In this issue of Blood, Li et al report their finding that the small molecule compound lasalocid A specifically targets mutated MYD88 L265P protein in diffuse large B-cell lymphoma (DLBCL).1 Extensive analysis shows that lasalocid A selectively targets and degrades mutated MYD88, thereby overcoming ibrutinib resistance. It also synergizes with the BCL2 inhibitor venetoclax, offering a promising targeted therapy for MYD88 L265P-mutant DLBCL, particularly in the high-risk activated B-cell (ABC) subtype.
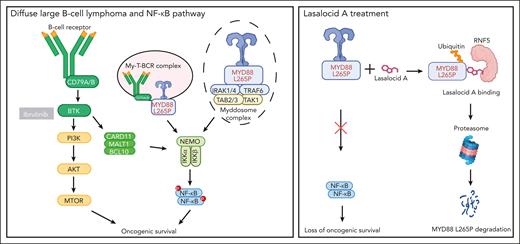
The MYD88 L265P mutation significantly increases the activity of MYD88 and the formation of the myddosome complex (see figure). This leads to constitutive activation of downstream nuclear factor κB (NF-κB) signaling, which provides survival stimuli for the lymphoma B cells. The MYD88 L265P mutation is commonly found in DLBCLs with the ABC phenotype, including primary cutaneous, central nervous system, intravascular, and testicular large B-cell lymphomas, all of which have poorer survival compared with other DLBCL subtypes.2-4 Studies have shown that the MYD88 L265P mutation independently confers additional risk, beyond the risk shown by clinical prognostic scores such as the International Prognostic Index.5
Lasalocid A targets mutated MYD88 in DLBCL.NF-κB activation in DLBCL can occur via the myddosome complex formed by MYD88, the B-cell receptor (BCR) pathway, and the MYD88, TLR9, and BCR (My-T-BCR) complex. The MYD88 L265P mutation drives constitutive activation of this pathway, promoting oncogenic is survival in lymphoma B cells. Although BCR pathway inhibitors like ibrutinib can block this pathway, lasalocid A specifically targets mutated MYD88. Acting as a “molecular glue,” lasalocid A binds MYD88 L265P to E3 ligase RNF5, leading to ubiquitination and proteasomal degradation of MYD88 and selectively removing survival signals in tumor B cells, and thus is an emerging specifically targeted therapeutic approach.
Lasalocid A targets mutated MYD88 in DLBCL.NF-κB activation in DLBCL can occur via the myddosome complex formed by MYD88, the B-cell receptor (BCR) pathway, and the MYD88, TLR9, and BCR (My-T-BCR) complex. The MYD88 L265P mutation drives constitutive activation of this pathway, promoting oncogenic is survival in lymphoma B cells. Although BCR pathway inhibitors like ibrutinib can block this pathway, lasalocid A specifically targets mutated MYD88. Acting as a “molecular glue,” lasalocid A binds MYD88 L265P to E3 ligase RNF5, leading to ubiquitination and proteasomal degradation of MYD88 and selectively removing survival signals in tumor B cells, and thus is an emerging specifically targeted therapeutic approach.
Over recent years, various targeted therapies have been explored to disrupt the activity of the aberrant signaling pathway resulting from this mutation, including inhibitors of the myddosome complex formation (eg, IRAK1/4 or TAK1 inhibitors) and the B-cell receptor pathway (eg, Bruton's tyrosine kinase [BTK] inhibitor ibrutinib).2
Only ibrutinib has shown promise as a targeted therapy for these patients, as demonstrated in the phase 3 Phoenix trial. Patients with mutational clusters MCD (MYD88 and CD79B) and N1 (NOTCH1) exhibited improved survival outcomes with R-CHOP (rituximab, cyclophosphamide, doxorubicin, oncovin [vincristine], and prednisone) + ibrutinib treatment.6 In addition, the Guidance trial, the first molecularly driven study, randomized patients with DLBCL with MCD or BN2 clusters to immunochemotherapy including ibrutinib, showing improved survival compared with R-CHOP.7 Although effective in MYD88 L265P-mutated DLBCL, ibrutinib inhibits the B-cell receptor pathway in both healthy and tumor B cells, potentially leading to therapy resistance and toxicity.8 Alongside B-cell receptor pathway inhibition with ibrutinib, other agents targeting MYD88 or downstream IRAK proteins are under investigation, although they may similarly impact healthy B cells.2
The authors highlight lasalocid A as a “molecular glue” or a proteolysis targeting chimera that selectively binds to L265P-mutated MYD88 (see figure). This binding likely occurs through a conformational change, facilitating interaction with residues R201 and K203. Lasalocid A subsequently recruits the endoplasmic reticulum-associated E3 ligase RNF5 to the mutated MYD88 protein. RNF5, which recognizes misfolded proteins, likely detects the conformational change in mutated MYD88, thus promoting its selective ubiquitination and proteasomal degradation. Previous research has demonstrated that ABC DLBCL relies on mutated MYD88 for survival.9 Selective degradation of this protein would therefore effectively impair tumor B cells.
DLBCL is typically treated with a combination of CD20-targeted antibodies (eg, rituximab) and chemotherapy, leading to complete remission in most cases. This treatment can cause adverse events, particularly myelosuppression, which increase the risk for infections. A therapy that selectively targets tumor B cells, rather than healthy B cells, would be highly advantageous. Although this therapy cures approximately 70% of patients, the majority of those who are chemorefractory or relapse have the MCD genotype DLBCL, highlighting the need for novel therapeutic approaches for these patients.
Furthermore, patients with primary central nervous system lymphoma (PCNSL) are often treated with high-dose methotrexate. Unfortunately, these patients still have an inferior prognosis. Given the high prevalence of MYD88 L265P mutations in PCNSL, these patients might greatly benefit from lasalocid A, provided it can cross the blood-brain barrier. Another disease that could be highly responsive to lasalocid A treatment is lymphoplasmacytic lymphoma (LPL, or Waldenström macroglobulinemia), as MYD88 L265P drives oncogenesis in approximately 85% of cases. Already, a rational molecular-driven approach that includes BTK inhibitors is often used to treat patients with LPL. Ibrutinib refractoriness is a key factor in patient mortality, with limited alternative therapeutic targets available. For diseases like mantle cell lymphoma or chronic lymphocytic leukemia, it will be interesting to see whether lasalocid A can overcome ibrutinib resistance, as BTK mutations often drive refractoriness in the absence of a MYD88 L265P mutation. This warrants further exploration in in vitro studies.
Lastly, the synergistic effect of lasalocid A with venetoclax is noteworthy. A recent study demonstrated that the combination of venetoclax, ibrutinib, prednisone, obinutuzumab, and lenalidomide (ViPOR) achieved durable remission in relapsed or refractory DLBCL, particularly in ABC-DLBCL and MYD88 L265P-mutant DLBCL (phase 1b/2 trial).10 These findings suggest that adding lasalocid A to the ViPOR regimen for ABC-DLBCL and MCD genotype DLBCL, particularly in cases with the MYD88 L265P mutation, could enhance treatment efficacy. However, additional studies are required to assess potential toxicity and confirm survival benefits.
Clinical trials evaluating lasalocid A as monotherapy or in combination with chemotherapy and targeted agents (eg, venetoclax) are anticipated in patients with lymphoma, especially those with chemorefractory PCNSL or ibrutinib-resistant LPL, who have limited treatment options and poor prognoses. If lasalocid A demonstrates efficacy, manageable adverse events, and improved survival outcomes, it could become a valuable addition to the treatment arsenal for aggressive B-cell lymphomas. We eagerly await the results of these trials.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal