In this issue of Blood, Brinkmann et al investigated the potential benefits of combined administration of CD20-targeting bispecific antibodies (CD20-BsAbs) together with CD19-directed chimeric antigen receptor (CAR) T cells by coculturing bispecific antibodies and CAR T cells with primary samples from various lymphoma types as well as coadministration in an immunocompetent murine chronic lymphocytic leukemia (CLL) model.1 The authors report that the addition of CD20-BsAbs supports CD19-directed CAR T cells by promoting CAR T-cell expansion and enhances the activity of endogenous T cells. These observations are remarkable and may challenge the design of current clinical trials, for example, in multiple myeloma, investigating the sequential use of CAR T-cell treatment followed by BsAb treatment.2 Instead, they propose a rational for the combined use of the 2 modalities.
The introduction of CAR T cells directed against the B-cell lineage marker CD19 has led to improved outcomes for patients with relapsed or refractory (r/r) B-cell neoplasms.3,4 A number of CD19 CAR T-cell products have been registered for such patients, including tisagenlecleucel, axicabtagene ciloleucel, and lisocabtagene maraleucel. Despite high initial response rates within the first months after treatment, B-cell malignancies relapse in more than half of the patients following CAR T-cell treatment. Therefore, enhancement of the procedure is an unmet clinical need for patients who relapse or fail to respond to treatment. A combined effort against different B-cell antigens, for example, CD19 and CD20, may improve the efficacy of CAR T-cell therapy. CAR T cells targeting in parallel CD19 and CD20 may later be shown to be helpful, but CAR T-cell production is time-consuming and CAR T-cell expansion of such dual constructs has been problematic.
Reasons for failing CAR T-cell treatment have been associated with a variety of factors. They include (among others) loss of target antigen expression in the B-cell malignancies, molecular alterations of the target such as CD19 polymorphisms, abundance of target presentation leading to exhaustion of the CAR T-cell product, and the varying composition of the CAR T-cell product itself due to the previous treatment.5-7
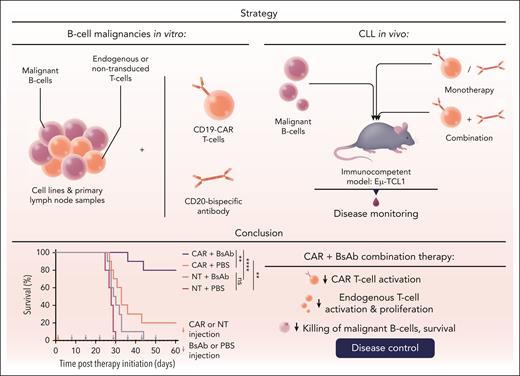
A conditio sine qua non, however, of effective CAR T-cell therapy is the requirement of a sufficient expansion and persistence of effective CAR T cells.8 Increasing the number and boosting the kinetics of circulating and tumor-infiltrating CAR T cells has therefore been a matter of ongoing research in the field. Consequently, the findings of Brinkmann et al are remarkable as they suggest that coadministering CAR T-cell therapy and BsAb treatment may both enhance the expansion of CAR T cells while also increasing the activity of endogenous T cells. This was observed when CD20-BsAbs were added to cocultures of CD19-CAR and primary B-cell malignancies, and also in an immunocompetent mouse model of CLL. In the mouse model, relapse was frequently observed after CD19 CAR T-cell monotherapy, whereas the combination with CD20 BsAbs started 1 week after CAR T-cell infusion resulted in a cure rate of 80% of the mice 8 weeks after start of treatment (see figure).
Combined administration of CD20-BsAbs with CD19-directed CAR T-cell treatment was investigated in primary samples from lymphoma types and in an immunocompetent murine CLL model. The addition of CD20-BsAbs supports CD19-directed CAR T cells by promoting CAR T-cell expansion and enhances the activity of endogenous T cells. NT, nontransduced T cells; PBS, phosphate-buffered saline. Professional illustration by Somersault18:24.
Combined administration of CD20-BsAbs with CD19-directed CAR T-cell treatment was investigated in primary samples from lymphoma types and in an immunocompetent murine CLL model. The addition of CD20-BsAbs supports CD19-directed CAR T cells by promoting CAR T-cell expansion and enhances the activity of endogenous T cells. NT, nontransduced T cells; PBS, phosphate-buffered saline. Professional illustration by Somersault18:24.
These results are supported by previous observations in patients with lymphoma that the sequential use of BsAbs after CAR T-cell therapy can enhance levels of circulating CAR T cells. However, the clinical significance of the finding remained unclear.9 Here, Brinkmann et al found that the levels of increased CAR T-cell expansion after simultaneous use of anti-CD19 CAR T-cell therapy and anti-CD20-directed BsAbs did lead to improved outcomes in a murine model compared with single use of CAR T-cell therapy or BsAb treatment. These findings also support the increasing perception that efficacy of CAR T-cell therapy is not impaired by (previous) BsAb treatment in diffuse large B-cell lymphoma (DLBCL), and the opposite (ie, improved) might eventually be shown to be the case.10
Targeting more than 1 antigen (CD19 and CD20) has become a promising approach to increase the efficacy of CAR T-cell therapy in patients with B-cell malignancies. Open questions regarding this approach are both the sequencing and the mechanistic mode of such dual targeting. Options include dual targeting using modified CAR T-cell constructs, antibody targeting with constructs directed against more than 1 epitope, and combinations of BsAbs and CAR T-cell treatment, given simultaneously or sequentially. The sequencing of CAR T-cell therapy followed by BsAb treatment is currently being studied in trials for patients with r/r myeloma and DLBCL. This strategy reflects the tendency of oncologists to administer the modality presumed to be most effective (CAR T cells) followed by what else is available. The work of Brinkmann et al may lead to reconsidering whether the sequential use of CAR T-cell therapy followed by BsAbs is the most effective approach of combining these 2 modalities, which may require a head-to-head comparison trial to determine the optimum sequencing.
A number of questions arise from such a concept: CAR T-cell therapy provides a definite cure for up to 40% of patients with r/r B-cell malignancies. The additional treatment with BsAbs would lead to overtreatment of such patients and presumably expose them to increased toxicities. However, identifying these patients in advance remains an unresolved issue. Also, one may speculate that the infection risk after such combined use of CAR T cells and BsAbs may be increased as compared with CAR T-cell monotherapy due to an even deeper or longer B-cell depletion. Moreover, cytokine release syndrome or immune effector cell–associated neurotoxicity may be more pronounced after the combined approach. Finally, the economic burden arising from the combined use of CAR T cells and BsAbs should be considered, which is a very complex calculation. The clear rational for combined therapy trials has been established by the pioneer work of Brinkmann et al.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal