (CI, 0.02-0.39) in type 3. The response in HA carriers was 0.90 (CI, 0.68-0.98), mild HA 0.54 (CI, 0.43-0.64), mild and moderate HA 0.45 (CI, 0.31-0.61), and moderate HA only 0.16 (CI, 0.11-0.22). We observed significant differences in proportions of complete response between disease subtypes in patients with HA with a lower response in moderate patients. VWD type 1 and 2 also showed large differences in proportion of response between subtypes although most subtypes responded quite well to DDAVP according to the “study definition.”
We analyzed the increase from baseline in VWF:Ag in patients with VWD and observed a large increase in VWF:Ag in all patients with type 1 VWD and in patients affected by types 2A, 2B, and 2M VWD (Figure 2C). Median VWF:Ag levels (U/dL) with standard deviation 1 hour after DDAVP administration are 121.20 ± 35.39 in type 1 VWD; in type 2A, 2B, and 2M, the levels are 140.28 ± 59.77, 170.79 ± 65.23 and 151.30 ± 54.82, respectively. All subtypes increase to >100 U/dL. However, levels of VWF:Act only increase strongly in VWD type 1 and VWD type 2B (106.93 ± 42.38 and 73.06 ± 32.41). Although limited data were available, the average platelet count in patients with type 2B (12 patients in 4 articles31,57,76,104) was reported to be reduced after DDAVP (90 × 109/L) compared with before administration (172 × 109/L). In VWD type 2A and 2M, median levels remain low (59.36 ± 37.84 and 56.20 ± 10.84). Both barely reach levels >50 U/dL. In VWD type 2B, VWF:Act levels do increase, which makes sense, given that type 2B mutations do not cause less activity of VWF, but rather increased binding affinity to platelets. VWD type 2N was not included in this analysis because only 2 studies had data on both VWF:Ag and VWF:Act.
Determinants of DDAVP response in patients with bleeding disorders
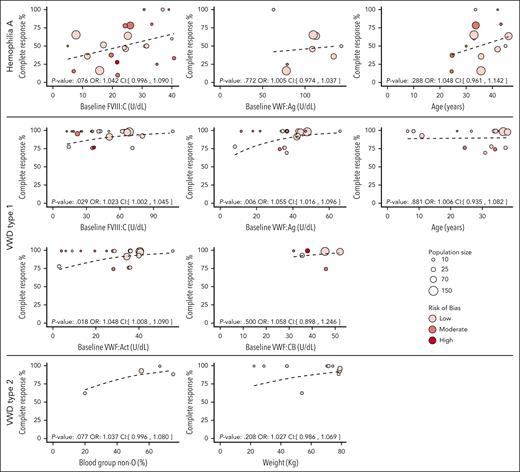
Based on the meta-regression analyses, determinants of DDAVP response, according to the “study definition” differed per disease type (Figure 3). For patients with HA, higher baseline factor levels of FVIII:C (U/dL) showed a borderline significant association (odds ratio [OR], 1.042 per U/dL; 95% CI, 0.996-1.090) and female sex showed a significant association with higher proportion of complete response (OR, 1.020 per percentage point more women; 95% CI, 1.004-1.037). Given that all females were carriers of HA, they logically had higher baseline FVIII levels than males, which explains the difference in response. Although increased baseline VWF:Ag levels per unit and higher age per year showed a positive association with complete response, these were not significant (OR, 1.005 per U/dL; 95% CI, 0.974-1.037; OR, 1.048 per year; 95% CI 0.961-1.142 respectively). In patients with VWD type 1, VWF:Ag (OR, 1.055 per U/dL; 95% CI 1.016-1.096), VWF:Act (OR ,1.048 per U/dL; 95% CI, 1.008-1.090), and FVIII:C (OR, 1.023 per U/dL; 95% CI 1.002-1.045) were associated with the proportion of complete response; age (OR, 1.006 per U/dL; 95% CI, 0.935-1.082) and VWF collagen binding were not (OR, 1.058 per U/dL; 95% CI, 0.896-1.246). No determinants showed a significant association with response in patients with VWD type 2. However, blood group non-O and weight did show some trend (OR, 1.037; 95% CI, 0.996-1.080; OR, 1.027 per kg; 95% CI, 0.986-1.069, respectively). Route of administration is presented in supplemental Figure 2. However, meta-regression was not performed on the route of administration because not enough data of different routes were available. Other determinants such as bleeding time and sex in VWD showed no positive nor negative effect in our study. All estimated ORs with 95% CI of the determinants per disease type are presented in supplemental Table 3.
Baseline factor levels and other determinants affect DDAVP response. Complete response rate according to the study definition is plotted against possible determinants for DDAVP response per disease. Bubble size indicates the population size per study. Color indicates RoB of the study: low (light red), moderate (red), or high (dark red) RoB. The fitted values of the meta-regression are indicated by the black dotted line. Odds ratio with confidence interval and P value are shown above the x-axes.
Baseline factor levels and other determinants affect DDAVP response. Complete response rate according to the study definition is plotted against possible determinants for DDAVP response per disease. Bubble size indicates the population size per study. Color indicates RoB of the study: low (light red), moderate (red), or high (dark red) RoB. The fitted values of the meta-regression are indicated by the black dotted line. Odds ratio with confidence interval and P value are shown above the x-axes.
Assessing FVIII and VWF mutations as determinants of DDAVP response
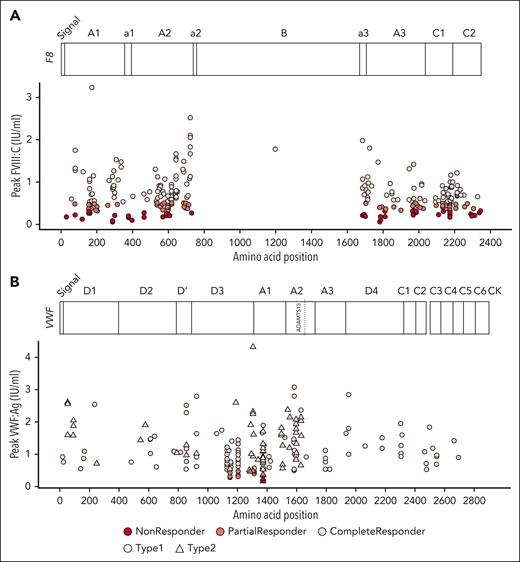
Genetic variants of FVIII and VWF may affect folding, storage, secretion, and interaction with other proteins, thereby affecting DDAVP response. We collected patient mutation information from the articles when available (18 studies, 8 reported FVIII mutations and 10 VWF mutations). For HA, data of 377 patients with known FVIII genetic annotations were extracted. Of these patients, 203 were complete, 113 partial, and 61 nonresponders. In total, 159 distinct missense variants were recorded whereas the rest of the variants represented mutations at noncoding regions, repetitions, and possible exon deletions. We plotted peak FVIII:C levels against amino acid positions affected by missense mutations to investigate whether missense mutations at specific protein locations were associated with response (Figure 4A). This revealed that mutations were distributed over all FVIII domains, but scarcely along the B domain. Importantly, we observed that mutations in the same location were associated with different responses. For instance, in 26 subjects with the variant Arg2169His, 9 had a complete response, 13 partial response, and 4 were nonresponders.
Mutation landscape of individual responses to DDAVP. Each dot represents a patient. Colors represent no (dark red), partial (red), and complete (light red) response. Missense mutation position relative to the protein sequence are plotted on the x-axis against response. (A) Variants in HA; y-axis represents peak FVIII:C levels after DDAVP. (B) Variants in VWD; y-axis represents peak VWF:Ag levels after DDAVP. Shape depicts VWD type 1 (circle) and type 2 (triangle). Protein domains and relevant annotations are plotted at the top of each graph.
Mutation landscape of individual responses to DDAVP. Each dot represents a patient. Colors represent no (dark red), partial (red), and complete (light red) response. Missense mutation position relative to the protein sequence are plotted on the x-axis against response. (A) Variants in HA; y-axis represents peak FVIII:C levels after DDAVP. (B) Variants in VWD; y-axis represents peak VWF:Ag levels after DDAVP. Shape depicts VWD type 1 (circle) and type 2 (triangle). Protein domains and relevant annotations are plotted at the top of each graph.
For VWD, data on 209 individuals with genetic information were collected: 136 patients with VWD type 1 and 73 patients with VWD type 2. Of these patients, 189 had missense mutations, of whom 172 were complete, 13 were partial, and only 4 were nonresponders. Most of the response profiles originated from 2 studies.10,12 Altogether, 85 different VWF missense mutations were reported. To assess possible associations between protein structure and DDAVP response, peak VWF:Ag levels were plotted against amino acid position (Figure 4B).
RoB sensitivity analysis
The sensitivity analysis with removal of high RoB studies from the meta-analysis showed that high RoB studies did not substantially influence the results of the meta-analysis on proportion of response. The total proportion of response remained 0.71. The proportion per subtype stayed the same except for a small change in mild and moderate HA (0.45-0.48) and VWD type 2B (0.71-0.66). The results of the meta-regression on the influence of determinants also did not change significantly. An overview of the slightly changed results after removing high RoB articles is presented in supplemental Table 4.
Discussion
Patients with bleeding disorders experience frequent bleeding that is often treated by administration of DDAVP. However, despite that DDAVP has long been used, there is still an unexplained large interindividual heterogeneity in DDAVP response. It is still largely unknown which factors determine a complete DDAVP response. In this systematic review, we aimed to identify factors that determine DDAVP response in bleeding disorders from the collective data available in the literature. Our meta-analysis, which was based on 71 of 103 included articles and contained 1722 patients, found that subtype of disease is a strong determinant of response. Furthermore, we show higher baseline levels of FVIII:C increase response rate in HA and that higher FVIII:C, VWF:Ag, and VWF:Act baseline levels significantly increase response rate in VWD type 1. Furthermore, although not significant, a trend is shown that higher age (per year) and weight (per kg) correlate with better response in HA and VWD. Finally, comparison of mutations with response profiles revealed heterogeneous responses to DDAVP for patients carrying the same mutation. Remarkably, for both FVIII and VWF, we observed that the same amino acid substitution could be present in complete, partial, and nonresponders and that in VWD only mutations in the D3 and A1 domain led to nonresponders/partial responders.
Our results indicate that disease subtype is an important determinant, which aligns with previous reports.10,22 It is important to note that there is a large discrepancy in VWF:Ag and VWF:Act response for patients with VWD type 2. Especially VWD type 2A and 2M show a strong increase in antigen levels after DDAVP administration, whereas activity levels only rise slightly, indicating low hemostatic efficacy as the secreted protein is dysfunctional. However, in many patients, the activity level did rise >50 U/dL, which may be sufficient for milder bleeding episodes. Therefore, it is also important to test DDAVP effectivity in these patients. In addition, we show that patients with VWD type 2B respond quite well to DDAVP. However, due to the risk of thrombocytopenia, DDAVP is contraindicated.
Specific mutations, which are tied to disease type, have also been shown to influence DDAVP response based on activity levels of VWF10,12 and FVIII:C,7,14,16,21 even showing that similar mutations lead to similar responses to DDAVP.19 It may be explained by comparable baseline levels of FVIII that is present among patients with the same FVIII mutation.114 Our data showed that missense mutations were present along the whole VWF sequence. It also revealed that all partial and nonresponders had mutations in the D3, A1, and A2 domain (position 1149-1584). This region is nestled between the intra- and interchain disulfide bridges in D3 and the cleavage site of ADAMTS13 in A2. Interestingly, this section influences VWF multimer stability and includes the binding site for the platelet glycoprotein GP1bα, which is important for platelet plug formation.115 Notably, in both HA and VWD, we observed that mutations on the same location were associated with different responses to treatment. Recently, Guillet et al116 analyzed a link between desmopressin response and FVIII genotype in patients with HA. The authors proposed that mutations could be categorized in 4 groups of response effectiveness. Our analysis contains 5 similar mutations. Although those classified as groups 1 and 2 mutations seem to respond similarly, groups 3 and 4 mutations, labeled as moderately and frequently ineffective,116 also showed complete and partial responders in our data set. For a complete analysis, adjustments for other determinants should be performed in a multivariate model, but this was not possible in our analysis due to insufficient sample size. Furthermore, as a result of combining diverse groups of patients with the same mutations, we captured a more heterogeneous sample of other determinants that may outweigh the influence of mutations on DDAVP response. Taken together, our analyses suggest that FVIII and VWF mutations are not the main determinants of response, which precludes prediction of DDAVP response based on mutations alone.
Other determinants have been described in literature extensively. For instance, higher baseline factor levels have been associated with better response,11,14,17-19 which is confirmed by our meta-regression analysis in the case of FVIII:C, VWF:Ag, and VWF:Act in VWD type 1. This observation may be explained by lower clearance of VWF, higher production rate, or storage of VWF. Age was not found to be a significant determinant in our analysis. However, this has been shown previously in children17-19 and adults.14 This finding was recently confirmed by Atiq et al117 in patients with low VWF and type 1 VWD (not in systematic review). Our study shows no significant effect of blood group on response. Literature has shown a correlation between blood group and response in VWD type 1C (Vicenza),13 whereas no effect was seen in patients with platelet normal VWD23 and children with VWD type 1.18 This could be explained by the inherent higher VWF baseline levels of patients with blood group non-O.118 Previously, route of administration has been tested, but no differences in response were observed.15,20 It has also been reported that onset of effect after DDAVP does not differ significantly between administration routes.119,120 Unfortunately, in our study, administration route could not be analyzed due to insufficient data, although IV and subcutaneous administration seem to yield similar responses in HA. For the remaining determinant, weight, no effect was observed in our data and no effect has been described in the literature.
Our meta-analysis has some limitations. First, variation in the setting and route of administration of the DDAVP test might influence the response rate. Most studies reported DDAVP response measurements in steady state, but 21 studies reported measurements perioperatively or in the context of bleeding episodes. Second, none of the included articles excluded patients based on their DDAVP response. However, this does not exclude selection bias because some studies excluded patients with VWD type 3 or type 2B given that these patients were expected to have a weak response or where DDAVP is considered contraindicated. Third, the included studies ranged from 1980 to 2022. As such, the definitions of response as stated by the article laboratory tests used are exceptionally heterogeneous. Definitions of response varied with respect to time to peak, whether fold change was calculated, cutoff for complete response, and whether activity or antigen was measured. Due to this heterogeneity, we could not pool the extracted data for our analysis. Therefore, we strongly recommend standardized definitions of response should be maintained and data should be made available for other definitions of response to be calculated. Fourth, we calculated response based on the rise of VWF:Ag and FVIII >50 U/dL. Therefore, the “study definition” was not applicable to patients with baseline levels >50 U/dL. This was the case in patients with PFD and some HA carriers, which could have resulted in an overestimation of response rate, which were therefore not included in the meta-analysis. For these patient groups, an alternative definition of response should be used. For VWD type 2, some patients also have baseline VWF:Ag levels >50 U/dL, and therefore, we specifically performed an additional analysis on VWF:Act response in those patients. Fifth, the potential misdiagnosis between severity types in HA and VWD due to variation in assays could influence the response rate calculated in this study. Finally, given that we used aggregated data, we can only study the effect of determinants on the response rates between studies but not assess the effect on individuals within a study. For future research, we suggest performing studies using individual patient data.
Our study also has several strengths. First, to the best of our knowledge, this is the first meta-analysis to show the response rate of DDAVP in different bleeding disorders and identify determinants influence this response rate. Second, the high number of studies and disease types included allowed us to analyze many different aspects of DDAVP response. Furthermore, the large patient numbers with VWD type 1 and HA allowed for accurate analysis in these subtypes. We were able to extract mutation data of 377 patients with HA and 209 patients with VWD. Finally, after sensitivity analysis, we determined that removal of high RoB articles did not significantly change the analysis outcome.
This study offers a comprehensive overview of DDAVP response proportions in different bleeding disorders and which determinants might play a role in DDAVP response. Especially coagulation factor base levels have been found as an important determinant for the response to DDAVP. These factors should be kept in mind when performing DDAVP tests. Our analysis, which indicates that most of the patients with VWD type 1 (baseline VWF:Ag >30 U/dL) have a complete DDAVP response, lends support to the current guidelines regarding DDAVP testing in patients with VWD type 1.121 Furthermore, the relative low proportion of response in mild and mild/moderate HA indicates that DDAVP response should be tested in those patients to ensure a sufficient response. This information can be used as a guide by clinicians treating patients with bleeding disorders. However, despite the strong relationship between DDAVP response with baseline factor levels and disease subtype, individual DDAVP tests may still be required in these heterogeneous bleeding disorders. Furthermore, heterogeneity in article definition precluded meta-analysis, and therefore, we strongly recommend the use of a clear and uniform definition of response in future studies. Finally, our detailed analysis on mutations and DDAVP response can be used in future studies into the biological mechanisms of DDAVP response.
Acknowledgments
The authors thank J. Schoones from the Walaeus Library for his assistance in the setup of the search strategy.
S.L., J.D.C.A., I.v.M., R.B., J.E., and K.F. received research funding from SYMPHONY: grant NWO-NWA.1160.18.038.
The SYMPHONY consortium, which aims to orchestrate personalized treatment in patients with bleeding disorders, is a unique collaboration among patients, health care professionals, and translational and fundamental researchers specializing in inherited bleeding disorders, as well as experts from multiple disciplines.122 It aims to identify the best treatment choice for each individual based on bleeding phenotype. To achieve this goal, work packages (WPs) have been organized according to 3 themes (diagnostics [WPs 3-4], treatment [WPs 5-9], and fundamental research [WPs 10-12]). Principal investigator, M. H. Cnossen; project manager, S. H. Reitsma. Beneficiaries of the SYMPHONY consortium: Erasmus University Medical Center–Sophia Children’s Hospital, project leadership and coordination; Sanquin Diagnostics; Sanquin Research; Amsterdam University Medical Centers; University Medical Center Groningen; University Medical Center Utrecht; Leiden University Medical Center; Radboud University Medical Center; Netherlands Society of Hemophilia Patients; Netherlands Society for Thrombosis and Hemostasis; Bayer B.V.; CSL Behring B.V.; and Swedish Orphan Biovitrum (Belgium) Belgium Limited Liability Company/Société Privée à Responsabilité Limitée.
Authorship
Contribution: S.L., J.D.C.A., and I.v.M. performed article selection, screening, and data extraction, analyzed the data, and wrote the manuscript; and all authors participated in the design of the research, revised the manuscript, and approved the final manuscript.
Conflict-of-interest disclosure: S.L., J.D.C.A., I.v.M., R.B., J.E., and K.F. received research funding from SYMPHONY; the funding had no role in the writing or decision making of this manuscript. The remaining authors declare no competing financial interests.
A complete list of the members of the SYMPHONY consortium appears in “Appendix.”
Correspondence: Sebastiaan Laan, Department of Internal Medicine, Division of Thrombosis and Hemostasis, Leiden Univeristy Medical Centre, Leiden, The Netherlands; email: s.n.j.laan@lumc.nl.
Appendix
The members of the SYMPHONY consortium are: Martijn Brands, Sjoerd Koopman, Laura Bukkems, Michael Cloesmeijer, Alexander Janssen, Karin Fijnvandraat, Samantha Gouw, Ron Mathôt, Lotte Haverman, Emile van den Akker, Maartje van den Biggelaar, Masja de Haas, Sander Meijer, Jan Voorberg, Jessica Del Castillo Alferez, Huan Zhang, Johan Boender, Amsterdam, Noord Holland, The Netherlands; Stephan Meijer, Den Haag, Zuid Holland, The Netherlands; Karina Meijer, Groningen, The Netherlands; Sean de Jong, Hoofddorp, Noord Holland, The Netherlands; Geertje Goedhart, Anske van der Bom, Mettine Bos, Jeroen Eikenboom, Felix van der Meer, Sebastiaan Laan, Leiden, Zuid Holland, The Netherlands; Saskia Schols, Nijmegen, Gelderland, The Netherlands; Ruben Bierings, Lex Burdorf, Marjon Cnossen, Jan Hazelzet, Elise Huisman, Marieke Kruip, Frank Leebeek, Nikki van Leeuwen, Hester Lingsma, Moniek de Maat, Iris van Moort, Suzanne Polinder, Simone Reitsma, Eliza Roest, Ryanne Arisz, Lorenzo Romano, Wala Al Arashi, Shannon van Hoorn, Tine Goedhart, Caroline Mussert, Diaz Prameyllawati, Carin Uyl, Rotterdam, Zuid Holland, The Netherlands; Nathalie Jansen, Kathelijn Fischer, Hans Kristian Ploos van Amstel, Rolf Urbanus, Minka Zivkovic, Annelien Bredenoord, Rieke van der Graaf, Lieke Baas, Roger Schutgens, Mariëtte Driessens, Utrecht, The Netherlands.
References
Author notes
All extracted data are made available in the supplemental Data.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal