In this issue of Blood, Anjum et al1 elegantly show that aging platelets shift their functional profile from hemostatic to inflammatory. Platelets, the blood anucleate cellular components in charge of maintaining the body hemostasis, are also key players at the crossroads of hemostasis and immunity.2 More than 100 years ago, the first reports of platelets interacting with bacteria were published (reviewed by Clawson and White3), and in the past 40 years, major evidence has demonstrated the immunomodulatory functions of platelets. The detailed understanding of the interactions and feedback between these 2 systems and their impact at specific (patho)-physiological processes (such as immunothrombosis) is an increasing need.4
Platelets have a short lifespan (5-9 days), and the number of aged platelets that are cleared is in balance with the number of newly produced ones. Around 1011 platelets are produced every day by an average healthy adult, which is about 10% of the total amount of circulating platelets (in mice, the platelet lifespan is 3-5 days, and the production is around 1010 platelets per day). Thus, a normal platelet life cycle implies a balanced proportion of newly produced platelets, aging platelets, and aged platelets that are ready to be cleared.5
Platelets are very dynamic entities, with the capacity to recognize different ligands leading to their activation. On the one hand, the physiological context and initial natural ligand and tissue-cell interactions activating platelets and downstream signaling will determine the extent of the response and the contribution of platelets to a particular process, that is, endothelial repair, immune response, developmental separation of lymph from blood vessels, cancer metastasis, etc.6 On the other hand, not only external factors determine platelet responses. Platelets are not always “the same,” quality- and functionwise, which may be conditioned by the health status or age of the individual, or by treatment, as reported by various groups. These differences might be due to the production process itself (ie, stress or adaptive megakaryopoiesis) or caused by modifications occurring once in the circulation (ie, because of an infection or aging).7
Previously, omics analyses on newly produced (reticulated) and aged platelets have shown a general trend of reduction of the proteome and transcriptome (specific entries).7 In the study by Anjum and colleagues, a modified pulse-labeling method that allows tracing of platelets with different circulatory ages in a precise manner was essential to link qualitative differences at the proteome level to the functional profile shift of aging platelets. These findings have important implications for transfusion medicine and in the management of conditions where the platelet life cycle is altered or where antiplatelet treatment is required.
The authors show first that aged platelets are less responsive to hemostatic stimuli, exemplified by flow experiments where the recruitment of platelets to thrombi show that younger platelets are incorporated preferentially compared with aged ones. These results support previous reports of studies in humans that showed that in conditions in which the platelet clearance is accelerated, newly produced platelets (of which there is a surplus proportionwise) are hyperreactive, prothrombotic, and less responsive to antiplatelet treatment.8
The authors continue to describe in detail the functional characteristics of platelets aging in circulation, which concludes with a shift toward an immunomodulatory profile. Aged platelets, in comparison with young platelets, have relative increased expression of the receptors that mediate platelet immune functions, form more aggregates with leukocytes and neutrophils, kill bacteria more efficiently, and contribute more to pathophysiological events in murine preclinical models of inflammation. Furthermore, in murine models where the platelet life cycle is artificially extended, the aged platelets have hemostatic defects and inflammatory tendencies, which the authors demonstrate is due to their immune functional predilection.
Interestingly, the authors provide evidence that platelets stored for transfusion (in platelet concentrates) gradually shift their functional profile during storage, diminishing their hemostatic capacity while becoming more proinflammatory. In addition to the well-known platelet storage lesion, this finding has implications for the effectiveness of transfusion of older concentrates. The main populations receiving prophylactic platelet transfusions are patients with cancer and patients in the intensive care unit (ICU). However, these transfusions do not always result in the expected rise in the adjusted platelet count. An additional serious concern for patients in the ICU is the high risk of transfusion-related acute lung injury (1 in 5000), which is fatal in 5% to 24% of the cases, making it the leading cause of death after transfusion therapy.9 Unfortunately, there are few studies on this topic, and not all of them agree regarding the impact of the shelf life of transfusion products.10 The mechanistic insight provided by Anjum and colleagues opens new paths for the study and subsequent improvement of such therapeutic options.
The idea that several platelet subpopulations circulate in peripheral blood, and that they may be important for platelet production and physiology in health and disease, is now being explored. In normal conditions, there will be an equilibrated number of platelet subpopulations with different functional properties. In situations in which the platelet life cycle is challenged, those proportions will change. We are just beginning to understand the process and implications (see figure). As an example, newly produced platelets through stress megakaryopoiesis may not share the same qualitative and functional characteristics as platelets produced in the steady state. The application of single-cell omics under relevant health conditions, or in platelet concentrates during storage, will help with the identification of biomarkers that might aid in the personalized treatment of patients, especially those who are vulnerable (due to underlying inflammation, sepsis, or altered platelet life cycle), to be able to predict better responses to treatment or to avoid adverse events, as those that may result from platelet-mediated exacerbated immune responses.
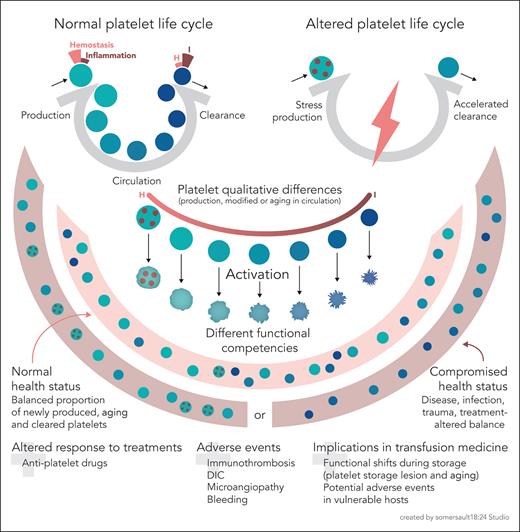
Aging platelets shift their hemostatic properties to inflammatory functions. Schematic representation of a normal platelet life cycle (top left), with platelet production in balance with platelet clearance and aging platelets in the circulation. The functional competency of platelets shifts, as proposed by Anjum et al, from a hemostatic to an inflammatory mode as they age. In general terms, an altered platelet life cycle (top right) occurs when, due to various reasons, there is stress megakaryopoiesis (with the release if immature platelets, represented as dotted circles) or accelerated platelet clearance. Platelet qualitative differences due to stress production, or modifications while in circulation, including aging, impact the platelet functional competency (center, focusing on aging). In summary, a normal platelet life cycle (physiological conditions of health) implies a balanced proportion of newly produced, aging, and cleared platelets. A disrupted platelet life cycle (due to a compromised health status or treatment) implies a dysbalanced proportion of platelets enriched for specific functional competencies. Finally, as platelets also age in platelet concentrates for transfusion, there will be functional shifts with storage time, in addition to potential changes driven by the platelet storage lesion. Older concentrates might potentially lead to transfusion-related adverse events in vulnerable hosts (with special concern when their platelet life cycle is altered or with underlying inflammation). DIC, disseminated intravascular coagulation; H, hemostasis; I, inflammation. The figure was created based on sketches by the author.
Aging platelets shift their hemostatic properties to inflammatory functions. Schematic representation of a normal platelet life cycle (top left), with platelet production in balance with platelet clearance and aging platelets in the circulation. The functional competency of platelets shifts, as proposed by Anjum et al, from a hemostatic to an inflammatory mode as they age. In general terms, an altered platelet life cycle (top right) occurs when, due to various reasons, there is stress megakaryopoiesis (with the release if immature platelets, represented as dotted circles) or accelerated platelet clearance. Platelet qualitative differences due to stress production, or modifications while in circulation, including aging, impact the platelet functional competency (center, focusing on aging). In summary, a normal platelet life cycle (physiological conditions of health) implies a balanced proportion of newly produced, aging, and cleared platelets. A disrupted platelet life cycle (due to a compromised health status or treatment) implies a dysbalanced proportion of platelets enriched for specific functional competencies. Finally, as platelets also age in platelet concentrates for transfusion, there will be functional shifts with storage time, in addition to potential changes driven by the platelet storage lesion. Older concentrates might potentially lead to transfusion-related adverse events in vulnerable hosts (with special concern when their platelet life cycle is altered or with underlying inflammation). DIC, disseminated intravascular coagulation; H, hemostasis; I, inflammation. The figure was created based on sketches by the author.
Conflict-of-interest disclosure: L.G. is chief scientific officer and cofounder of Platelet Biotechnologies S.L. (PlaBiTe) and declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal