Key Points
Hemochromatosis C282Y homozygotes had an increased risk of any infection and a markedly increased risk of sepsis and death from infections.
C282Y homozygotes with normal iron, transferrin saturation, or ferritin, not currently recommended for genotyping, had high infection risk.
Visual Abstract
It is unclear whether risk of infection is increased in individuals with hereditary hemochromatosis and in individuals with low or high plasma iron, transferrin saturation, or ferritin. Therefore, we tested whether high and low iron, transferrin saturation, and ferritin are associated with risk of infections observationally and genetically through HFE genotypes. We studied 142 188 Danish general population individuals. Iron, transferrin saturation, and ferritin were measured in 136 656, 136 599, and 38 020 individuals, respectively. HFE was genotyped for C282Y and H63D in 132 542 individuals. Median follow-up after study enrollment was 8 years (range, 0-38) for hospital and emergency room admissions with infections (n = 20 394) using the National Patient Register, covering all Danish hospitals. Hazard ratios for any infection were 1.20 (95% confidence interval [CI], 1.12-1.28) and 1.14 (95% CI, 1.07-1.22) in individuals with plasma iron ≤5th or ≥95th percentile compared with individuals with iron from 26th to 74th percentiles. Findings for transferrin saturation were similar, whereas infection risk was not increased in individuals with ferritin ≤5th or ≥95th percentile. Hazard ratios in C282Y homozygotes vs noncarriers were 1.40 (95% CI, 1.16-1.68) for any infection, 1.69 (95% CI, 1.05-2.73) for sepsis, and 2.34 (95% CI, 1.41-3.90) for death from infectious disease. Risk of infection was increased in C282Y homozygotes with normal plasma iron, transferrin saturation, or ferritin, and in C282Y homozygotes without liver disease, diabetes, and/or heart failure. In summary, low and high plasma iron and transferrin saturation were independently associated with increased infection risk. C282Y homozygotes had increased risk of any infection, sepsis, and death from infections. Even C282Y homozygotes with normal iron, transferrin saturation, or ferritin, not currently recommended for genotyping, had increased infection risk.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited with commendation by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at https://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 796.
Disclosures
CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declares no competing financial interests.
Learning objectives
Upon completion of this activity, participants will:
Describe associations of high and low iron, transferrin saturation, and ferritin with risk for infections, based on a Danish general population–based study
Determine associations of hemochromatosis genotypes with risk for infections, based on a Danish general population–based study
Identify clinical implications of possible associations of high and low iron, transferrin saturation, and ferritin and of hemochromatosis genotypes with risk for infections, based on a Danish general population–based study
Release date: August 15, 2024; Expiration date: August 15, 2025
Introduction
Iron is a life-essential micronutrient that is necessary for hemoglobin synthesis and function of immune cells but also a prerequisite for the growth of microorganisms.1,2 Serum iron parameters are often measured in routine blood samples in which plasma iron and transferrin saturation indicate how much iron is available in the bloodstream, whereas plasma ferritin is a measure of total iron accumulated in the body.3-5 It is unclear whether high levels of plasma iron, transferrin saturation, and/or ferritin increase susceptibility to infections, because recent studies have shown conflicting results.3,5
Individuals with the condition hemochromatosis have increased accumulation of iron in the body, which can lead to liver cirrhosis, hepatocellular cancer, diabetes mellitus, heart failure, and arthritis.6,7 Hereditary hemochromatosis is most often caused by homozygosity or compound heterozygosity for single nucleotide polymorphism variants in the HFE hemochromatosis gene encoding the human homeostatic iron regulator protein. These variants are C282Y (rs1800562) and H63D (rs1799945), either as C282Y homozygosity (C282Y/C282Y) having a prevalence of 0.25% to 1.0% in Northern Europe or as compound heterozygosity (C282Y/H63D) with a prevalence of about 2% in Northern Europe.8,9 On average, C282Y homozygotes have higher levels of plasma iron, transferrin saturation, and ferritin than noncarrier individuals,10-13 but results on penetrance have varied substantially between studies, because clinical penetrance has been reported in between 1% and 60% of C282Y homozygous individuals.7,9,14 In Northern Europe, 80% to 95% of individuals diagnosed with hereditary hemochromatosis are homozygous for the HFE C282Y variant.11,15,16
It is unclear whether homozygosity and/or heterozygosity for the C282Y or H63D variants cause increased risk of infections. This question is important due to possible clinical consequences especially for C282Y homozygous individuals, but also because it offers a method to study potentially causal effects on the risk of infections from iron accumulation.17
We tested the hypothesis that high and low plasma iron, transferrin saturation, and ferritin are associated with increased risk of infections observationally and genetically through HFE C282Y and H63D variants. For this purpose, we studied 142 188 individuals from the White Danish general population. All individuals had blood samples drawn and were followed for hospitalization and death due to infections.
Methods
We studied 142 188 individuals from 3 cohort studies of the Danish general population: The Copenhagen City Heart Study, the Copenhagen General Population Study, and the Danish General Suburban Population Study. From the Copenhagen City Heart Study,18 we included 13 661 individuals examined between 1981 and 1983 or between 1991 and 1994. From the Copenhagen General Population Study,19 we included 108 061 individuals examined between 2003 and 2015. From the Danish General Suburban Population Study,20 we included 20 466 individuals examined between 2010 and 2013. Details on enrollment procedures and participation rates are described in the supplemental Methods, available on the Blood website. Age at enrollment was 20 to 100 years. At the day of enrollment, all individuals had blood samples drawn, filled out a questionnaire regarding health and lifestyle, and had a physical examination performed. Study procedures were similar for the 3 general population studies. More than 99% of individuals were White and of Danish descent. Individuals were only included into 1 of the 3 studies, and none were lost to follow-up. The studies were approved by Danish ethical committees (De Videnskabsetiske Komitéer for Københavns og Frederiksberg Kommuner, Region Sjællands forskningsfortegnelse), and all participants provided written, informed consent.
Covariates
Covariates associated with risk of infection and/or with levels of plasma iron, transferrin saturation, or ferritin were chosen based on an a priori literature review.21 Information on age and sex was obtained from the Danish Civil Registration System. Smoking status, cumulative smoking in pack-years, alcohol intake, and menopausal status were derived from the questionnaire, whereas body mass index was from the physical examination (details in supplemental Methods). C-reactive protein levels were measured using high sensitivity standard laboratory assays.
Plasma iron, transferrin saturation, and ferritin
All individuals had blood samples drawn at the day of study enrollment, which was used for measuring iron, transferrin saturation, and ferritin. In the Copenhagen City Heart Study and the Copenhagen General Population Study, plasma iron and transferrin were measured using a Konelab autoanalyzer (ThermoFisher assays “IRON” and “TRANSFERRIN”), whereas ferritin was measured using an Advia Centaur (Siemens assay “FER”). In the Danish General Suburban Population Study, iron, transferrin, and ferritin were measured using a Cobas 6000 (Roche assays “IRON2,” “TRANS,” and “Ferritin”).
For a total of 32 141 individuals, a second blood sample for a repeat measurement of iron and/or transferrin saturation was obtained at a median of 10 years after the first blood sample, as described in supplemental Methods along with details on sample storage, calibration, and quality control.
Genotypes
We genotyped the 2 hemochromatosis variants C282Y and H63D in the HFE gene, both known to increase levels of iron, transferrin saturation, and ferritin.6 For details on genotyping and Hardy-Weinberg equilibrium, see supplemental Methods.
Infectious disease and vital status
All hospital inpatient admissions in Denmark since 1977 and all emergency room visits and outpatient visits since 1994 have been registered in the Danish National Patient Register.22 We obtained information from this register on each individual regarding all inpatient admissions with an infectious disease as the main diagnosis from 1 January 1977 until 31 December 2018. Similarly, we obtained information on all emergency room visits with an infectious disease as the main diagnosis from 1 January 1994 until 31 December 2018. Categorization of infectious disease hospitalizations are described in supplemental Methods and supplemental Table 1. Information on bloodstream infections from 1 January 2010 to 31 December 2019 was retrieved from the national Danish Microbiology Database23 as described in supplemental Methods.
We have previously ascertained validity of infectious disease diagnoses from the Danish National Patient Register through review of detailed clinical information from hospital charts on 141 admissions coded as infectious diseases. In 139 of 141 admissions (99%), hospital charts documented relevant signs and symptoms of infection, a positive culture from a sterile site or relevant specimen, and/or treatment with antibiotics.18
Information on cause of death until 31 December 2018 was retrieved from the national Danish Cause of Death Register containing all diagnoses that a physician listed on the death certificate for all deaths occurring in Denmark from 1970 onward.24 Death from infectious disease was defined as any death with an infection as the underlying cause of death, immediate cause of death, or the disease linking the underlying and immediate cause of death. Information about vital status and emigration until 31 December 2019 was retrieved from the national Danish Civil Registration System.
Comorbidities
Noninfectious comorbidities can be associated with levels of plasma iron, transferrin saturation, and ferritin but also with risk of infection.25,26 Therefore, we retrieved information on comorbidities at study enrollment from the Danish National Patient Register. Comorbidities were defined using the Charlson comorbidity index (see supplemental Methods).
Statistical analysis
Statistical analyses were performed using Stata17.0. All statistical tests were 2-sided. Risk of infection and risk of death from infection was modeled by Cox proportional hazards regression for the main analyses, whereas Fine-Gray competing risk regression with risk of infection as main end point and death as a competing risk were performed as supplementary analyses.27 We adjusted for age by using left-truncated age (time since birth with delayed entry) as time scale, which is often recommended for studies of the general population because the date of study enrollment is largely chosen at random, meaning that age is generally a stronger predictor for risk of future disease than time since study enrollment.28,29
When analyzing the risk of hospitalization with infection according to levels of iron, transferrin saturation, and ferritin, follow-up began at the date of study enrollment. We divided individuals into 5 categories of measured iron, transferrin saturation, and ferritin, with focus on extreme high and extreme low levels: 0 to 5th, 6th to 25th, 26th to 74th, 75th to 94th, and 95th to 100th percentiles. The 26th to 74th percentile was used as the reference group. When analyzing the risk of hospitalization with infection according to HFE genotype, follow-up began at age 20 or 1 January 1977, whichever came last. When analyzing the risk of infectious disease death according to levels of iron, transferrin saturation, ferritin, or HFE genotype, follow-up began at the date of study enrollment. Further details on follow-up (including follow-up for the analysis on risk of bloodstream infections), statistical models, and handling of missing values are described in supplemental Methods and supplemental Figure 1. Infections before the start of follow-up were ignored in all analyses.
Results
Table 1 presents baseline characteristics of the 142 188 general population individuals according to study cohort, whereas baseline characteristics according to levels of plasma iron, transferrin saturation, and ferritin are presented in supplemental Tables 2-4. From study enrollment until the end of follow-up (median, 8 years; range, 0-38), 20 394 individuals were hospitalized due to infections. The rate of infections was 1144 infections per 100 000 individuals per year in the first year after study enrollment, with an increasing trend until the 36th year after study enrollment when the rate peaked at 5055 infections per 100 000 individuals per year (supplemental Figure 2).
Baseline characteristics of 142 188 individuals from the general population according to study cohort
| . | Cohort . | ||
|---|---|---|---|
| Copenhagen City Heart Study . | Copenhagen General Population Study . | Danish General Suburban Population Study . | |
| Individuals, n | 13 661 | 108 061 | 20 466 |
| Plasma iron, n (%) | 8 884 (65)∗ | 107 343 (99) | 20 429 (100) |
| Plasma transferrin saturation, n (%) | 8 871 (65)∗ | 107 302 (99) | 20 426 (100) |
| Plasma ferritin, n (%) | 8 999 (66)∗ | 8 632 (8) | 20 389 (100) |
| Hemochromatosis genotyped, n (%) | 9 174 (67)∗ | 103 276 (96) | 20 092 (98) |
| Age (IQR), y | 61 (49-70) | 58 (48-67) | 57 (46-67) |
| Year of birth (IQR) | 1928 (1919-1940) | 1951 (1942-1961) | 1954 (1945-1966) |
| Men, n (%) | 6 438 (47) | 48 640 (45) | 9 344 (46) |
| Premenopausal women, n (% of women) | 2 527 (35) | 19 298 (32) | 3 962 (36) |
| Ever smokers, n (%) | 10 817 (79) | 62 463 (58) | 11 470 (56) |
| Cumulative smoking (IQR)†, pack-years | 22 (9-38) | 16 (6-30) | 18 (8-31) |
| Alcohol consumption >168/84 g per wk, n (%)‡ | 3 742 (27) | 42 232 (39) | 4 852 (24) |
| Body mass index (IQR), kg/m2 | 24.9 (22.5-27.8) | 25.6 (23.2-28.4) | 26.1 (23.5-29.2) |
| Any comorbidity, n (%)§ | 1 570 (11) | 23 209 (21) | 4 355 (21) |
| Plasma C-reactive protein (IQR), mg/L | 1.7 (1.3-3.0) | 1.4 (0.9-2.3) | 1.4 (0.7-3.0) |
| . | Cohort . | ||
|---|---|---|---|
| Copenhagen City Heart Study . | Copenhagen General Population Study . | Danish General Suburban Population Study . | |
| Individuals, n | 13 661 | 108 061 | 20 466 |
| Plasma iron, n (%) | 8 884 (65)∗ | 107 343 (99) | 20 429 (100) |
| Plasma transferrin saturation, n (%) | 8 871 (65)∗ | 107 302 (99) | 20 426 (100) |
| Plasma ferritin, n (%) | 8 999 (66)∗ | 8 632 (8) | 20 389 (100) |
| Hemochromatosis genotyped, n (%) | 9 174 (67)∗ | 103 276 (96) | 20 092 (98) |
| Age (IQR), y | 61 (49-70) | 58 (48-67) | 57 (46-67) |
| Year of birth (IQR) | 1928 (1919-1940) | 1951 (1942-1961) | 1954 (1945-1966) |
| Men, n (%) | 6 438 (47) | 48 640 (45) | 9 344 (46) |
| Premenopausal women, n (% of women) | 2 527 (35) | 19 298 (32) | 3 962 (36) |
| Ever smokers, n (%) | 10 817 (79) | 62 463 (58) | 11 470 (56) |
| Cumulative smoking (IQR)†, pack-years | 22 (9-38) | 16 (6-30) | 18 (8-31) |
| Alcohol consumption >168/84 g per wk, n (%)‡ | 3 742 (27) | 42 232 (39) | 4 852 (24) |
| Body mass index (IQR), kg/m2 | 24.9 (22.5-27.8) | 25.6 (23.2-28.4) | 26.1 (23.5-29.2) |
| Any comorbidity, n (%)§ | 1 570 (11) | 23 209 (21) | 4 355 (21) |
| Plasma C-reactive protein (IQR), mg/L | 1.7 (1.3-3.0) | 1.4 (0.9-2.3) | 1.4 (0.7-3.0) |
n (%) is displayed for categorical variables, whereas median (IQR) is displayed for continuous variables.
IQR, interquartile range.
In the Copenhagen City Heart Study, 8999 individuals were included from the 1981 to 1983 examination, and 9725 individuals were included from the 1991 to 1994 examination, of whom 5063 individuals attended both examinations. Only individuals who attended the 1991 to 1994 examination had iron (8884 individuals of 10 135 attending [88%]), transferrin saturation (8871 individuals of 10 135 attending [88%]), and hemochromatosis genotype (9174 individuals of 10 135 attending [91%]) measured. Only individuals who attended the 1981 to 1983 examination had ferritin measured (8999 individuals of 12 698 attending [71%]).
Ever smokers only.
>168 g/wk for men and >84 g/wk for women.
Any comorbidity as defined by the Charlson comorbidity index.
Risk of infection according to levels of iron, transferrin saturation, and ferritin
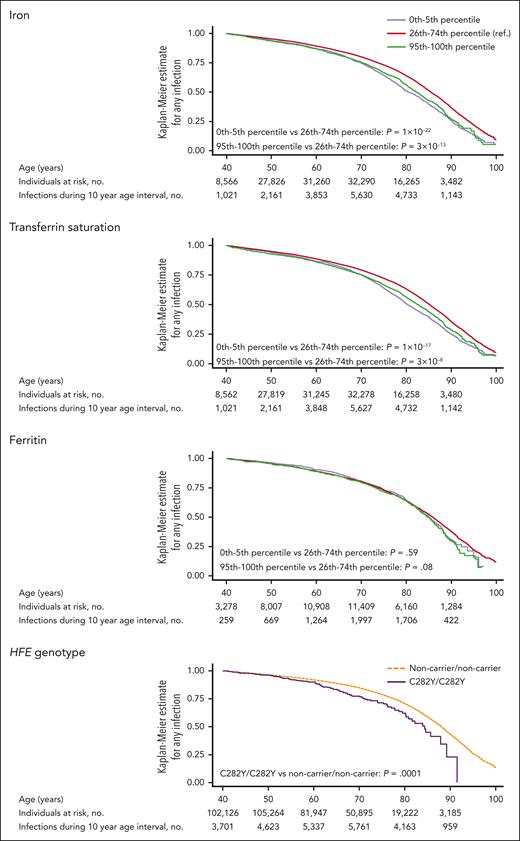
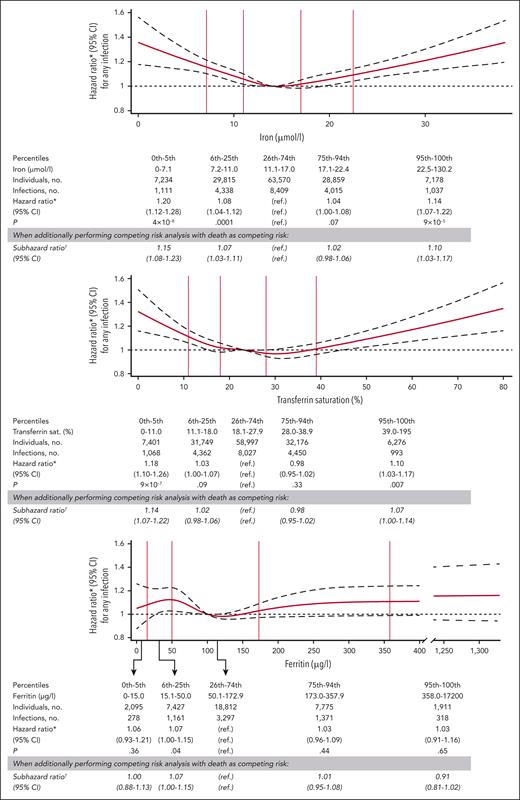
Kaplan-Meier curves for risk of any infection according to levels of iron, transferrin saturation, and ferritin are shown in Figure 1. After multivariable adjustment, low plasma iron was associated with increased risk of any infection with a hazard ratio of 1.20 (95% confidence interval [CI], 1.12-1.28) for individuals with iron at or below the fifth percentile (7.1 μmol/L), compared with individuals with iron between the 26th and 74th percentiles (Figure 2). High plasma iron was also associated with increased risk of any infection with a hazard ratio of 1.14 (95% CI, 1.07-1.22) for iron at or above the 95th percentile (22.5 μmol/L) vs between the 26th and 74th percentiles. Similarly, both low transferrin saturation (hazard ratio, 1.18; 95% CI, 1.10-1.26, for transferrin saturation at or below the fifth percentile [11%]) and high transferrin saturation (hazard ratio, 1.10; 95% CI, 1.03-1.17, for transferrin saturation at or above the 95th percentile [39%]) were associated with increased risk of any infection. For ferritin, we found no association between low (≤5th percentile) or high levels (≥95th percentile) and risk of any infection (Figure 2). Results were similar to those described above when performing competing risk analysis for risk of any infection according to levels of iron, transferrin saturation, and ferritin, with death as a competing risk (Figure 2). Similarly, results were similar to those presented in Figure 2 when additionally including household income and education level in the multivariable adjusted model or when only including individuals who were noncarriers for both C282Y and H63D (noncarrier/noncarrier; supplemental Table 5).
Kaplan-Meier curves for risk of any infection according to plasma iron, transferrin saturation, ferritin, and HFE genotype. The number of individuals at risk and number of infections shown are for all individuals who have measurements of iron, transferrin saturation, ferritin, or HFE genotype, respectively. The graphical curves depict solely the 0 to 5th percentile, 26th to 74th percentile, and 95th to 100th percentile when studying the risk of infection according to iron, transferrin saturation, or ferritin, whereas the curves depict solely C282Y/C282Y and noncarrier/noncarrier individuals when studying risk of infection according to HFE genotype. Statistical power is limited in individuals aged <40 years as a result of relatively few individuals enrolled before age 40. Therefore, curves are shown from age 40 years and onward to avoid single infection events in younger individuals leading to large fluctuations in the graphical depiction of Kaplan-Meier estimates due to low statistical power. P values were calculated using a log-rank test. no., number. ref., reference.
Kaplan-Meier curves for risk of any infection according to plasma iron, transferrin saturation, ferritin, and HFE genotype. The number of individuals at risk and number of infections shown are for all individuals who have measurements of iron, transferrin saturation, ferritin, or HFE genotype, respectively. The graphical curves depict solely the 0 to 5th percentile, 26th to 74th percentile, and 95th to 100th percentile when studying the risk of infection according to iron, transferrin saturation, or ferritin, whereas the curves depict solely C282Y/C282Y and noncarrier/noncarrier individuals when studying risk of infection according to HFE genotype. Statistical power is limited in individuals aged <40 years as a result of relatively few individuals enrolled before age 40. Therefore, curves are shown from age 40 years and onward to avoid single infection events in younger individuals leading to large fluctuations in the graphical depiction of Kaplan-Meier estimates due to low statistical power. P values were calculated using a log-rank test. no., number. ref., reference.
Risk of any infection according to levels of plasma iron, transferrin saturation, and ferritin. Solid red lines are hazard ratios obtained using Cox regression, broken black lines indicate 95% CIs. Analyses were multivariable adjusted for age, year of birth, sex, smoking status, cumulative smoking in pack-years, alcohol consumption, body mass index, menopausal status, study cohort, Charlson comorbidity index, and level of C-reactive protein. Reference for the continuous models (splines) is the median value of plasma iron (14 μmol/L), transferrin saturation (23%), or ferritin (100 μg/L) in the respective analyses. Total number of individuals in the analyses on risk of any infection according to iron, transferrin saturation, and ferritin were 136 656, 136 599, and 38 020, respectively. All individuals who had a measurement of iron, transferrin saturation, or ferritin were included in the categorical analyses and in modeling of the splines, but when drawing the spline curves the x-axis was capped at the 99.9th percentile. ∗Hazard ratio, 95% CI, and P value obtained using Cox regression.†Subhazard ratio and 95% CI obtained using Fine-Gray competing risk regression on risk of any infection with death from any cause as competing risk. ref., reference group; sat., saturation.
Risk of any infection according to levels of plasma iron, transferrin saturation, and ferritin. Solid red lines are hazard ratios obtained using Cox regression, broken black lines indicate 95% CIs. Analyses were multivariable adjusted for age, year of birth, sex, smoking status, cumulative smoking in pack-years, alcohol consumption, body mass index, menopausal status, study cohort, Charlson comorbidity index, and level of C-reactive protein. Reference for the continuous models (splines) is the median value of plasma iron (14 μmol/L), transferrin saturation (23%), or ferritin (100 μg/L) in the respective analyses. Total number of individuals in the analyses on risk of any infection according to iron, transferrin saturation, and ferritin were 136 656, 136 599, and 38 020, respectively. All individuals who had a measurement of iron, transferrin saturation, or ferritin were included in the categorical analyses and in modeling of the splines, but when drawing the spline curves the x-axis was capped at the 99.9th percentile. ∗Hazard ratio, 95% CI, and P value obtained using Cox regression.†Subhazard ratio and 95% CI obtained using Fine-Gray competing risk regression on risk of any infection with death from any cause as competing risk. ref., reference group; sat., saturation.
Because levels of iron and transferrin saturation at or below the fifth percentile are common in acute infections, chronic inflammatory disorders, and other comorbidities, we performed separate analyses on the risk of any infection according to levels of iron, transferrin saturation, and ferritin excluding individuals who had any infection within the first 180 days after study enrollment and excluding individuals who at study enrollment had anemia, neutropenia, any comorbidity, or a C-reactive protein level >10 mg/L (supplemental Table 5). All these analyses gave results similar to those presented in Figure 2.
When stratified according to follow-up intervals, risk estimates for any infection were similar for the first 5 years after study enrollment and for the time interval from 5 years after study enrollment and onward (supplemental Figure 3), indicating that the increased risk was not likely caused by latent infections already present at study enrollment. Because non–transferrin bound iron is often considered to occur when transferrin saturation exceeds 70%,30 we also examined the risk of any infection after excluding the 254 individuals who had a transferrin saturation ≥70% at the day of study enrollment, which gave results similar to those presented in Figure 2 (supplemental Table 5). When comparing the 254 individuals with transferrin saturation ≥70% with the 58 997 individuals with transferrin saturation between the 26th and 74th percentiles, the hazard ratio for any infection was 1.23 (95% CI, 0.91-1.65). These results indicate that non–transferrin bound iron is not likely the primary cause of the increased risk of any infection in individuals with high iron or high transferrin saturation.
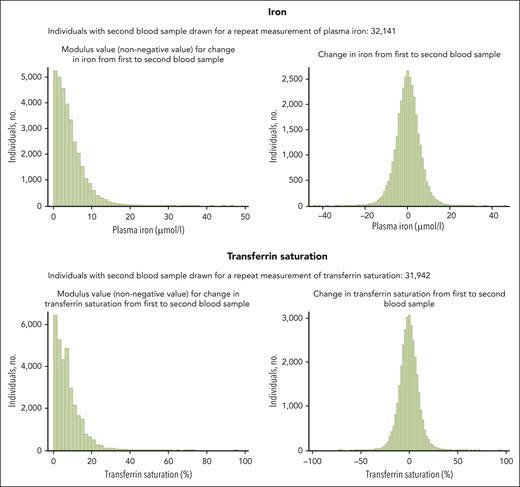
Because levels of iron and transferrin saturation may change over time,31,32Figure 3 shows changes in iron and transferrin saturation in 32 141 individuals who had a second blood sample obtained for repeat measurements after a median of 10 years. Among these, 26 240 individuals had a repeat measurement performed before the end of follow-up for infectious disease hospitalizations on 31 December 2018. When studying the risk of any infection according to the first measurements of iron and transferrin saturation only in individuals with repeat measurements available (supplemental Figure 4), the risk estimates were generally similar to those observed for the whole study population (Figure 2) but with substantially lower statistical power and therefore wider CIs due to the limited number of individuals with repeat measurements available. Similarly, when studying the risk of any infection according to the second (repeat) measurements of iron and transferrin saturation, the risk estimates were generally similar to those observed when using the first measurement (supplemental Figure 4; Figure 2).
Change in plasma iron and transferrin saturation for each individual between first blood sample drawn at study enrollment and second blood sample drawn for a repeat measurement of plasma iron or transferrin saturation. For a total of 32 141 individuals, a second blood sample for a repeat measurement of iron and/or transferrin saturation was obtained at a median of 10 years (IQR, 9.6-10.7; range, 0.7-15.8) after the first blood sample. Median modulus value (nonnegative value) for change in plasma iron was 3.2 μmol/L (IQR, 1.7-5.9). For transferrin saturation median modulus value (nonnegative value) for change was 6% (IQR, 3%-10%). Importantly, all main analyses on risk of infections were based on the blood samples drawn at the day of study enrollment (first blood sample), except for supplemental Figure 4 in which the second blood sample for repeat measurements of iron and transferrin saturation was used to model risk of infection. No individuals had repeat measurements of ferritin. IQR, interquartile range.
Change in plasma iron and transferrin saturation for each individual between first blood sample drawn at study enrollment and second blood sample drawn for a repeat measurement of plasma iron or transferrin saturation. For a total of 32 141 individuals, a second blood sample for a repeat measurement of iron and/or transferrin saturation was obtained at a median of 10 years (IQR, 9.6-10.7; range, 0.7-15.8) after the first blood sample. Median modulus value (nonnegative value) for change in plasma iron was 3.2 μmol/L (IQR, 1.7-5.9). For transferrin saturation median modulus value (nonnegative value) for change was 6% (IQR, 3%-10%). Importantly, all main analyses on risk of infections were based on the blood samples drawn at the day of study enrollment (first blood sample), except for supplemental Figure 4 in which the second blood sample for repeat measurements of iron and transferrin saturation was used to model risk of infection. No individuals had repeat measurements of ferritin. IQR, interquartile range.
Because >90% of infection-related deaths are due to pneumonia or sepsis,19 results on the risk of pneumonia and sepsis are presented in detail (Figure 4). Both low iron (hazard ratio, 1.20; 95% CI, 1.10-1.32) and high iron (hazard ratio, 1.22; 95% CI, 1.11-1.34) were associated with an increased risk of pneumonia. Similarly, low transferrin saturation (hazard ratio, 1.20; 95% CI, 1.09-1.32) and high transferrin saturation (hazard ratio, 1.11; 95% CI, 1.01-1.22) were associated with an increased risk of pneumonia. For sepsis, both low iron (hazard ratio, 1.18; 95% CI, 1.01-1.38) and high iron (hazard ratio, 1.38; 95% CI, 1.20-1.58) were associated with increased risk (Figure 4). Low transferrin saturation was not associated with the risk of sepsis (hazard ratio, 1.12; 95% CI, 0.95-1.32), but high transferrin saturation was associated with an increased risk of sepsis (hazard ratio, 1.27; 95% CI, 1.11-1.46). When studying the risk of any bloodstream infection, defined as any episode in which ≥1 microorganisms grew in a blood culture, results were similar to those found for sepsis with an increased risk of any bloodstream infection observed in individuals with low iron (hazard ratio, 1.23; 95% CI, 1.05-1.44), high iron (hazard ratio, 1.26; 95% CI, 1.08-1.46), and high transferrin saturation (hazard ratio, 1.24; 95% CI, 1.07-1.44; supplemental Figure 5). The increased risk of bloodstream infections was primarily due to gram-positive bacteria with hazard ratios of 1.45 (95% CI, 1.20-1.77) for low iron, 1.31 (95% CI, 1.07-1.59) for high iron, 1.27 (95% CI, 1.02-1.58) for low transferrin saturation, and 1.38 (95% CI, 1.13-1.67) for high transferrin saturation (supplemental Figure 6). The levels of iron, transferrin saturation, and ferritin were not associated with the risk of gram-negative bloodstream infections (supplemental Figure 6).
Risk of pneumonia and sepsis according to levels of plasma iron, transferrin saturation, and ferritin. Solid red lines are hazard ratios, broken black lines indicate 95% CIs. Analyses were multivariable adjusted for age, year of birth, sex, smoking status, cumulative smoking in pack-years, alcohol consumption, body mass index, menopausal status, study cohort, Charlson comorbidity index, and level of C-reactive protein. Reference for the continuous models (splines) is the median value of plasma iron (14 μmol/L), transferrin saturation (23%), or ferritin (100 μg/L) in the respective analyses. All individuals who had a measurement of iron, transferrin saturation, or ferritin were included in the categorical analyses and in modeling of the splines, but when drawing the spline curves, the x-axis was capped at the 99.9th percentile. ref., reference.
Risk of pneumonia and sepsis according to levels of plasma iron, transferrin saturation, and ferritin. Solid red lines are hazard ratios, broken black lines indicate 95% CIs. Analyses were multivariable adjusted for age, year of birth, sex, smoking status, cumulative smoking in pack-years, alcohol consumption, body mass index, menopausal status, study cohort, Charlson comorbidity index, and level of C-reactive protein. Reference for the continuous models (splines) is the median value of plasma iron (14 μmol/L), transferrin saturation (23%), or ferritin (100 μg/L) in the respective analyses. All individuals who had a measurement of iron, transferrin saturation, or ferritin were included in the categorical analyses and in modeling of the splines, but when drawing the spline curves, the x-axis was capped at the 99.9th percentile. ref., reference.
When investigating the risk of other specific types of infection, low iron was associated with an increased risk of urinary tract infection (hazard ratio, 1.20; 95% CI, 1.06-1.36) and skin infection (hazard ratio, 1.18; 95% CI, 1.02-1.36). For transferrin saturation, low levels were associated with an increased risk of urinary tract infection (hazard ratio, 1.18; 95% CI, 1.03-1.34), whereas high levels were associated with an increased risk of diarrheal disease (hazard ratio, 1.27; 95% CI, 1.04-1.56; supplemental Figure 7). The risk estimates for any infection, pneumonia, and sepsis according to low or high levels of iron, transferrin saturation, and ferritin were similar for men and women, with no indication of interaction between sex and any of the 3 biomarkers on the risk of any infection, pneumonia, or sepsis (supplemental Figure 8).
When examining the risk of death from infectious disease, low iron (hazard ratio, 1.31; 95% CI, 1.14-1.52) and low transferrin saturation (hazard ratio, 1.31; 95% CI, 1.12-1.53) were associated with increased risk, whereas the risk was not increased in individuals with high iron (hazard ratio, 1.04; 95% CI, 0.88-1.23), high transferrin saturation (hazard ratio, 1.09; 95% CI, 0.93-1.27), high ferritin (hazard ratio, 0.95; 95% CI, 0.74-1.22), or low ferritin (hazard ratio, 1.28; 95% CI, 0.98-1.68; supplemental Figure 9). When stratified according to follow-up intervals, the risk estimates for death from infectious disease were similar for the first 5 years after study enrollment and for the time interval from 5 years after study enrollment and onward (supplemental Figure 9).
Risk of infections according to hemochromatosis genotypes
Among 132 542 individuals genotyped for HFE C282Y and H63D, 422 individuals were homozygous for the C282Y variant (228 women and 194 men). Supplemental Table 6 presents the baseline characteristics according to genotypes, showing potential associations with genotype for year of birth (P for trend = .03) and neutrophil count (P for trend = .04), however, when adjusting for 12 multiple comparisons using the Bonferroni method, these findings were no longer significant at the P value <.05 level (required P value <.004 = .05/12). Follow-up for the analysis on risk of infections according to hemochromatosis genotypes began at age 20 or 1 January 1977, whichever came last (illustrated in supplemental Figure 1). During follow-up (median, 39 years; range, 0-42), 29 936 of the genotyped individuals were hospitalized due to infections, of which 14 747 were hospitalized before study enrollment, and 15 189 were hospitalized after.
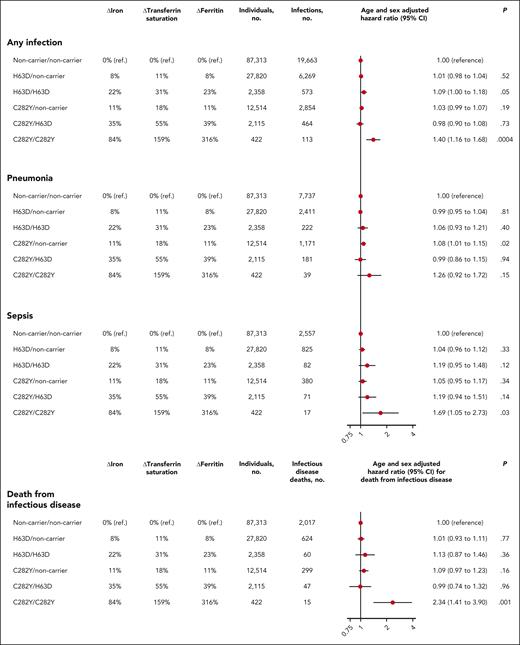
Individuals with C282Y homozygosity (C282Y/C282Y) had increased risk of any infection (hazard ratio, 1.40; 95% CI, 1.16-1.68), compared with individuals who were noncarrier for both C282Y and H63D (noncarrier/noncarrier; Figures 1 and 5). Results were similar when performing competing risk analysis for risk of any infection with death as a competing risk (hazard ratio, 1.36; 95% CI, 1.13-1.64, for C282Y homozygotes vs noncarriers; supplemental Figure 10). For pneumonia, the hazard ratio was 1.26 (95% CI, 0.92-1.72) in C282Y homozygotes. Risk of sepsis was markedly increased in C282Y homozygotes with a hazard ratio of 1.69 (95% CI, 1.05-2.73). For other specific types of infection in C282Y homozygotes, risk was only increased at the P value <.05 level for skin infection (hazard ratio, 1.50; 95% CI, 1.10-2.03; P = .009), although pneumonia (1.26; 95% CI, 0.92-1.72; P = .15), urinary tract infection (hazard ratio, 1.20; 95% CI, 0.78-1.84; P = .41), and endocarditis (hazard ratio, 2.98; 95% CI, 0.95-9.30; P = .06) may also contribute to the increased risk of any infection (supplemental Figure 11). Risk of any bloodstream infection was not significantly increased at the P value <.05 level for C282Y homozygotes (hazard ratio, 1.31; 95% CI, 0.72-2.36; P = .38); however, statistical power was limited, because only 11 C282Y homozygotes had bloodstream infections (supplemental Figure 12). When stratified by sex, the risk estimates for any infection were more pronounced for C282Y homozygous women than men (hazard ratio, 1.68; 95% CI, 1.32-2.14, for C282Y homozygous women compared with noncarrier women; hazard ratio, 1.15; 95% CI, 0.86-1.53, for homozygous men compared with noncarrier men; P for interaction with sex = .03; supplemental Figure 13). Risk of any infection (hazard ratio, 0.99; 95% CI, 0.90-1.08) was not increased in compound heterozygous individuals (C282Y/H63D; Figure 5).
Risk of any infection, pneumonia, sepsis, and death from infectious disease according to hemochromatosis genotypes C282Y and H63D. ΔIron, ΔTransferrin saturation, and ΔFerritin indicate the mean difference as percentage of iron, transferrin saturation, and ferritin, respectively, compared with that of noncarrier/noncarrier individuals. Dots indicate hazard ratio; solid vertical lines indicate 95% CI. C282Y/C282Y, homozygous for the C282Y variant; C282Y/H63D, compound heterozygous for the C282Y and H63D variants; C282Y/noncarrier, heterozygous for the C282Y variant; H63D/H63D, homozygous for the H63D variant; H63D/noncarrier, heterozygous for the H63D variant; noncarrier/noncarrier, noncarrier for both C282Y and H63D.
Risk of any infection, pneumonia, sepsis, and death from infectious disease according to hemochromatosis genotypes C282Y and H63D. ΔIron, ΔTransferrin saturation, and ΔFerritin indicate the mean difference as percentage of iron, transferrin saturation, and ferritin, respectively, compared with that of noncarrier/noncarrier individuals. Dots indicate hazard ratio; solid vertical lines indicate 95% CI. C282Y/C282Y, homozygous for the C282Y variant; C282Y/H63D, compound heterozygous for the C282Y and H63D variants; C282Y/noncarrier, heterozygous for the C282Y variant; H63D/H63D, homozygous for the H63D variant; H63D/noncarrier, heterozygous for the H63D variant; noncarrier/noncarrier, noncarrier for both C282Y and H63D.
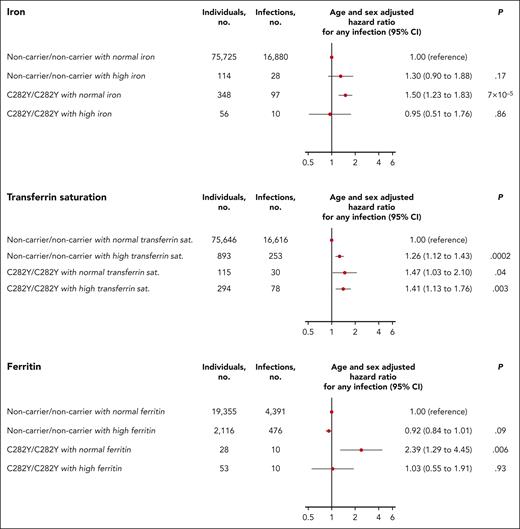
Clinical guidelines from several countries including Denmark and the United Kingdom recommend testing patients for the hemochromatosis variants C282Y and H63D only when both transferrin saturation and ferritin level are increased.8,10,11,33 If either transferrin saturation or ferritin is normal, testing for hemochromatosis is not usually recommended. Therefore, we examined the risk of infection in C282Y homozygotes not currently recommended for hemochromatosis genotype testing by stratifying C282Y homozygotes according to levels of iron, transferrin saturation, and ferritin at study enrollment. Surprisingly, the risk of any infection was increased in C282Y homozygotes with normal plasma iron (hazard ratio, 1.50; 95% CI, 1.23-1.83, for C282Y homozygotes with normal iron vs noncarrier individuals with normal iron), normal transferrin saturation (hazard ratio, 1.47; 95% CI, 1.03-2.10), or normal ferritin (hazard ratio, 2.39; 95% CI, 1.29-4.45; Figure 6).
Risk of any infection for C282Y homozygotes (C282Y/C282Y) compared with individuals noncarrier for both C282Y and H63D (noncarrier/noncarrier) stratified by levels of plasma iron, transferrin saturation, and ferritin at study enrollment. Normal iron was defined as iron from 9 to 34 μmol/L and high iron defined as iron >34 μmol/L. Normal transferrin saturation was defined as transferrin saturation from 10% to 45% for women aged <50 years and 15% to 45% for women aged >50 years and men regardless of age. High transferrin saturation was defined as transferrin saturation >45% regardless of sex and age. Normal ferritin was defined as ferritin from 12 to 200 μg/L for women and 12 to 300 μg/L for men. High ferritin was defined as ferritin >200 μg/L for women and >300 μg/L for men. Dots indicate hazard ratio; solid vertical lines indicate 95% CI. sat., saturation.
Risk of any infection for C282Y homozygotes (C282Y/C282Y) compared with individuals noncarrier for both C282Y and H63D (noncarrier/noncarrier) stratified by levels of plasma iron, transferrin saturation, and ferritin at study enrollment. Normal iron was defined as iron from 9 to 34 μmol/L and high iron defined as iron >34 μmol/L. Normal transferrin saturation was defined as transferrin saturation from 10% to 45% for women aged <50 years and 15% to 45% for women aged >50 years and men regardless of age. High transferrin saturation was defined as transferrin saturation >45% regardless of sex and age. Normal ferritin was defined as ferritin from 12 to 200 μg/L for women and 12 to 300 μg/L for men. High ferritin was defined as ferritin >200 μg/L for women and >300 μg/L for men. Dots indicate hazard ratio; solid vertical lines indicate 95% CI. sat., saturation.
A total of 89 C282Y homozygotes had a second blood sample for a repeat measurement of iron and transferrin saturation obtained at a median of 10 years after the first blood sample (which was obtained at study enrollment). Among the 89 C282Y homozygotes with repeat blood samples, 76 had a normal iron level at study enrollment, and 88.2% (n = 67) of these still had a normal iron level after a median of 10 years. Likewise, among 24 C282Y homozygotes with normal transferrin saturation at study enrollment, 62.5% (n = 15) still had normal transferrin saturation after a median of 10 years. No individuals had repeat measurements of ferritin performed.
The risk of any infection was increased in C282Y homozygotes without liver disease, diabetes, or heart failure diagnosed at any time before or after study enrollment (hazard ratio, 1.32; 95% CI, 1.07-1.64; supplemental Figure 14). When stratifying C282Y homozygotes according to whether or not they had been diagnosed with hemochromatosis at any time before or after study enrollment, we found similar risk estimates for any infection in C282Y homozygotes diagnosed with hemochromatosis (hazard ratio, 1.43; 95% CI, 0.94-2.17, when compared with noncarrier individuals) and in C282Y homozygotes never diagnosed with hemochromatosis (hazard ratio, 1.39; 95% CI, 1.13-1.71; supplemental Figure 15).
To examine whether C282Y homozygotes also had increased risk of fatal infections, we studied the risk of death from infectious disease according to hemochromatosis genotype. After age and sex adjustment, individuals with C282Y homozygosity had an increased risk of death from infectious disease compared with noncarrier individuals (hazard ratio, 2.34; 95% CI, 1.41-3.90; Figure 5).
Discussion
In this study of 142 188 individuals from the general population, we found that high and low plasma iron and high and low transferrin saturation were associated with an increased risk of any infection. Furthermore, individuals with hemochromatosis C282Y homozygosity had an increased risk of any infection and a markedly increased risk of sepsis and death from infectious disease. Finally, despite normal levels of iron, transferrin saturation, or ferritin, C282Y homozygotes had a high risk of any infection. These are novel findings.
The increased risk of infection in individuals with both low and high iron indicates a U-shaped relationship between iron available in the bloodstream and the risk of infections. This could hypothetically be explained by increased proliferation of invading pathogens in iron-rich environments34 and decreased proliferation in individuals with low-normal levels of iron essential for various biochemical processes in the pathogen.2,34 Thus, decreasing levels of iron could possibly reduce the risk of infection until a certain low level at which iron-dependent lymphocyte and/or neutrophil proliferation and activation becomes impaired, theoretically increasing susceptibility to infections.2,35-39
Our finding that high levels of iron and high transferrin saturation were associated with increased risk of infections including increased risk of sepsis and bloodstream infections is novel. Our results contrasts a Norwegian study of 61 852 individuals from the general population of whom 1738 had bloodstream infections,3 because the Norwegian study observed a high risk of bloodstream infections in individuals with low levels of plasma iron or transferrin saturation but not in individuals with high levels. The different results between the Danish and Norwegian studies may be explained by higher statistical power in our study of 142 188 individuals, of whom 20 394 were hospitalized with any infection, and 3711 were hospitalized with sepsis after study enrollment.
Our finding that hemochromatosis C282Y homozygotes are at an increased risk of any infection and at a markedly increased risk of sepsis and death from infectious disease is likewise novel. That said, this finding is partly supported by a study based on the UK Biobank, examining the risk of pneumonia in 451 243 general population individuals genotyped for C282Y and H63D.13 In the UK study, increased risk of pneumonia was observed among men homozygous for C282Y but not among women, which has been proposed to be due to menstrual bleeding in women leading to less accumulation of iron.13,40 In contrast, when stratifying the analyses by sex, our study found lower risk estimates for any infection in C282Y homozygous men than in homozygous women, whereas we found a similarly increased risk of sepsis in C282Y homozygous men and women.
Our findings that even C282Y homozygous individuals with normal levels of iron, transferrin saturation, or ferritin had an increased risk of infection and that risk of infection was also high in C282Y homozygotes not diagnosed with organ damage (liver disease, diabetes, or heart failure) potentially caused by iron overload could perhaps influence decisions on which individuals should be tested for hereditary hemochromatosis. Clinical guidelines from several countries recommend testing patients for the HFE variants C282Y and H63D only when both transferrin saturation and ferritin level are increased.8,10,33 Therefore, our finding that even C282Y homozygous individuals with normal levels of iron, transferrin saturation, or ferritin had an increased risk of infection indicate that C282Y homozygotes not currently recommended for genetic testing had an increased risk of infection.
Mechanistically, the increased infection risk in C282Y homozygotes with normal levels of iron, transferrin, or ferritin and the especially high risk in C282Y homozygous women could hypothetically be explained by the lower levels of the hepcidin hormone in C282Y homozygotes. Hepcidin is the major hormone regulating iron uptake from the intestine, but it also carries antimicrobial properties.1,41 Impairment in inducing hepcidin through the HFE-dependent pathway in C282Y homozygotes could infer suboptimal response to infections regardless of each individual’s steady-state level of iron, transferrin saturation, or ferritin. Other potential mechanisms include HFE variants causing altered intracellular iron levels in macrophages, with potential importance in intracellular infections,42 or the increased infection risk might be due to a linked variant, since the genomic neighborhood of HFE contains several immune-related genes encoding antigen-presenting molecules and cytokines, including tumor necrosis factor α.43-45 However, we are not aware of any specifically linked variants, and all the above-mentioned hypothetical mechanisms need further investigation.
Strengths of this study include the large cohort, our comprehensive data on health and lifestyle, and the nationwide Danish registries without any losses to follow-up enabling detailed information on comorbidities and infectious disease hospitalizations. Among the limitations of our study is the observational nature when examining the risk of infection according to plasma iron, transferrin saturation, and ferritin. Because the maximum follow-up after study enrollment was 38 years, levels of iron, transferrin saturation, and ferritin for each individual could have changed between the time of measurement and the time of infection, which represents a potential weakness of the study. Furthermore, although the multivariable adjusted model included C-reactive protein and comorbidities, and we performed several stratified analyses excluding individuals with indices of conditions affecting iron parameters or risk of infection, we cannot rule out residual confounding, which limits interpretation of causality. Importantly, however, the association between hemochromatosis C282Y genotype and the risk of infections is likely causal, because genes are assorted at random in the gamete state, ensuing random distribution of confounding factors, making reverse causality impossible and confounding unlikely.
Further studies are needed to elucidate whether prophylactic interventions could limit severe infections in C282Y homozygotes. For example, wider use of pneumococcal vaccination or wider use of antibiotic treatment in case of fever may hypothetically limit the risk of sepsis and death from infections in C282Y homozygotes; however, this is speculative, and further studies are needed. The need for further studies is underlined by our finding that individuals with C282Y homozygosity diagnosed with hemochromatosis at a hospital at any time before or after study enrollment had similar risk of infection as C282Y homozygotes never diagnosed with hemochromatosis. Although the effect of therapeutic phlebotomy cannot directly be assessed in this observational study, our results might imply that the current treatment of hemochromatosis, in which phlebotomy is performed to reduce overall iron storage, may possibly not sufficiently reduce infection risk.
In summary, low and high iron and transferrin saturation were associated with increased infection risk. C282Y homozygotes had an increased risk of any infection, sepsis, and death from infections. Even C282Y homozygotes with normal iron, transferrin saturation, or ferritin, not currently recommended for genotyping, had increased infection risk.
Acknowledgments
This study was conducted using data from the Copenhagen City Heart Study, the Copenhagen General Population Study, and the Danish General Suburban Population Study. The authors thank all participants and staff of the studies. The authors also thank Jørgen Kurtzhals from the University of Copenhagen for his invaluable assistance in obtaining essential microbiology data for this study. Furthermore, the authors express their gratitude to the 3 anonymous reviewers for their helpful and constructive suggestions during the review process.
This work was supported by research grants from the Capital Region of Denmark, Karla og Verner Sørensens Almennyttige Fond, Beckett-Fonden, and the Independent Research Fund Denmark. The Copenhagen General Population Study and the Copenhagen City Heart Study are supported by the Danish Heart Foundation and Copenhagen University Hospital–Herlev and Gentofte. C.E. is partly funded by the Laboratory Medicine Endowment Fund of Boston Children’s Hospital.
Authorship
Contribution: M.M., A.G., and J.H. conceived and designed the study; M.M., B.G.N., C.E., S.E.B., and J.H. collected the data and assembled the databases; M.M., A.G., B.G.N., C.E., J.P., S.E.B., and J.H. analyzed and interpreted the data; M.M. and J.H. wrote the manuscript drafts; M.M., A.G., B.G.N., C.E., J.P., S.E.B., and J.H. performed manuscript revision, had full access to all data, and approved the final manuscript; and M.M. and J.H. accessed and verified the underlying data reported in the manuscript.
Conflict-of-interest disclosure: The authors declare no support from any commercial organization for the submitted work. A.G. has done consultancy for Pharmacosmos A/S; received research funding from Sanofi A/S; and received research funding and payment for consultancy/advisory board work from Novo Nordisk A/S. The remaining authors declare no competing financial interests.
Correspondence: Jens Helby, Department of Haematology, Copenhagen University Hospital, Rigshospitalet, Blegdamsvej 9, DK-2100 Copenhagen, Denmark; email: jens.helby.petersen.02@regionh.dk.
References
Author notes
Presented in abstract form at the European Hematology Association 2022 Hybrid Congress, Vienna, Austria, 9-17 June 2022.
Technical details and statistical code can be made available on request from the corresponding author, Jens Helby (jens.helby.petersen.02@regionh.dk).
Only through collaborative agreement or locally managed access can further data be made available for additional analysis.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal