Iron is crucial for host and pathogen. In this issue of Blood, Mottelson et al1 show that low or high plasma iron, and homozygosity for the hemochromatosis mutation C282Y, is associated with risk of infection in a cohort of >140 000 Danish people.
Genetic evidence demonstrates availability of iron in the body can determine the outcome of infection. Rare mutations in Tfrc that partially disable cellular iron uptake cause severe immunodeficiency,2 whereas pathogenic iron overload due to HFE hemochromatosis brings susceptibility to siderophilic bacteria.3 In these opposing and relatively extreme cases, either iron deficit to immune cells or overaccumulation of body iron predisposes to serious, even fatal, infections. How the spectrum of iron parameters across the general population relates to the likelihood of infection has been less certain, and is comprehensively explored by Mottelson et al.
Hepcidin regulates iron delivery into the circulation from recycling macrophages, hepatocytes, and duodenal enterocytes, and so determines body iron stores and compartmentalization of iron between plasma and organs. Hemochromatosis is an autosomal-recessive condition, wherein most affected individuals are homozygous for the p.Cys282Tyr variant in HFE, which results in reduced hepcidin production and high plasma iron.3 Although not all individuals homozygous for the p.Cys282Tyr variant develop hepatic iron overload,4 the genetic defect in HFE impairs hepcidin synthesis.
A recent study from the UK Biobank found that the risk for urinary tract and skin infections is higher among p.Cys282Tyr homozygous men, but trends toward higher infection rates were also found for other infections, including pneumonia, respiratory tract infections, and sepsis. These findings suggest that either the HFE gene variant itself or the associated impaired hepcidin-mediated iron regulation causes impaired immune function. The finding that the effects were more pronounced in men than in women suggest that the underlying mechanism is iron dependent.5
The study in this issue of Blood further supports the concept that in individuals homozygous for p.Cys282Tyr, the increased infection risk is associated with an imbalance in the availability of plasma iron. The study shows that hypoferremia and hyperferremia were both associated with an increased infection risk. This U-shaped relationship was even more pronounced when infection risk was analyzed with transferrin saturation, whereas the association with ferritin (a marker of iron stores, but affected by inflammation) was much less pronounced. These data suggest that the plasma iron compartment rather than total body iron is most relevant for this infection risk. In particular, the risk for pneumonia, sepsis, and death was significantly increased at a transferrin saturation (TSAT) of >45% to 50%, which well reflects the upper limit of normal. This threshold is lower than the reported cutoff at which non–transferrin-bound labile iron species (NTBI) become detectable in serum.6 The large patient numbers result in high statistical significance, but the interval between the time point when serum iron parameters were determined and the study end point poses a limitation, because the changes in serum iron parameters over time were only assessed in a subgroup of patients. In this subgroup, most study participants maintained fairly stable serum iron parameters. These results support findings from genome-wide association studies that show that serum iron parameters are genetically determined.
The strongest genetic determinant of serum iron parameters is HFE, and the present study shows that homozygosity for p.Cys282Tyr is also associated with an increased risk of infections. Further analysis showed an interaction between genetics and transferrin saturation, where patients with high TSAT and homozygosity for p.Cys282Tyr conferred a significantly higher infection risk. Nevertheless, and unexpectedly, p.Cys282Tyr homozygotes with normal TSAT, and without liver disease, diabetes, or heart failure, diagnosed at any time before or after study enrollment, and regardless of whether they had been diagnosed with hemochromatosis, also all had increased risk of infection.
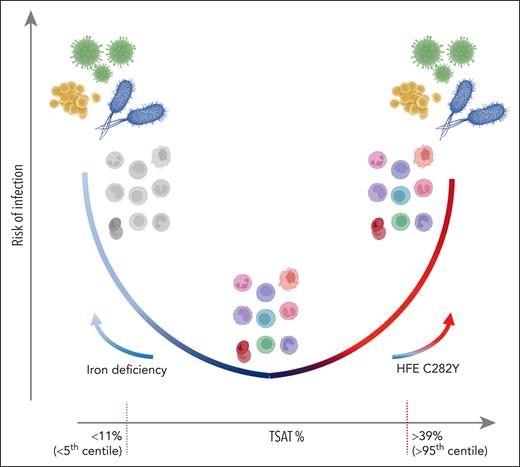
These findings raise several issues. It is not clear why both low and high plasma iron confer risk of infection (see figure). For high iron, this is likely due to increased availability of iron for pathogens, allowing them to colonize rapidly and overwhelm host immunity; however, an important unknown is how TSAT relates to iron concentrations and availability (including NTBI) in tissue, interstitial fluid, and barrier sites, such as the skin (many recoded infections were dermatological). Notably, the increased risk of p.Cys282Tyr homozygotes with normal TSAT implies control of plasma iron fluxes, including in response to infection, and not plasma iron concentration per se, is critical. This idea supports an emerging concept that hemochromatosis itself is more characterized by deregulation of plasma iron than by iron overload. Accordingly, classification of hemochromatosis has been recently revised to highlight that hemochromatosis is a disease of hepcidin deficiency7 and loss of control of plasma iron homeostasis. An insightful study has shown that high TSAT is also associated with increased fatigue and more days absent from work.8 Plausibly, low TSAT could lead to susceptibility to infections by impairing host innate and adaptive immune functions, a concept supported by work in mice,9 but which needs additional investigations in humans. Specifically, which immune functions are impaired below which TSAT thresholds are unknown, and whether this predisposes to certain pathogens (presumably not siderophilic bacteria) is unclear.
Schematic of risk of infection vs TSAT, with values given as indicative of <5th and >95th centile, based on Mottelson et al. At low TSAT, driven by iron deficiency, immune dysfunction (colorless cells) may lead to infection susceptibility. At higher TSAT or unregulated plasma iron concentrations, potentially caused by homozygosity for HFE C282Y, increased iron availability may favor pathogen outgrowth despite iron supply to immune cells. Stable and regulated TSAT in the normal range is associated with immune control of infection.
Schematic of risk of infection vs TSAT, with values given as indicative of <5th and >95th centile, based on Mottelson et al. At low TSAT, driven by iron deficiency, immune dysfunction (colorless cells) may lead to infection susceptibility. At higher TSAT or unregulated plasma iron concentrations, potentially caused by homozygosity for HFE C282Y, increased iron availability may favor pathogen outgrowth despite iron supply to immune cells. Stable and regulated TSAT in the normal range is associated with immune control of infection.
In terms of patient management, the work from Mottelson et al indicates that phlebotomy as the cornerstone treatment of hemochromatosis could evolve in the context of novel therapies that could suppress TSAT in hemochromatosis to protect against infections. Such strategies are available with the hepcidin mimetic rusfertide, TMPRSS6 inhibition, or base editing to correct the genetic defect in HFE. Beyond iron overload, the present work is also a strong reminder that iron deficiency should be corrected not only to prevent anemia but to ensure defense against infection. Defects in iron homeostasis and iron delivery to immune cells can associate with chronic sequelae in the context of inflammation (eg, with severe acute respiratory syndrome coronavirus 2 and long COVID-19).10 Therefore, to ensure health, plasma iron needs stability at the bottom of the U-shaped curve.
Conflict-of-interest disclosure: H.D. has received grant support from Procter & Gamble and consultancy fees from Pharmacosmos. H.Z. has received consultancy fees from Novo Nordisk and Pharmacosmos.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal