Key Points
Platelets change their phenotype as they age in circulation, skewing toward inflammatory function over time.
Genetic models of increased platelet half-life and transfusion of in vitro–aged platelets enhance inflammatory responses.
Visual Abstract
Platelets are crucial players in hemostasis and thrombosis but also contribute to immune regulation and host defense, using different receptors, signaling pathways, and effector functions, respectively. Whether distinct subsets of platelets specialize in these diverse tasks is insufficiently understood. Here, we used a pulse-labeling method in Mus musculus models for tracking in vivo platelet aging and its functional implications. Using in vitro and in vivo assays, we reveal that young, reticulated platelets show heightened responses in the setting of clot formation, with corresponding, increased responses to agonists, adhesion, and retractile function. Unexpectedly, aged platelets lose their hemostatic proficiency but are more prone to react to inflammatory challenge: compared with reticulated platelets, this cohort was more likely to form platelet-leukocyte aggregates and showed increased adhesion to neutrophils in vitro, as well as enhanced bactericidal function. In vivo, this was reflected in increased pulmonary recruitment of aged platelets in an acute lung injury model. Proteomic analyses confirmed the upregulation of immune pathways in this cohort, including enhanced procoagulant function. In mouse models of prolonged platelet half-life, this resulted in increased pulmonary leukocyte infiltration and inflammation upon acute lung injury. Similarly, human platelet concentrates decreased their hemostatic function and elevated their putative immunomodulatory potential in vitro over time, and in a mouse model of platelet transfusion, aged platelet concentrates resulted in augmented inflammation. In summary, we show that platelets exhibit age-dependent phenotypic shifts, allowing them to fulfill their diverse tasks in the vasculature. Because functional alterations of aging platelets extend to platelet concentrates, this may hold important implications for transfusion medicine.
Introduction
Platelets are the major cellular component of hemostasis and are critical for forming a stable plug to prevent bleeding at sites of vascular injury. Because they are also important mediators of thrombosis, this cell type is an attractive and clinically proven pharmacological target in cardiovascular disease. Beyond clot formation, it has become evident that platelets are also important mediators of immunity that arrive first at sites of inflammation, form a tight partnership with innate immune cells, and are instrumental for leukocyte vascular adhesion, immunosurveillance, and extravasation.1-5 In addition, they serve as guardians of the vasculature, maintaining vascular and lymphatic integrity under steady-state and inflammatory conditions.4,6-8 Furthermore, platelets have been implicated in cancer development and metastasis, as well as neo-angiogenesis, tissue repair, and even adaptive immunity.9-12 Other cell types with similarly diverse (patho)physiological functions, for example fibroblasts or lymphocytes, have specific subsets that mediate their respective effector function.13,14 This specialization is frequently fluid and exhibits dynamic plasticity. Even short-lived neutrophils show distinct phenotypes depending on age and context.15-18 In platelets, it remains insufficiently understood whether there are subsets that mediate the diverse functions outlined earlier. Recently, some environmental influence on platelet biogenesis has been proposed, because there is a contribution of lung and splenic megakaryocytes (MKs) to circulating platelet pools.19,20
One subset with ample evidence in basic research but also clinical medicine are young, newly produced platelets. These are termed reticulated platelets due to their elevated content of ribosomal RNA and have been recognized as a highly reactive subset that can contribute to (re-)thrombosis and failure of platelet inhibitory therapies in cardiovascular disease.21-24 It is thought that platelets lose their functions as they age, and this functional decline leads to defects in hemostasis and thrombosis in models of prolonged platelet half-life.25,26 Importantly, most of these findings were generated using indirect indicators of platelet age, which leaves key questions, including the dynamics of clearance, phenotype, and function of circulating platelets from birth to their decay, unanswered. Here, using innovative pulse-labeling techniques, single-cell–based functional assays, genetic mouse models, and transfusion experiments, we precisely map phenotypic, functional, and proteomic changes of platelets as they age in circulation. We show that although in vivo aging platelets lose hemostatic potential in vitro and in vivo, they move toward proinflammatory function with important implications in inflammation.
Methods
Platelet pulse-labeling model for tracking specific age cohorts of platelets
To track young platelets as they age in circulation, C57BL/6J mice were injected twice IV (pulse-labeled with a 12-hour interval) with 2.0 μg of glycoprotein Ib (GPIb) antibody conjugated with different fluorophores (X649 and X488) consecutively. Repeated blood sampling (∼20 μL) from facial vein was performed to check for the percentage of pulse-labeled platelets (calculated by subtracting the percentage of platelets labeled with the first antibody from those of the second antibody; eg, [% of X488] to [% of X649] of all CD41+ platelets), surface marker expression, and platelet phenotype as they aged. To understand the functional difference between distinct platelet age cohorts, mice were pulse-labeled 108, 60, and 12 hours before blood sampling. Blood was collected terminally from the retro-orbital vein to perform multiple in vitro assays simultaneously.
Generation of young and old platelet age cohorts by ablating platelet production
The PF4-cre;RS26-iDTR model was used to control platelet age by ablating MKs using diphtheria toxin (DT).27 Administration of 250 ng per mouse of DT on day 1 and 2, and consecutive readministrations of 100 ng per mouse with a 48-hour interval resulted in complete thrombocytopenia after 6 days. A homogeneous population of platelets, aged at least 4 days, was collected by sampling blood via the retro-orbital vein 4 days after repetitive DT injections in Cre+ mice. Young platelets were isolated by collecting blood 8 days after sequential DT administration, after confirming platelet rejuvenation after a period of thrombocytopenia. A heterogeneous mixed-age (control) population was collected via the vein plexus after repeated DT injection in Cre– mice.
Sample preparation for mass spectrometry
Platelet-rich plasma samples were incubated with CD45 MicroBeads to deplete CD45+ population to ensure pure platelet populations. Platelet-rich plasma was then diluted 1:2 in Tyrode buffer and centrifuged for 5 minutes at 1200g twice. Platelet pellets were lysed with sodium deoxycholate buffer (2% sodium deoxycholate in 100 mM tris[hydroxymethyl]aminomethane pH 8.5) by boiling at 95°C immediately for 5 minutes, followed by 10 cycles of 30 seconds on and 30 seconds off in a Bioruptor Sonicator. Protein samples were reduced and alkylated for 5 minutes at 45°C by adding alkylation buffer (1:10 of 10 mM tris(2-carboxyethyl)phosphine hydrochloride, 40 mM 2-chloroacetamide, pH 7). Next, protein was digested by the addition of 1:100 (enzyme:protein) trypsin and endoproteinase LysC and incubation at 37°C overnight. Samples were supplemented with loading buffer (1% trifluoroacetic acid [TFA] in isopropanol) and loaded onto self-made styrenedivinylbenzene-reverse phase sulfonate StageTips (47 mm; catalog no. 2241; 3M Empore) by centrifugation at 700g for 8 minutes. After washing once with loading buffer and once with wash buffer (5% acetonitrile and 0.2% TFA), the peptides were eluted with elution buffer (80% acetonitrile and 0.3125% NH4OH), concentrated in SpeedVac at 45°C for ∼35 minutes and resuspended (2% acetonitrile and 0.1% TFA).
Acute lung injury model
Anesthetized mice were administered 20 μg of lipopolysaccharide (LPS) intranasally. Mice were scored every hour, and blood was sampled every 4 hours. Four or 8 hours after LPS treatment, mice were euthanized via cervical dislocation without damaging the trachea. Bronchoalveolar lavage fluid (BALF) or lung fluid was collected for analysis. BALF (>0.5 mL) was collected by intratracheal flushing with 1× phosphate-buffered saline (with 1% bovine serum albumin and 2 mM EDTA). BALFs were stained with antibodies for flow cytometric analysis. For lung histology, lungs were removed surgically, fixed in 4% paraformaldehyde for 1 hour, dehydrated in 30% sucrose overnight, and cryo-embedded. Lung sections were then stained with CD41 to check for labeled platelet recruitment.
Results
Tracking of platelet cohorts in vivo reveals dynamic changes over their lifetime
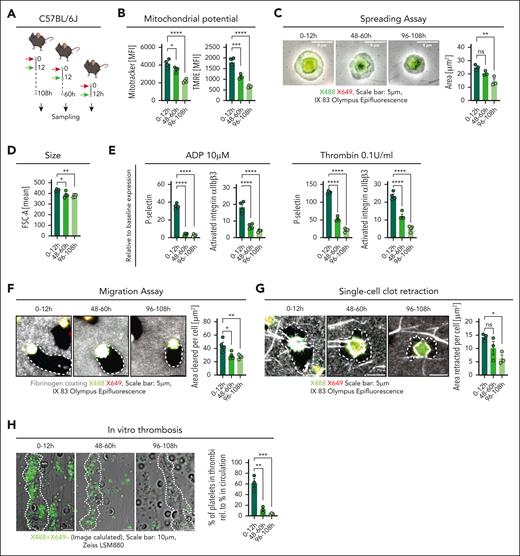
To gain insight into platelet aging and adaptation over their lifetime, we used a pulse-labeling method using 2 injections of nonactivating, nondepleting GPIb antibody tagged with a different fluorophore at 0 and 12 hours (Figure 1A-B). We established that the injection of 2.0 μg of antibody per mouse was a nonsaturating dose, leading to efficient labeling of circulating platelets while still allowing for unaffected secondary labeling 5 minutes later (supplemental Figure 1A-E, available on the Blood website). MKs were not labeled using this strategy (supplemental Figure 1F-I). Using platelet reporter mice, we showed that labeling with antibody did not affect phenotype, activation, or platelet-leukocyte aggregate (PLA) formation (supplemental Figure 2A-F). The pulse-labeling strategy resulted in a single-positive population of platelets produced in between the 2 injections, that could be followed over their lifetime (Figure 1C-D). This cohort showed an increased half-life and slowed initial clearance compared with the general platelet population, confirming differential clearance dynamics of platelets depending on age (Figure 1E). Furthermore, we found an initial increase in size and activation, as assessed by P-selectin expression, and a gradual increase in phosphatidylserine exposure, confirming prior, indirect findings (Figure 1F).
Tracking of platelet age cohorts in vivo. (A) Pulse-labeling scheme in C57BL/6J mice. (B) Representative image of isolated platelets from pulse-labeled mice spread on a fibrinogen-coated chamber. (C) Experimental outline depicting C57BL/6J mice pulse-labeled with X488 and X649 antibodies at 12-hour interval with repetitive blood sampling over time. (D) Representative gating strategy for flow cytometric analysis of pulse-labeled platelets in whole blood. (E) Graph showing percentage of labeled platelets (of all CD41+ platelets) over time, with 2-way repeated measures (RM) analysis of variance (ANOVA), comparison between labeled platelet groups (P = .0008), with the post hoc Šídák multiple comparisons test; bar graph depicting half-life of labeled platelets; paired t test, 2-tailed (P = .0021; n = 5). (F) Single-labeled platelet size; RM 1-way ANOVA (P < .0001); P-selectin expression over time in single-labeled platelets (RM 1-way ANOVA; P = .0289); phosphatidylserine exposure (RM 1-way ANOVA, P = .0178), with the post hoc Dunnett multiple comparisons test. (G) Scheme for pulse-labeling mice 108, 60, and 12 hours before sampling to determine platelet phenotype in different age cohorts simultaneously. (H) Graphs depicting single-labeled platelet percentage in circulation (n = 4 per group), platelet clearance rate (n = 5 per group), and platelet half-life (n = 4 per group); ordinary 1-way ANOVA for each graph, P < .0001; the post hoc Dunnett multiple comparisons test compared with the 0- to 12-hour group. (I) Platelet surface markers of single-labeled platelets: P-selectin mean fluorescence intensity (MFI; n = 4 per group), desialylation (RCA I binding MFI) relative to MFI of all platelets (n = 5 per group), and phosphatidylserine exposure measured by C1q binding (n = 4 per group); ordinary 1-way ANOVA, P = .0385, .0004, and .0063, respectively; the post hoc Dunnett multiple comparisons test compared with the 0- to 12-hour group. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; FSC-A, forward scatter; h, hours; ns, nonsignificant.; rel.RCA I, relative Ricinus Communis Agglutinin I binding; SSC-A, sideward scatter.
Tracking of platelet age cohorts in vivo. (A) Pulse-labeling scheme in C57BL/6J mice. (B) Representative image of isolated platelets from pulse-labeled mice spread on a fibrinogen-coated chamber. (C) Experimental outline depicting C57BL/6J mice pulse-labeled with X488 and X649 antibodies at 12-hour interval with repetitive blood sampling over time. (D) Representative gating strategy for flow cytometric analysis of pulse-labeled platelets in whole blood. (E) Graph showing percentage of labeled platelets (of all CD41+ platelets) over time, with 2-way repeated measures (RM) analysis of variance (ANOVA), comparison between labeled platelet groups (P = .0008), with the post hoc Šídák multiple comparisons test; bar graph depicting half-life of labeled platelets; paired t test, 2-tailed (P = .0021; n = 5). (F) Single-labeled platelet size; RM 1-way ANOVA (P < .0001); P-selectin expression over time in single-labeled platelets (RM 1-way ANOVA; P = .0289); phosphatidylserine exposure (RM 1-way ANOVA, P = .0178), with the post hoc Dunnett multiple comparisons test. (G) Scheme for pulse-labeling mice 108, 60, and 12 hours before sampling to determine platelet phenotype in different age cohorts simultaneously. (H) Graphs depicting single-labeled platelet percentage in circulation (n = 4 per group), platelet clearance rate (n = 5 per group), and platelet half-life (n = 4 per group); ordinary 1-way ANOVA for each graph, P < .0001; the post hoc Dunnett multiple comparisons test compared with the 0- to 12-hour group. (I) Platelet surface markers of single-labeled platelets: P-selectin mean fluorescence intensity (MFI; n = 4 per group), desialylation (RCA I binding MFI) relative to MFI of all platelets (n = 5 per group), and phosphatidylserine exposure measured by C1q binding (n = 4 per group); ordinary 1-way ANOVA, P = .0385, .0004, and .0063, respectively; the post hoc Dunnett multiple comparisons test compared with the 0- to 12-hour group. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; FSC-A, forward scatter; h, hours; ns, nonsignificant.; rel.RCA I, relative Ricinus Communis Agglutinin I binding; SSC-A, sideward scatter.
Next, we deployed this method to generate differently aged cohorts of platelets and compare their phenotype and function at different time points of their life cycle. For this, we collected blood samples 108, 60, and 12 hours after initial pulse labeling, corresponding to platelet cohorts with a mean age of 6 hours, 2 days, and 4 days (Figure 1G).
The aged cohort (mean age, 4 days) showed a significant decline in counts, decreased remaining half-life, and heightened clearance rate (Figure 1H). Correspondingly, this was accompanied by a decrease in P-selectin expression and an increase in desialylation and phosphatidylserine expression, markers of platelet clearance and apoptosis (Figure 1I). In summary, these data directly show dynamic changes in platelets over their lifetime in circulation and allow for functional and phenotypic analysis of age subsets.
Aged platelets exhibit decreased hemostatic and thrombotic potential in vivo
We then used this strategy to compare the function of different platelet age cohorts in vitro. For this, we used assays that allowed for 2-color–based separation of single-positive platelets as well as simultaneous single-cell–based functional analysis (see “Methods”; Figure 2A). Platelets exhibited a time-dependent loss of mitochondrial abundance and function, as measured by MitoTracker intensity (CMTMRos) and tetramethylrhodamine uptake, respectively (Figure 2B).26 Upon spreading on fibrinogen, younger platelets showed increased surface area coverage, corresponding to their increased size (Figure 2C-D).
Aged platelets display diminished hemostatic and thrombotic potential in vitro. (A) Schematic outline showing pulse-labeled C57BL/6J mice (red arrow, X649; green arrow, X488). (B) MitoTracker and tetramethylrhodamine MFI of platelet age cohorts analyzed via flow cytometry (n = 4 per group; both P < .0001). (C) Representative micrographs of spread platelets and analysis of platelet size by area (n = 3 per group; P = .0060). (D) Platelet size measured by FSC-A (n = 4 per group; P = .0085). (E) Flow cytometric measurements of P-selectin expression (MFI) and GPIIbIIIa (αIIbβ3) integrin activation (MFI) in washed platelets after treatment with agonists relative to their expression after phosphate-buffered saline (PBS) treatment (n = 4 per group; all P < .0001). (F) Representative images of single-labeled platelet migration on labeled fibrinogen substrate and quantification of migration as cleared area (n = 4 per group; P = .0094); outlined area showing cleared substrate. (G) Representative micrographs showing single-cell clot retraction assay of pulse-labeled platelets with fibrinogen and platelet poor plasma (n = 3 per group; P = .0406); outlined area showing retracted substrate. (H) Representative micrographs of single-labeled platelets (using Image Calculator in ImageJ: subtracting X649 labeled from X488) showing in vitro thrombus formation with whole blood on collagen I (n = 3 per group); white dotted lines enclose area depicting thrombi; bar graph depicting percentage of single-labeled platelets in thrombus relative to the percentage of single-labeled platelets in blood (P = .0005). Statistical tests for all, ordinary 1-way ANOVA with the post hoc Dunnett multiple comparisons test compared with 0- to 12-hour group. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; h, hour; ns, nonsignificant.
Aged platelets display diminished hemostatic and thrombotic potential in vitro. (A) Schematic outline showing pulse-labeled C57BL/6J mice (red arrow, X649; green arrow, X488). (B) MitoTracker and tetramethylrhodamine MFI of platelet age cohorts analyzed via flow cytometry (n = 4 per group; both P < .0001). (C) Representative micrographs of spread platelets and analysis of platelet size by area (n = 3 per group; P = .0060). (D) Platelet size measured by FSC-A (n = 4 per group; P = .0085). (E) Flow cytometric measurements of P-selectin expression (MFI) and GPIIbIIIa (αIIbβ3) integrin activation (MFI) in washed platelets after treatment with agonists relative to their expression after phosphate-buffered saline (PBS) treatment (n = 4 per group; all P < .0001). (F) Representative images of single-labeled platelet migration on labeled fibrinogen substrate and quantification of migration as cleared area (n = 4 per group; P = .0094); outlined area showing cleared substrate. (G) Representative micrographs showing single-cell clot retraction assay of pulse-labeled platelets with fibrinogen and platelet poor plasma (n = 3 per group; P = .0406); outlined area showing retracted substrate. (H) Representative micrographs of single-labeled platelets (using Image Calculator in ImageJ: subtracting X649 labeled from X488) showing in vitro thrombus formation with whole blood on collagen I (n = 3 per group); white dotted lines enclose area depicting thrombi; bar graph depicting percentage of single-labeled platelets in thrombus relative to the percentage of single-labeled platelets in blood (P = .0005). Statistical tests for all, ordinary 1-way ANOVA with the post hoc Dunnett multiple comparisons test compared with 0- to 12-hour group. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; h, hour; ns, nonsignificant.
Activation with agonists adenosine 5′-diphosphate and thrombin led to greatly enhanced GPIIbIIIa activation and P-selectin expression, a marker of alpha granule release, in the young cohort, with a significant decline in aged platelets (Figure 2E; supplemental Figure 3). Single-cell migration and retraction analyses revealed age-dependent decreases in both assays (Figure 2F-G). This culminated in a significant increase in the recruitment of young platelets in a flow chamber–based thrombosis assay, in which 0- to 12-hour-old platelets were enriched compared with their overall blood concentrations (Figure 2H). These data show maximum hemostatic/clot-forming potential of platelets early after release, which then declines within 48 hours. This substantiates the indirect findings made in humans that reticulated platelets contribute a disproportionately large share to thrombotic risk.
Platelets shift toward thromboinflammatory potential as they age in circulation
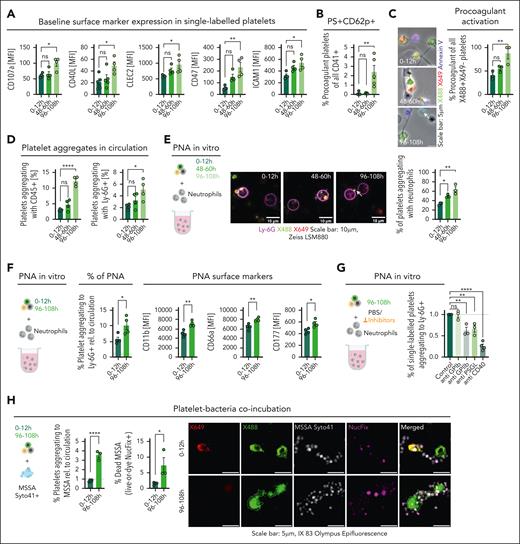
Platelet function is not restricted to clot formation but also encompasses the recruitment of the coagulation cascade as well as immunomodulatory function. Interestingly, our pulse-labeling method revealed increased expression of receptors known to be involved in platelet immune function, including C-type lectin-like receptor 2 (CLEC-2), CD40L, and ICAM-1, upon progressive aging in circulation (Figure 3A). Aged platelets already showed heightened phosphatidylserine exposure under steady state (compare with Figure 1I), and this was further enhanced by agonists, in particular, thrombin and convulxin (supplemental Figure 4A). These platelets were P-selectin and Annexin V positive, indicating a bona fide procoagulant phenotype (Figure 3B; supplemental Figure 4B). These results could be reproduced on a collagen matrix, which also triggers procoagulant activation.8 Again, the 4-day-old cohort showed an almost twofold increase in procoagulant activation compared with young platelets (Figure 3C). Platelet procoagulant activity has been shown to be crucially involved in inflammatory responses.4,8,28 Another important mechanism of platelet immune function is the formation of PLA. The aged platelet cohort showed a striking increase in PLA formation and in particular, platelet-neutrophil aggregate (PNA) formation in the circulation (Figure 3D; supplemental Figure 4C-D). These observations could solely be based on the longer circulation time and therefore a stochastic increase in the likeliness of interaction with leukocytes in the aged cohort. To address this, we coincubated isolated platelets from the 3 labeled age cohorts with neutrophils in vitro. Again, the 4-day-old cohort showed an increase in interaction, indicating an elevated propensity for interaction upon aging (Figure 3E). Given the increased procoagulant potential of aged platelets, we assessed whether this pathway is also responsible for their heightened interaction with immune cells. Cyclophilin D–deficient platelets still showed unaltered heightened PLA formation and interaction with neutrophils upon aging, despite a decrease in procoagulant transformation on collagen matrices (supplemental Figure 5A-I). This underscores that procoagulant activation is not solely responsible for the observed phenotype, while also underlining that the observed procoagulant propensity is cyclophilin D (CypD) dependent and therefore not solely indicating increased apoptosis of the aged cohort. Interestingly, healthy neutrophils coincubated and forming aggregates with aged platelets showed a more proinflammatory phenotype, indicated by upregulated CD11b, CD66a, and CD177 (Figure 3F). This points toward an enhanced proinflammatory effect of aged compared with young platelets on neutrophils. Next, we assessed whether enhanced leukocyte interaction could be blocked by interfering with established heterotypic platelet-leukocyte receptor-ligand pairs. Blockade of GPIIbIIIa, P-selectin glycoprotein ligand-1 (PSGL-1), and CD40L led to reduced PNA formation, whereas GPIb blockade had no effect (Figure 3G). The strongest effect could be seen with blockade of CD40L, correlating well with the enhanced expression of this receptor by aged platelets (Figure 3G).
Aged platelets show increased thromboinflammatory potential in vitro. (A) Bar graphs representing flow cytometric analysis of baseline surface markers of platelet age cohorts in whole blood (n = 4 per group; P = .0106, .0291, .0484, .0216, .0047, and .0216, respectively). (B) Bar graph depicting the percentage of single-labeled platelets that are PS+P-selectin–positive (P = .0023). (C) Representative micrographs and quantification of procoagulant activation of single-labeled platelets seeded on collagen I/fibrinogen matrix (n = 3 per group); graph showing percentage of single-labeled procoagulant platelets (P = .0145). (D) Bar graphs showing PLA (percentage of single-labeled platelets aggregating with leukocytes; P < .0001) and PNA (percentage of single-labeled platelets aggregating with neutrophils; P = .0318) in mouse whole blood (n = 4 per group). (E) Schematic outline, representative micrographs, and quantification of isolated platelets from pulse-labeled mice coincubated with isolated neutrophils (n = 3 per group); bar graph representing percentage of single-labeled platelets of total platelets aggregating with neutrophils (P = .0038). (F) Schematic outline of isolated platelet-rich plasma from pulse-labeled C57BL/6J mice coincubated (n = 4 per group) with isolated neutrophils (nonlabeled C57BL/6J mice, n = 2); bar graph representing the percentage of single-labeled platelets out of total platelets aggregating with neutrophils relative to their percentage in circulation (P = .0322); bar graphs depicting surface marker expressions in neutrophils post aggregation with pulse-labeled platelets: CD11b (P = .0030), CD66a (P = .0054), CD177 (P = .0143); statistical tests, unpaired t tests, 2-tailed. (G) Platelets isolated from mice pulse-labeled 108 hours before experiment treated with anti-GPIb, anti-GPIIbIIIa, anti-PSGL, and anti-CD40 Fab fragments/antibodies coincubated with isolated neutrophils; quantification of single-labeled platelets aggregating with neutrophils relative to control depicted in a bar graph (P < .0001). Statistical tests for panels A-E and 3G, ordinary 1-way ANOVA with the post hoc Dunnett multiple comparisons test. (H) Schematic outline shows pulse-labeled platelets coincubated with methicillin-susceptible S aureus prestained with SYTO 41 dye (5 μM), followed by staining with Live-or-Dye NucFix of killed bacteria; bar graphs depicting percentage of pulse-labeled platelets aggregating to methicillin-susceptible S aureus (MSSA) relative to their percentage in circulation (<0.0001) and the percentage of dead bacteria represented by the percentage of MSSA that are positive for NucFix (0.0408); statistical tests, unpaired t tests, 2-tailed. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. h, hour; ns, nonsignificant.
Aged platelets show increased thromboinflammatory potential in vitro. (A) Bar graphs representing flow cytometric analysis of baseline surface markers of platelet age cohorts in whole blood (n = 4 per group; P = .0106, .0291, .0484, .0216, .0047, and .0216, respectively). (B) Bar graph depicting the percentage of single-labeled platelets that are PS+P-selectin–positive (P = .0023). (C) Representative micrographs and quantification of procoagulant activation of single-labeled platelets seeded on collagen I/fibrinogen matrix (n = 3 per group); graph showing percentage of single-labeled procoagulant platelets (P = .0145). (D) Bar graphs showing PLA (percentage of single-labeled platelets aggregating with leukocytes; P < .0001) and PNA (percentage of single-labeled platelets aggregating with neutrophils; P = .0318) in mouse whole blood (n = 4 per group). (E) Schematic outline, representative micrographs, and quantification of isolated platelets from pulse-labeled mice coincubated with isolated neutrophils (n = 3 per group); bar graph representing percentage of single-labeled platelets of total platelets aggregating with neutrophils (P = .0038). (F) Schematic outline of isolated platelet-rich plasma from pulse-labeled C57BL/6J mice coincubated (n = 4 per group) with isolated neutrophils (nonlabeled C57BL/6J mice, n = 2); bar graph representing the percentage of single-labeled platelets out of total platelets aggregating with neutrophils relative to their percentage in circulation (P = .0322); bar graphs depicting surface marker expressions in neutrophils post aggregation with pulse-labeled platelets: CD11b (P = .0030), CD66a (P = .0054), CD177 (P = .0143); statistical tests, unpaired t tests, 2-tailed. (G) Platelets isolated from mice pulse-labeled 108 hours before experiment treated with anti-GPIb, anti-GPIIbIIIa, anti-PSGL, and anti-CD40 Fab fragments/antibodies coincubated with isolated neutrophils; quantification of single-labeled platelets aggregating with neutrophils relative to control depicted in a bar graph (P < .0001). Statistical tests for panels A-E and 3G, ordinary 1-way ANOVA with the post hoc Dunnett multiple comparisons test. (H) Schematic outline shows pulse-labeled platelets coincubated with methicillin-susceptible S aureus prestained with SYTO 41 dye (5 μM), followed by staining with Live-or-Dye NucFix of killed bacteria; bar graphs depicting percentage of pulse-labeled platelets aggregating to methicillin-susceptible S aureus (MSSA) relative to their percentage in circulation (<0.0001) and the percentage of dead bacteria represented by the percentage of MSSA that are positive for NucFix (0.0408); statistical tests, unpaired t tests, 2-tailed. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. h, hour; ns, nonsignificant.
Platelets can also directly interact with and kill bacteria.29 We, therefore, coincubated pulse-labeled platelets with methicillin-susceptible Staphylococcus aureus. We observed a strongly enhanced binding of 4-day-old platelets, compared with young platelets, to methicillin-susceptible S aureus (Figure 3H; supplemental Figure 6A-D). Moreover, aged platelets showed an increased proficiency in killing S aureus (Figure 3H).
These data highlight that platelets do not show unidirectional functional decline upon aging but upregulate certain receptors and effector functions, including procoagulant activation, binding and killing of bacteria, and enhanced interaction with leukocytes.
Specialized platelet subsets are recruited in clot formation and inflammation in vivo
We next aimed to assess whether this age-dependent skewing of effector functions is mirrored in vivo under thrombotic or inflammatory conditions. First, we used a mesenteric thrombosis model to track pulse-labeled platelets using intravital microscopy. Indeed, we confirmed a strong increase in the recruitment of 0- to 12-hour-old platelets in the early phases of thrombus formation, whereas the aged cohort showed a relative defect in recruitment dynamics compared with blood abundance (Figure 4A-C).
Recruitment of specialized age subsets in thrombosis and inflammation in vivo. (A-C) C57BL/6J mice (n = 3 per group) were pulse labeled 108 hours and 12 hours before blood sampling and mesentery vein imaging; experimental outline (A); percentage of single-labeled platelets in circulation (B; unpaired t test, 2-tailed P = .0492); Representative micrographs depicting thrombi initiated by exposing mesentery vein to FeCl3 (C); bar graphs depicting the percentage of area covered in thrombus by single-labeled platelets relative to platelet percentage in circulation in panel B (unpaired t test, 2-tailed, P = .0097). (D-H) Pulse-labeled C57BL/6J mice (E-G; n = 4 per group) were subjected to acute lung injury; experimental outline (D); percentage decline of single-labeled platelets in circulation (E; 2-way RM ANOVA, comparison between labeled platelet groups: P = .0022, with the post-hoc Dunnett multiple comparisons test); PLA formation in circulation 8 hour after acute lung injury (ALI) and in BALF (F; both P < .0001); percentage of single-labeled platelets recruited in BALF relative to the percentage in circulation 8 hour after ALI (G; P = .0002); C57BL/6J mice (n = 3 per group) were euthanized 8 hour after acute lung injury (H); lung histology was performed via staining with CD41; percentage of single-labeled platelets recruitment depicted in the bar graph (P = .0072); For panels F-H, the reported P values are from ANOVA summary; statistical tests, ordinary 1-way ANOVA with the post-hoc Dunnett multiple comparisons test compared with the 0- to 12-hour group. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; h, hours; ns, nonsignificant.
Recruitment of specialized age subsets in thrombosis and inflammation in vivo. (A-C) C57BL/6J mice (n = 3 per group) were pulse labeled 108 hours and 12 hours before blood sampling and mesentery vein imaging; experimental outline (A); percentage of single-labeled platelets in circulation (B; unpaired t test, 2-tailed P = .0492); Representative micrographs depicting thrombi initiated by exposing mesentery vein to FeCl3 (C); bar graphs depicting the percentage of area covered in thrombus by single-labeled platelets relative to platelet percentage in circulation in panel B (unpaired t test, 2-tailed, P = .0097). (D-H) Pulse-labeled C57BL/6J mice (E-G; n = 4 per group) were subjected to acute lung injury; experimental outline (D); percentage decline of single-labeled platelets in circulation (E; 2-way RM ANOVA, comparison between labeled platelet groups: P = .0022, with the post-hoc Dunnett multiple comparisons test); PLA formation in circulation 8 hour after acute lung injury (ALI) and in BALF (F; both P < .0001); percentage of single-labeled platelets recruited in BALF relative to the percentage in circulation 8 hour after ALI (G; P = .0002); C57BL/6J mice (n = 3 per group) were euthanized 8 hour after acute lung injury (H); lung histology was performed via staining with CD41; percentage of single-labeled platelets recruitment depicted in the bar graph (P = .0072); For panels F-H, the reported P values are from ANOVA summary; statistical tests, ordinary 1-way ANOVA with the post-hoc Dunnett multiple comparisons test compared with the 0- to 12-hour group. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; h, hours; ns, nonsignificant.
Acute lung injury is the common final path of multiple systemic inflammatory syndromes and carries high mortality rates. Moreover, platelets play a decisive role in its pathophysiology by contributing to immune cell recruitment and also maintaining vascular integrity.5,30,31 We, therefore, assessed pulmonary recruitment dynamics of platelets aged 0 to 12 hours, 48 to 60 hours, and 96 to 108 hours in acute lung injury, using our pulse-labeling approach (Figure 4D). Acute lung injury caused a rapid decline of particularly the aged cohort over 8 hours, which could indicate heightened recruitment (Figure 4E). Particularly, leukocytes aggregated with aged platelets showed a strong decline 8 hours after induction (Figure 4F). Indeed, analysis of bronchoalveolar lavage confirmed a strong relative increase in pulmonic extravasation of aged platelets, as well as abundance of PLA in the bronchoalveolar fluid (Figure 4F-G). Lung histology also indicated increased relative recruitment of aged platelets 8 hours after LPS instillation (Figure 4H). In summary, these experiments underpin the obtained in vitro results and highlight that platelet cohorts engage differently upon thrombotic or inflammatory challenges, depending on functional aging.
Aged platelets show proteomic changes corresponding to heightened immunomodulatory function
To better understand the underlying cellular changes accompanying platelet aging in vivo, we aimed to perform state-of-the-art shotgun proteomics. Sorting of marked cohorts led to insufficient protein content as well as signs of platelet activation (data not shown). We, therefore, used the PF4-cre;RS26-iDTR model.27 This model allows for targeted deletion of MKs via DT application, without affecting already circulating platelets. Platelet counts rebound after 7 days (sampled on day 8) with heightened production, as indicated by increased MK counts (supplemental Figure 7A-C). The produced platelets functionally resemble steady-state young platelets with increased platelet size, heightened recruitment of young platelets in a flow chamber–based thrombosis assay, and an increased mitochondrial potential, whereas the 4-day-old platelets show reduced P-selectin expression and increased CD40L and PS exposure (supplemental Figure 7D-H). We sampled platelets within 4 days of DT injection from both control Cre– wild-type (WT) mice and Cre+ mice, representing the aged cohort, because DT treatment led to abolished platelet production; we also sampled rejuvenated platelets after thrombocytopenia from Cre+ and control platelets from Cre– on day 8 (Figure 5A; supplemental Figure 8A).
Platelet aging proteomics. (A-E) Rosa26-DTRxPF4cre mice were administered with DT, intraperitoneally every 48 hours; mixed-aged platelet cohorts were isolated from Cre– mice 4 days after serial DT injections (n = 4); platelets aged >4 days were collected 4 days after serial DT injections in Cre+ mice (n = 4); platelets aged <1 day were collected 8 days after serial DT injections in Cre+ mice during platelet recovery phase (n = 4); schematic outline (A); principal component analysis of all 2062 proteins quantified (n = 4) (B); volcano plot (C) of a student t test (P < .05; |log2 fold change| > 2) comparing 3124 proteins in the young (left) and old (right) cohorts; heat map (D) of the 447 significant proteins from panel C; Fisher exact test enrichment analysis (false discovery rate <0.02; count ≥10, top 10) of gene ontology biological pathway terms (E) among differential proteins from panel C. (F) Proteomic findings were confirmed by analyzing surface marker expression of single-labeled platelets in pulse-labeled C57BL/6J mice (n = 4 per group) via whole blood flow cytometry; CD36 (P = .0009), C3 (P = .0013) and fibrinogen (P = .0035); statistical tests, ordinary 1-way ANOVA with the post hoc Dunnett multiple comparisons test compared with the 0- to 12-hour group. ∗∗P < .01; ∗∗∗P < .001; FC, fold change; h, hour; LFQ, label-free quantification; ns, nonsignificant; rRNA, ribosomal RNA.
Platelet aging proteomics. (A-E) Rosa26-DTRxPF4cre mice were administered with DT, intraperitoneally every 48 hours; mixed-aged platelet cohorts were isolated from Cre– mice 4 days after serial DT injections (n = 4); platelets aged >4 days were collected 4 days after serial DT injections in Cre+ mice (n = 4); platelets aged <1 day were collected 8 days after serial DT injections in Cre+ mice during platelet recovery phase (n = 4); schematic outline (A); principal component analysis of all 2062 proteins quantified (n = 4) (B); volcano plot (C) of a student t test (P < .05; |log2 fold change| > 2) comparing 3124 proteins in the young (left) and old (right) cohorts; heat map (D) of the 447 significant proteins from panel C; Fisher exact test enrichment analysis (false discovery rate <0.02; count ≥10, top 10) of gene ontology biological pathway terms (E) among differential proteins from panel C. (F) Proteomic findings were confirmed by analyzing surface marker expression of single-labeled platelets in pulse-labeled C57BL/6J mice (n = 4 per group) via whole blood flow cytometry; CD36 (P = .0009), C3 (P = .0013) and fibrinogen (P = .0035); statistical tests, ordinary 1-way ANOVA with the post hoc Dunnett multiple comparisons test compared with the 0- to 12-hour group. ∗∗P < .01; ∗∗∗P < .001; FC, fold change; h, hour; LFQ, label-free quantification; ns, nonsignificant; rRNA, ribosomal RNA.
Proteomic analysis of isolated, purified platelets quantified 3495 proteins overall, with 71% proteins quantified in all cohorts (supplemental Figure 8B), and showed clear separation of the respective cohorts in a principal component analysis (Figure 5B). Next, we directly compared the aged platelet cohort with the young platelet cohort with a 2-sample student t test (P < .05; |log2 fold change| > 1.6), which revealed 296 upregulated proteins in young and 151 upregulated proteins in aged platelets (Figure 5C-D). An enrichment analysis of gene ontology biological processes showed a clear overrepresentation of proteins involved in ribosomal translation in young, reticulated platelets (Figure 5E). Interestingly, in aged platelets, we found inflammation, immunity, and the regulation of coagulation as upregulated processes (Figure 5E). At the protein level, aged platelets showed increases in multiple immunoglobulins, complement factors C3, C5, B, H, and I, coagulation factors II, XII, and XIII, and plasma proteins such as fibrinogen and fibronectin (supplemental Figure 8C-D). These proteins have, in part, been shown to be taken up by circulating platelets, which could explain why aged platelets contain more of these proteins. Furthermore, scavenger receptor CD36 was upregulated (Figure 5C). We confirmed the upregulation of CD36, C3, and fibrinogen via flow cytometry using our pulse-labeling method in unperturbed WT mice (Figure 5F). Furthermore, aged platelets showed increased carbonic anhydrase 1 and 2 content, known to be involved in platelet procoagulant transformation and ballooning, which corresponds well with the heightened procoagulant potential we observed (supplemental Figure 8C-D).32 Interestingly, aged platelets also showed an increase in multiple serpins, which is a family of protease inhibitors with important roles in regulating vascular function. These included plasma protease C1 inhibitor, alpha-2- antiplasmin, and antithrombin-III, as well as Serpin H1 (supplemental Figure 8C-D).
Taken together, proteomics data confirm changes in protein content over the platelet lifetime, with a relative increase in proteins involved in innate immune function and coagulation over time.
Increased platelet half-life fosters inflammation
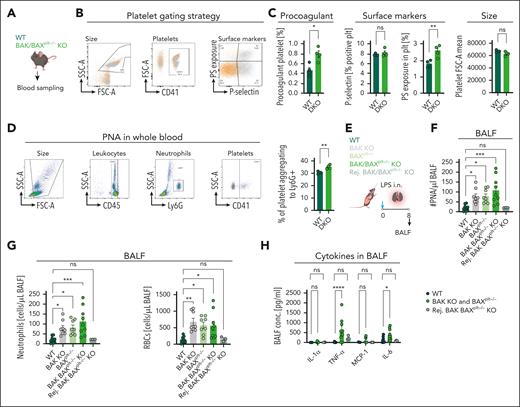
Our data indicate that aged platelets are skewed toward immune effector functions and could therefore alter inflammatory disease outcomes. To investigate this further, we used mouse models of increased platelet half-life, the BAK-KO, BAXPlt KO and BAK-KO BAXPlt double KO (DKO) mouse lines.33 Platelet half-life is significantly increased in these mouse lines, with an almost doubled life span in BAK-KO and DKO strains.34 These mice show defects in hemostasis and thrombus formation associated with platelet aging (supplemental Figure 9A-B).25 Given our insights into platelet immune function upon aging, we hypothesized that increased half-life might skew global platelet function toward immune modulatory properties. As expected, we observed increased baseline procoagulant function and PNA formation (Figure 6A-D). We, therefore, sought to investigate whether these mice showed elevated inflammatory propensity in acute lung injury (Figure 6E). Eight hours after LPS application, BAK KO, BAXPlt KO, and DKO mice exhibited increased recruitment of immune cells and PLA in BAL fluid (Figure 6F-G). This was accompanied by an increase in alveolar hemorrhage, as measured by erythrocyte extravasation (Figure 6G). Moreover, proinflammatory cytokines, tumor necrosis factor α and interleukin-6, were significantly higher in BAL fluid from DKO mice than WT controls (Figure 6H; supplemental Figure 9C-D). Importantly, after the rejuvenation of platelets in DKO mice by injecting platelet-depletion antibody and recovery of counts with newly generated platelets, the proinflammatory effect was abrogated, with leukocyte counts, bleeding, and cytokine levels in BAL comparable with WT controls (supplemental Figure 9B-D; Figure 6F-H). This underlines that platelet aging is associated with functional changes rather than strict loss of function, and these functional changes in platelets upon aging can affect inflammation outcomes.
Increased platelet half-life fosters inflammation. (A) Schematic outline showing blood sampling from WT and BAK/BAXplt–/– DKO. (B) Gating strategy for procoagulant platelets. (C) Bar graphs depicting flow cytometric analysis of percentage (%) of procoagulant platelets (P = .0121), P-selectin–positive platelet percentage (0.4077), PS+ platelet percentage (0.0092), and platelet size forward scatter area (0.0915). (D) Gating strategy for PNA in whole blood; bar graph shows the percentage of CD41+ aggregated to Ly-6G+ cells (P = .0031). Statistical tests for panels A-D, unpaired t tests, 2-tailed. (E) Experimental outline of acute lung injury in WT (n = 10), BAK KO (n = 8), BAXplt–/– (n = 10), BAK BAXplt–/– KO (n = 9), and rejuvenated BAK BAXplt–/– KO (n = 4) after antibody-mediated platelet depletion. (F) Bar graphs showing flow cytometric analysis of PNA (P = .0010). (G) RBC count (P = .0017) and neutrophil counts (P = .0009) in BALF. For panels F-G, ordinary 1-way ANOVA with post hoc Dunnett multiple comparisons test compared with WT. (H) Assessment of cytokine measurements in BALF, 2-way ANOVA comparison between mouse strains (P < .0001), with the post hoc Dunnett multiple comparisons test compared with WT. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; FSC-A; h, hour; IL, interleukin; LPS i.n., lipopolysaccaride intranasally; ns, nonsignificant; SSC-A, sideward scatter; TNF-α, tumor necrosis factor α.
Increased platelet half-life fosters inflammation. (A) Schematic outline showing blood sampling from WT and BAK/BAXplt–/– DKO. (B) Gating strategy for procoagulant platelets. (C) Bar graphs depicting flow cytometric analysis of percentage (%) of procoagulant platelets (P = .0121), P-selectin–positive platelet percentage (0.4077), PS+ platelet percentage (0.0092), and platelet size forward scatter area (0.0915). (D) Gating strategy for PNA in whole blood; bar graph shows the percentage of CD41+ aggregated to Ly-6G+ cells (P = .0031). Statistical tests for panels A-D, unpaired t tests, 2-tailed. (E) Experimental outline of acute lung injury in WT (n = 10), BAK KO (n = 8), BAXplt–/– (n = 10), BAK BAXplt–/– KO (n = 9), and rejuvenated BAK BAXplt–/– KO (n = 4) after antibody-mediated platelet depletion. (F) Bar graphs showing flow cytometric analysis of PNA (P = .0010). (G) RBC count (P = .0017) and neutrophil counts (P = .0009) in BALF. For panels F-G, ordinary 1-way ANOVA with post hoc Dunnett multiple comparisons test compared with WT. (H) Assessment of cytokine measurements in BALF, 2-way ANOVA comparison between mouse strains (P < .0001), with the post hoc Dunnett multiple comparisons test compared with WT. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; FSC-A; h, hour; IL, interleukin; LPS i.n., lipopolysaccaride intranasally; ns, nonsignificant; SSC-A, sideward scatter; TNF-α, tumor necrosis factor α.
Platelet concentrates shift toward proinflammatory properties over time in vitro and in vivo
Platelet concentrates are fundamental to prevent excessive hemorrhage in patients with thrombocytopenia in a range of diseases. Importantly, it has recently become clear that these transfusion products also confer immunomodulatory function.35-37 We, therefore, asked whether platelet concentrates would also show functional changes toward decreased hemostatic ability and heightened immune responsiveness over time in vitro. Human platelet concentrates kept under stable conditions in vitro exhibited mostly similar phenotypic changes as observed in murine platelets in vivo, with increased CLEC2, phosphatidylserine exposure, and CD36 and CD47 expression and a decline in RNA content (supplemental Figure 10A; Figure 7A-B). In contrast to in vivo aging, P-selectin expression increased over time, potentially indicating storage-associated activation (supplemental Figure 10B). Functionally, platelets exhibited a significant decline in spreading, aggregation, alpha granule secretion, and adhesion in an in vitro flow chamber–based thrombosis assay (Figure 7C-D; supplemental Figure 10C-D).
Platelets shift toward inflammatory function during in vitro storage. (A) Schematic outline of platelet concentrate sampling scheme. (B) Flow cytometric analysis of baseline surface marker expression PS exposure (0.0170), CD36 (0.0028), and CD47 (0.0045) relative to time point 1 [TP1]; percentage of reticulated platelets depicted by thiazole orange positive cells (n = 3; P < .0001; RM 1-way ANOVA with the post hoc Dunnett multiple comparisons test compared with TP1). (C) Representative micrographs and analysis of spreading of unactivated or stimulated (4-μM adenosine 5′-diphosphate [ADP] + 2-μM U46619) platelets (n = 3); 2-way RM ANOVA (comparison between PBS and ADP, P = .0064) with post hoc Holm-Šídák multiple comparisons test compared with TP1. (D) Representative micrographs showing in vitro thrombus formation (n = 5); bar graph showing the percentage of thrombus area per field of view (P = .0058). (E) Representative micrographs of procoagulant activation of platelets seeded on collagen I/fibrinogen matrix; graphs showing percentage of procoagulant platelets (P = .0107) and percentage of ballooned procoagulant platelets relative to TP1 (P = .001). (F) Flow cytometric analysis showing percentage of P-selectin–positive PS+ platelets (n = 4; P = .0105). (G) Schematic outline of platelet-neutrophil coincubation (n = 3); flow cytometric analysis showing percentage of platelets aggregating with neutrophils (0.0274); statistical tests for panels D-G, RM 1-way ANOVA with the post hoc Dunnett multiple comparisons test compared with TP7. (H) In vitro aged donor C57BL/6J platelets, freshly isolated (day 0 [D0], n = 4) or stored for 2 days in DSD (day 2 [D2], n = 7) were transfused into thrombocytopenic C57BL/6J recipient mice (n = 4 per group); LPS was given intranasally to induce acute lung injury (ALI) in recipients; blood sampled at 0 and 6 hours after ALI and BALF was collected. (I) Bar graph depicting decline of transfused platelets, mixed-effects model (REML), and comparison between transfused groups (P = .0015 with the post hoc Šídák multiple comparisons test). (J) Bar graphs showing surface marker expression: CD11b (P = .0344) and CD66a (P = .0316) of neutrophils in circulation after ALI (unpaired t test, 2-tailed). (K) Bar graphs depicting the percentage of transfused in vitro aged platelets aggregating with CD45+ cells (P = .0203) and the percentage of platelets aggregating with Ly-6G+ cells (P = .0890) in BALF (unpaired t test, 2-tailed). (L) Assessment of cytokine measurements in BALF (2-way ANOVA, comparison between transfused groups; P = .0014, with the post hoc Šídák multiple comparisons test; additional cytokines shown in supplemental Figure 11H. (M) Clinical progression of ALI in recipients that received transfusion with D0 or D2 in vitro–aged platelets (2-way RM ANOVA, comparison between transfused groups; P < .0001, with the post hoc Šídák multiple comparisons test). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; h, hour; IL, interleukin; LPS I.N., lipopolysaccharide intranasally; ns, nonsignificant; TNF-α, tumor necrosis factor α.
Platelets shift toward inflammatory function during in vitro storage. (A) Schematic outline of platelet concentrate sampling scheme. (B) Flow cytometric analysis of baseline surface marker expression PS exposure (0.0170), CD36 (0.0028), and CD47 (0.0045) relative to time point 1 [TP1]; percentage of reticulated platelets depicted by thiazole orange positive cells (n = 3; P < .0001; RM 1-way ANOVA with the post hoc Dunnett multiple comparisons test compared with TP1). (C) Representative micrographs and analysis of spreading of unactivated or stimulated (4-μM adenosine 5′-diphosphate [ADP] + 2-μM U46619) platelets (n = 3); 2-way RM ANOVA (comparison between PBS and ADP, P = .0064) with post hoc Holm-Šídák multiple comparisons test compared with TP1. (D) Representative micrographs showing in vitro thrombus formation (n = 5); bar graph showing the percentage of thrombus area per field of view (P = .0058). (E) Representative micrographs of procoagulant activation of platelets seeded on collagen I/fibrinogen matrix; graphs showing percentage of procoagulant platelets (P = .0107) and percentage of ballooned procoagulant platelets relative to TP1 (P = .001). (F) Flow cytometric analysis showing percentage of P-selectin–positive PS+ platelets (n = 4; P = .0105). (G) Schematic outline of platelet-neutrophil coincubation (n = 3); flow cytometric analysis showing percentage of platelets aggregating with neutrophils (0.0274); statistical tests for panels D-G, RM 1-way ANOVA with the post hoc Dunnett multiple comparisons test compared with TP7. (H) In vitro aged donor C57BL/6J platelets, freshly isolated (day 0 [D0], n = 4) or stored for 2 days in DSD (day 2 [D2], n = 7) were transfused into thrombocytopenic C57BL/6J recipient mice (n = 4 per group); LPS was given intranasally to induce acute lung injury (ALI) in recipients; blood sampled at 0 and 6 hours after ALI and BALF was collected. (I) Bar graph depicting decline of transfused platelets, mixed-effects model (REML), and comparison between transfused groups (P = .0015 with the post hoc Šídák multiple comparisons test). (J) Bar graphs showing surface marker expression: CD11b (P = .0344) and CD66a (P = .0316) of neutrophils in circulation after ALI (unpaired t test, 2-tailed). (K) Bar graphs depicting the percentage of transfused in vitro aged platelets aggregating with CD45+ cells (P = .0203) and the percentage of platelets aggregating with Ly-6G+ cells (P = .0890) in BALF (unpaired t test, 2-tailed). (L) Assessment of cytokine measurements in BALF (2-way ANOVA, comparison between transfused groups; P = .0014, with the post hoc Šídák multiple comparisons test; additional cytokines shown in supplemental Figure 11H. (M) Clinical progression of ALI in recipients that received transfusion with D0 or D2 in vitro–aged platelets (2-way RM ANOVA, comparison between transfused groups; P < .0001, with the post hoc Šídák multiple comparisons test). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; h, hour; IL, interleukin; LPS I.N., lipopolysaccharide intranasally; ns, nonsignificant; TNF-α, tumor necrosis factor α.
Importantly, and in line with our data from mice, in vitro–stored human platelets were polarized toward inflammatory functions: we observed an increase in the procoagulant phenotype at baseline and upon spreading on a collagen matrix, with increased PS+ P-selectin–positive platelets (Figure 7E-F). Furthermore, morphological analysis revealed increased ballooning, confirming bona fide procoagulant phenotype (Figure 7E). Additionally, coincubation at indicated time points with isolated neutrophils showed heightened aggregate formation upon prolonged storage time (Figure 7G; supplemental Figure 10E). In summary, these experiments illustrate that platelets also gradually shift toward immune effector functions in vitro, which affects the phenotype and the effect of platelet concentrates.
To investigate whether in vitro aging of platelet concentrates would affect inflammatory responses in vivo, we optimized in vitro storage of murine platelets and subsequently transfused in vitro–aged and freshly isolated platelets into thrombocytopenic mice and assessed their immune responses in acute lung injury (Figure 7H). In vitro–aged platelets showed an immunomodulatory phenotype (supplemental Figure 11A-H) and exhibited faster decline upon acute lung injury in vivo (Figure 7I). This was accompanied by a proinflammatory phenotype of circulating neutrophils (Figure 7J). In BAL fluid, we observed increased PNA and a stronger cytokine response, indicated by higher interleukin-6 and tumor necrosis factor α levels, similar to the effect witnessed in BAK-KO BAXPlt mice (Figure 7K-L; supplemental Figure 11E-I). In contrast to our genetic models of increased platelet half-life, the effect of transfusing in vitro–aged platelets had a stronger and complex systemic effect on inflammation, causing a more severe clinical disease course (Figure 7M).
This shows that in vitro aging recapitulates major elements of in vivo platelet aging, while also highlighting transfusion-specific effects that might depend on platelet isolation and in vitro storage. It also supports the notion that proinflammatory effects of platelet transfusions could be partly dependent on the age of transfused platelets.
Discussion
Here, using pulse labeling as well as genetically modified mouse models, and combining translational, in vitro, and in vivo studies, we for the first time, to our knowledge, precisely define platelet phenotype and function over their lifetime in the circulation. We confirm previous, mostly indirect findings that young, reticulated platelets newly released from MKs show a distinct phenotype with enhanced hemostatic and thrombotic potential.21-23 The prowess to form blood clots diminishes as platelets age, and previous studies have therefore described a “functional decline” of platelets, which is exacerbated by interfering with intrinsic apoptosis via Bak/Bax deficiency, leading to enhanced platelet half-life.25,26
We demonstrate that platelets change their phenotype and function over time and are, indeed, more prone to certain effector functions that are associated with immunity, including platelet leukocyte aggregate formation, procoagulant transformation, binding and killing of bacteria, and expression of immunoreceptor tyrosine-based activation motif receptors such as CLEC-2 and scavenger receptor CD36, as well as CD40L and C5aR. Importantly, this is reflected in vivo by an increased relative recruitment of aged platelets in a model of lung inflammation. However, it is important to note that the observed changes most likely do not represent bona fide separate populations but rather gradual, relative changes in phenotype over their lifetime in circulation.
Genetic ablation of platelet apoptosis pathways (BAK KO, BAXPlt, or BAK KO/BAXPlt KO), extending platelet half-life, led to a similar, proinflammatory platelet phenotype that enhanced pulmonary leukocyte recruitment and markers of inflammation. Importantly, this could be reversed upon platelet rejuvenation, excluding that immune or tissue alterations in this model play a major role in mediating these effects. These data hold multiple important implications. First, they point toward a potential division of labor by platelet subsets, depending on respective cellular age. It has long been speculated that diverse subsets of platelets exist, which were mostly thought to be derived from specialized MKs. The description of lung-derived platelets, with a distinct transcriptional profile of lung-resident MKs, as well as CD40Lhi splenic platelets arising after sepsis, have further driven the idea of a diverse platelet pool in the circulation.19,20,38 However, recent work has implicated that platelets are overwhelmingly derived from the bone marrow.39 Moreover, although single-cell RNA sequencing has revealed surprising diversity of MKs, this diversity seems mainly to be based on their roles as immune cells and regulators of stem cell quiescence, whereas the platelet-producing phenotype is relatively homogeneous.40-42
Production of a uniform platelet cohort with dynamic, gradual, and cell-intrinsic changes in phenotype and function over time might represent a more constructive strategy to fulfill the diverse requirements that the platelet lineage must meet in the vascular system. Indeed, similar adaptation has also been shown for short-lived neutrophils, with CXCR4hi aged neutrophils showing heightened immune functions such as reactive oxygen species production and NETosis, which are crucial for host defense.17,43 Importantly, neutrophils contain a nucleus and fully functional transcriptional and translation machinery, making adaptation over their life span more flexible and dynamic than changes observed in anucleate platelets.18
Coupling of the aging process with specific effector functions could also be linked to the energetic capacity of platelets. We confirm that young platelets have a superior mitochondrial function and show improved force generation, as assessed by retractile function.26 Therefore, it is well conceivable that this cohort is more suitable to form a stable plug to prevent bleeding, a process that requires energy-consuming clot retraction and stabilization, particularly under high-shear conditions.44 On the contrary, the baseline increase in phosphatidylserine exposure in aging platelets contributes to the clearance of this population, while potentially also predisposing to procoagulant function, which we found upregulated in this cohort, with important functional implications in thromboinflammation.28,45 Beyond inflammation, it has also been found that platelet subsets are generated in thrombosis, with some stabilizing the clot via GPIIbIIIa-mediated aggregation, whereas other platelets lose adhesive function, turn procoagulant, and mediate fibrin formation.46 Whether platelet age contributes to this dichotomous response in thrombosis remains an important question.
Our innovative proteomics approach revealed important differences between platelets aged 0 to 12 hours and those aged 96 to 108 hours. Aged platelets showed a relative increase in proteins involved in procoagulant transformation. Interestingly, some of the functional specialization could be derived by the uptake of plasma proteins, because this cohort showed an increased content of immunoglobulins, as well as complement and coagulation factors. Whether this increase is mediated by specific uptake by this cohort or simply depends on the prolonged circulation time is not clear. Moreover, whether active protein translation plays a role remains to be investigated. Furthermore, it is important to bear in mind that shotgun proteomics captures relative changes in abundance, which limits its value. However, we did confirm crucial hits via flow cytometry, confirming absolute increases in expression. The detected upregulation of Serpins in aged platelets is also of potential relevance. This class of proteins is important in regulating coagulation and fibrinolytic pathways and could hint toward an additional regulatory role of aging platelets.47 Taken together, our proteome data set is an important resource for further analyses and could help to identify additional targets that are critical in defining platelet (immune) effector function. It remains to be clarified whether there are further subsets within young and aging platelets, a question that could be answered by single-cell omics technology, for example platelet-adapted single-cell proteomics, in the future.
Our findings are potentially relevant for transfusion medicine. It has recently been highlighted that platelet concentrates should be recognized as immunomodulatory treatment.35-37,48 Because our data show additional proinflammatory changes occurring in platelets stored in vitro over time, it might be beneficial to transfuse freshly isolated, supposedly less proinflammatory platelets in vulnerable populations. Importantly, observational studies examining platelet concentrate age with inflammatory markers will be necessary to contextualize these findings in humans. Along these lines, it will be important to define the cellular and molecular mechanisms that are responsible for the upregulation of their proinflammatory profile. These insights could potentially be used to dampen this effect in vitro and modify the immune profile of stored platelet concentrates.
Acknowledgments
The authors thank all patients included for participation in this study, all laboratory members for technical support, and Benjamin Kile for the Bax/Bak transgenic mice provided in this study. They also thank Hellen Ishikawa-Ankerhold, Zeljka Sisic, Anna Titova, Michael Lorenz, Dominic van den Heuvel, Sebastian Helmer, Nicole Blount, and Beate Jant for excellent technical assistance.
This study was supported by the Deutsche Herzstiftung e.V., Frankfurt am Main (individual grants to L.N.), Deutsche Forschungsgemeinschaft (DFG), the DFG Sonderforschungsbereich (SFB) 1123 (L.N., S. Massberg [B06], and C.S. [A07]) and SCHU 2297/1-1, the DFG SFB 1525 (B.N. [A06]), the German Center for Cardiovascular Research (Clinician Scientist Program [L.N.] and 1.4VD [S.M.]), the DFG Clinician Scientist Program PRIME (413635475, R.K. and K.P.), and the FP7 program (project 260309, PRESTIGE [S. Massberg]). This work was also supported by the Ludwig Maximilians Universität (LMU)excellent program (R.K.), the Else Kröner-Fresenius-Stiftung (R.K.), the European Research Council (ERC-2018-ADG “IMMUNOTHROMBOSIS” [S. Massberg]), and the Corona foundation (L.N.). Work of M.S.R. was supported by LMU Munich’s Institutional Strategy LMUexcellent within the framework of the German Excellence Initiative, the German Research Foundation DFG (INST 86/1800-1 FUGG). Additional funding for this work was supported by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (R01HL160808 and R01HL163019; R.C.), and the American Heart Association (21POST830138; F.D.).
Authorship
Contribution: L.N. initiated and conceptualized the study; L.N. and A. Anjum created the methodology; A. Anjum, M. Mader, S. Mahameed, F.D., A.M., F.P.K., R.K., K.P., D.R., S.A., L.D., M. Mulkers, L. Laun, L. Li, N.K., K.Y., M-L.H., A. Akhalkatsi, Q.L, J.P., R.E., R.C., M.S.R., and L.N. conducted the investigation; R.K., S. Massberg, B.N., E.S., C.W., C.S., and L.N. collected the resources; A. Anjum, M. Mader, F.P.K., M.S.R., and L.N. conducted formal analysis; L.N. and A. Anjum wrote the original draft; A. Anjum and L.N. handled data curation and software; A. Anjum, F.P.K., and L.N. visualized the study; L.N. provided supervision and project administration, and administered the funding; and all authors edited the draft.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Leo Nicolai, Department of Medicine I, Ludwig Maximilian University Hospital, Ludwig Maximilian University Munich, Marchioninistr 15, 81377 Munich, Germany; email: leo.nicolai@med.uni-muenchen.de.
References
Author notes
M. Mader, S. Mahameed, and A.M. contributed equally to this study.
Data are available on request from the corresponding author, Leo Nicolai (leo.nicolai@med.uni-muenchen.de).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.





![Platelets shift toward inflammatory function during in vitro storage. (A) Schematic outline of platelet concentrate sampling scheme. (B) Flow cytometric analysis of baseline surface marker expression PS exposure (0.0170), CD36 (0.0028), and CD47 (0.0045) relative to time point 1 [TP1]; percentage of reticulated platelets depicted by thiazole orange positive cells (n = 3; P < .0001; RM 1-way ANOVA with the post hoc Dunnett multiple comparisons test compared with TP1). (C) Representative micrographs and analysis of spreading of unactivated or stimulated (4-μM adenosine 5′-diphosphate [ADP] + 2-μM U46619) platelets (n = 3); 2-way RM ANOVA (comparison between PBS and ADP, P = .0064) with post hoc Holm-Šídák multiple comparisons test compared with TP1. (D) Representative micrographs showing in vitro thrombus formation (n = 5); bar graph showing the percentage of thrombus area per field of view (P = .0058). (E) Representative micrographs of procoagulant activation of platelets seeded on collagen I/fibrinogen matrix; graphs showing percentage of procoagulant platelets (P = .0107) and percentage of ballooned procoagulant platelets relative to TP1 (P = .001). (F) Flow cytometric analysis showing percentage of P-selectin–positive PS+ platelets (n = 4; P = .0105). (G) Schematic outline of platelet-neutrophil coincubation (n = 3); flow cytometric analysis showing percentage of platelets aggregating with neutrophils (0.0274); statistical tests for panels D-G, RM 1-way ANOVA with the post hoc Dunnett multiple comparisons test compared with TP7. (H) In vitro aged donor C57BL/6J platelets, freshly isolated (day 0 [D0], n = 4) or stored for 2 days in DSD (day 2 [D2], n = 7) were transfused into thrombocytopenic C57BL/6J recipient mice (n = 4 per group); LPS was given intranasally to induce acute lung injury (ALI) in recipients; blood sampled at 0 and 6 hours after ALI and BALF was collected. (I) Bar graph depicting decline of transfused platelets, mixed-effects model (REML), and comparison between transfused groups (P = .0015 with the post hoc Šídák multiple comparisons test). (J) Bar graphs showing surface marker expression: CD11b (P = .0344) and CD66a (P = .0316) of neutrophils in circulation after ALI (unpaired t test, 2-tailed). (K) Bar graphs depicting the percentage of transfused in vitro aged platelets aggregating with CD45+ cells (P = .0203) and the percentage of platelets aggregating with Ly-6G+ cells (P = .0890) in BALF (unpaired t test, 2-tailed). (L) Assessment of cytokine measurements in BALF (2-way ANOVA, comparison between transfused groups; P = .0014, with the post hoc Šídák multiple comparisons test; additional cytokines shown in supplemental Figure 11H. (M) Clinical progression of ALI in recipients that received transfusion with D0 or D2 in vitro–aged platelets (2-way RM ANOVA, comparison between transfused groups; P < .0001, with the post hoc Šídák multiple comparisons test). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; h, hour; IL, interleukin; LPS I.N., lipopolysaccharide intranasally; ns, nonsignificant; TNF-α, tumor necrosis factor α.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/145/14/10.1182_blood.2024024901/2/m_blood_bld-2024-024901-gr7.jpeg?Expires=1769078399&Signature=FANTme4aoQd6cmGfYFeJuXK01PrsR5-dnK2dFjAbPgtXKPwPHPF~EShqVjKXUCEufV5RzJEhRfetiVUXqr33nbdoBd1BP5l-K8xc0O4zdeYBD0SYyHLdVbRjz-WW7nEPA6nYkdlz9AcXdjlQ7H9as~qd2f9S9yM3LIF8SZSrfT0IeygRwXdGayqWpPzgbwYf9OL74Op1EF3UwtDs75irToPHGC5KtFWWPO8h5~LPRdR1QNGlPOhzU654lX4VVOYmNs0bg1KPyT0lbu~24i7vhgqPL6cVB4QyupY40zCNlk2p2MlXnQaMF3cLnwiYB6NIzD1pkltR90QiG-2H0jAbyQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal