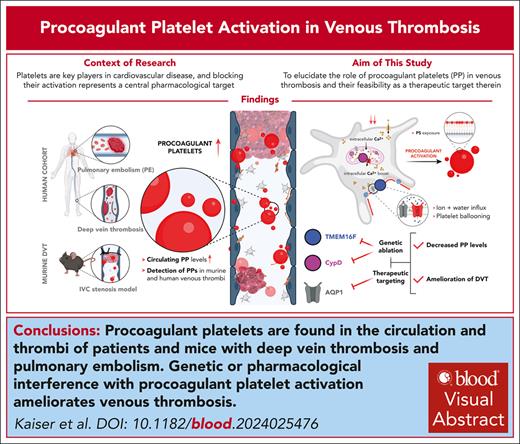
Key Points
Procoagulant platelets are found in the circulation and thrombi in patients and mice with DVT and PE.
Genetic or pharmacologic interference with procoagulant platelet activation ameliorates DVT formation.
Visual Abstract
Platelets are key players in cardiovascular disease, and platelet aggregation represents a central pharmacologic target, particularly in secondary prevention. However, inhibition of adenosine diphosphate and thromboxane signaling has low efficacy in preventing venous thromboembolism, necessitating the inhibition of the plasmatic coagulation cascade in this disease entity. Anticoagulation carries a significantly higher risk of bleeding complications, highlighting the need of alternative therapeutic approaches. We hypothesized that procoagulant activation (PA) of platelets promotes venous thrombus formation and that targeting PA could alleviate venous thrombosis. Here, we found elevated levels of procoagulant platelets in the circulation and in thrombi of patients with deep vein thrombosis (DVT) and pulmonary embolism, and in mice developing DVT following inferior vena cava stenosis. Furthermore, we detected PA of recruited platelets within murine venous thrombi and human pulmonary emboli. Mice with platelet-specific deficiency in central pathways of PA—cyclophilin D and transmembrane protein 16F—were more resistant toward low flow–induced venous thrombosis. Finally, we found that a clinically approved carbonic anhydrase inhibitor, methazolamide, reduced platelet procoagulant activity and alleviated murine thrombus formation without affecting trauma-associated hemostasis. These findings identify an essential role of platelet procoagulant function in venous thrombosis and delineate novel pharmacologic strategies targeting platelets in the prevention of venous thromboembolism.
Introduction
Deep vein thrombosis (DVT) and concomitant pulmonary embolism (PE) are among the most frequent cardiovascular diseases. Affecting >1 million individuals annually in the United States alone, venous thrombosis and thromboembolism account for a significant burden of cardiovascular morbidity and mortality.1 Both plasmatic coagulation cascade and platelets significantly contribute to thrombus development.2 Although previous work has shown the early local recruitment of platelets in venous thrombus generation, it is unclear whether distinct platelet substates differentially impact on DVT initiation and progression in vivo.3 Clinically, anticoagulation is at the center of therapy and prevention in DVT, with classic antiplatelet therapeutics being rarely used despite evidence suggesting clinical utility of platelet inhibition in preventing venous thrombosis.4,5 Although their antithrombotic efficacy is well documented, anticoagulants carry a significant (and compared with platelet inhibition increased) risk of bleeding, necessitating the development of alternative therapeutic strategies.6
Procoagulant platelets (PPs) constitute a highly activated subset of the platelet pool. Procoagulant activation (PA) occurs when platelets are simultaneously stimulated with canonical agonists, like thrombin and glycoprotein (GP)VI ligands, are exposed to high levels of mechanical shear stress, or encounter inflammation-associated cues, like neutrophil-derived S100A8/9 or immune complexes.7-10 Functionally, PPs act as a cellular bridge between primary and secondary hemostasis: Following stimulation, these platelets exhibit supramaximal calcium levels, undergo a morphologic change by taking on a balloon-like shape, and expose high amounts of the negatively charged phospholipid phosphatidylserine (PS) on their surface.7 PS exposure then promotes binding of calcium and other procoagulant factors, like fibrin(ogen), thrombin, and tissue factor.11 Cyclophilin D (CypD) and transmembrane protein 16F (TMEM16F) critically contribute to PP formation through regulating cytosolic calcium levels and the scrambling of PS from the inner to the outer plasma membrane leaflet, respectively.11,12 PPs have been implicated in arterial thrombosis, as well as in thromboinflammation and inflammatory hemostasis.9,13-15 However, the role of this subset in venous thromboembolism remains unclear.
Study design
Detailed methods are provided in the supplemental Data, available on the Blood website.
Flow cytometry of human whole blood
Patients with suspected DVT or PE, as assessed by clinical estimation and elevated D-dimer levels, presenting to the emergency department of Ludwig Maximilian University Hospital were prospectively recruited if either computed tomography pulmonary angiography or duplex compression sonography was scheduled to confirm the suspected diagnosis by imaging. Blood was drawn by phlebotomy into heparinized tubes. All study participants provided written informed consent in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committee of Ludwig Maximilian University Munich (number 20-0809). Participants were not compensated. Blood samples were stained with fluorescent antibodies (supplemental Table 1), lysed with BD fluorescence-activated cell sorting lysing buffer without paraformaldehyde, and run on a BD LSRFortessa. PPs were defined as triple-positive for CD41, PS, and P-selectin/CD62P, according to International Society on Thrombosis and Haemostasis, Inc guidelines16 (supplemental Figure 1A-B provides gating strategies).
Murine DVT model
DVT was induced by partial ligation of the inferior vena cava (IVC), as described previously.3,17 Briefly, mice were anesthetized using medetomidine/midazolam/fentanyl, and the IVC was exposed and partially ligated below the renal veins using a surgical suture and a placeholder (diameter, 0.5 mm), which was removed after ligation to allow for residual IVC flow across the stenosis. After 48 hours, mice were sacrificed after facial blood collection, and both thrombus incidence and thrombus weight were assessed. Pharmacologic blockade of platelet PA was achieved by intraperitoneal injections of 20 mg/kg body weight twice daily of the carbonic anhydrase inhibitor methazolamide (MZA; SML0720, Sigma) solved in sterile phosphate-buffered saline, beginning 12 hours before surgery.
Live imaging of stenosis-induced thrombi in the IVC
Mice were anaesthetized using medetomidine/midazolam/fentanyl and injected with platelet-labeling antibodies (×488, emfret), the mC1-multimer (15 μL, corresponding to 7 μg), and fluorescent fibrinogen (AF546 coupled; ThermoFisher), as previously described.13,18,19 For imaging the IVC, a surgical suture (including a 0.5-mm placeholder) was placed below the renal veins after exposing the IVC, as described above. Resulting thrombi were live imaged using an upright Zeiss LSM 980 confocal/2-photon microscope in superresolution mode (20×/1.0 differential interference contrast [free working distance = 1.8 mm], water immersion) between 3 to 4 hours following suture placement. PPs were identified by staining positive for both CD42b and PS and a balloon-like shape, as previously described.13
Results and discussion
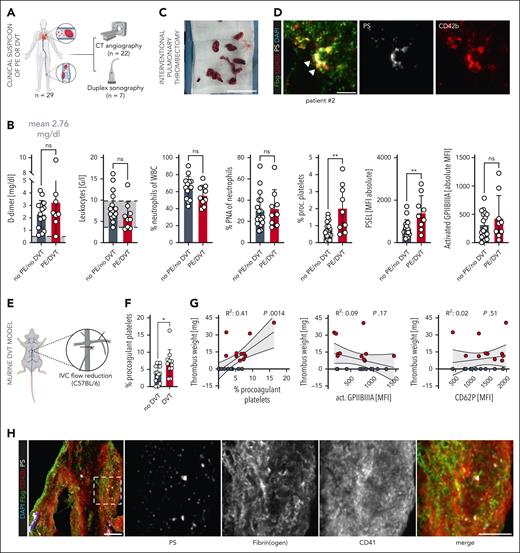
The activation state of circulating platelets has diagnostic and prognostic implications in cardiovascular disease.20 To investigate platelet activation in venous thromboembolism, we performed flow cytometry on blood samples of patients who were scheduled for either computed tomographic angiography (n = 22 patients) or Doppler sonography (n = 7 patients) due to clinically suspected DVT and/or PE and elevated D-dimer levels (mean D-dimer, 2.76 mg/dl; n = 29) (Figure 1A-B, Table 1). We tested classic platelet activation markers, including activated GPIIBIIIA and P-selectin (CD62P), and specifically the fraction of circulating PPs (as assessed by CD41+ CD62P+ PS+ platelets16) (supplemental Figure 1A-B). Although we found no significant differences in clinical parameters, including D-dimer and C-reactive protein levels, as well as platelet activation markers, like surface GPIIBIIIA activation, patients with confirmed DVT or PE (n = 9) had significantly higher PP levels compared with those without evidence of thrombosis on computed tomographic angiography or sonography studies (Figure 1B; supplemental Figure 1C). We next stained human pulmonary emboli that had been retrieved using a transfemoral mechanical thrombectomy device (n = 5) (supplemental Figure 2A-B). All thromboemboli stained not only positive for CD41/CD42b and fibrin(ogen), but also showed accumulation of PS+ platelets (Figure 1C-D; supplemental Figure 2C-F; supplemental Table 2).
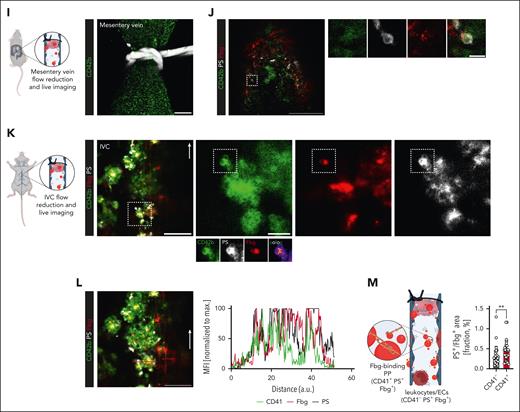
Procoagulant platelet activation is a hallmark of venous thrombosis in human individuals and mice. (A) Scheme of clinical cohort and performed imaging entities to confirm DVT or PE. (B) Quantification of systemic D-dimer levels, leukocyte counts, percentage neutrophils of leukocytes, percentage platelet-neutrophil aggregates (PNA) of circulating neutrophils, circulating procoagulant platelets (PPs), and platelet surface expression of activated GPIIBIIIA and P-selectin in patients with suspected DVT or PE, comparing those without (black) vs those with confirmed thrombosis or thromboembolism (red). Student t-test, 2-tailed, unpaired. Gray boxes indicate normal range. (C) Representative specimen of interventionally retrieved human pulmonary embolus (white line) that was retrieved using a transfemoral mechanical thrombectomy device. Scale bar = 5 cm. See supplemental Figure 2A and supplemental Video 1 for further imaging data on the same patient. (D) Representative IF staining of a human pulmonary embolus retrieved through interventional thrombectomy and stained for CD42b, fibrinogen, and phosphatidylserine (PS). Scale bar = 10 μm (left) and 3 μm (right panel). See supplemental Figure 2C-D for individual channels, further examples, and unstained controls. (E) Experimental scheme of inferior vena cava (IVC) stenosis model. (F) Quantification of circulating PPs in wild-type mice undergoing the stenosis-induced vena cava thrombosis model, comparing mice without vs those with evidence of caval thrombosis. Student t-test, 2-tailed, unpaired. (G) Linear regression analysis to correlate thrombus weight in mice with percentage circulating PPs or the indicated platelet surface markers. (H) Representative IF staining of a murine IVC thrombus stained for CD42b, fibrinogen, and PS. The highlighted, framed area is shown in split channels on the right-hand side. Scale bars = 25 μm (left panel), 15 μm (other panels). See supplemental Figure 2G for unstained controls. (I) Scheme of flow reduction–induced mesenteric vein thrombosis and live-imaging approach and representative confocal overview (generated through maximum intensity projection) of a ligated mesenteric vein; the white silk thread is visualized in the upper part of the image. Scale bar = 50 μm. (J) Representative confocal image of a developing thrombus in a mesenteric vein 1 hour after flow reduction. Right: split channels showing a procoagulant platelet derived from the highlighted area (white dashed line) of the developing thrombus. Scale bars = left 50, right 5 μm. See supplemental Video 2 for correlating live microscopy. (K) Scheme of flow reduction–induced IVC thrombosis and live-imaging approach as well as representative confocal overview of a developing thrombus. Right: split channels showing a procoagulant platelet derived from the highlighted area (white dashed line) of the developing thrombus. Micrographs below the CD42b channel highlight the colocalization of CD42b and PS along with local binding of fibrin(ogen). Pseudocolored image depicts areas of colocalization between CD42b and PS. Scale bars = left 50, right 10 μm. White arrow indicates the direction of blood flow. Scale bar = 50 μm. (L) Representative confocal image of a developing thrombus in an IVC ≈3 hours after flow reduction. Scale bar = 50 μm. See supplemental Video 3 for correlating live microscopy. Graph to right: normalized intensity profiles of CD41, fibrin(ogen), and PS, corresponding to red line in the image to the left. (M) Explanatory scheme and quantification of triple-positive areas vs Fbg/PS-only positive areas in IVC thrombi, representing fibrin(ogen) recruitment of procoagulant platelets vs fibrin(ogen) recruitment by leukocytes and/or endothelial cells. Individual data points correspond to randomly acquired images of IVC thrombi (n = 30) derived from n = 4 mice. Unless otherwise stated, all statistical tests were Student t-test, 2-tailed, unpaired. P values corresponding to asterisks: ∗P < .05, ∗∗P < .01, ∗∗∗P < .005.
Procoagulant platelet activation is a hallmark of venous thrombosis in human individuals and mice. (A) Scheme of clinical cohort and performed imaging entities to confirm DVT or PE. (B) Quantification of systemic D-dimer levels, leukocyte counts, percentage neutrophils of leukocytes, percentage platelet-neutrophil aggregates (PNA) of circulating neutrophils, circulating procoagulant platelets (PPs), and platelet surface expression of activated GPIIBIIIA and P-selectin in patients with suspected DVT or PE, comparing those without (black) vs those with confirmed thrombosis or thromboembolism (red). Student t-test, 2-tailed, unpaired. Gray boxes indicate normal range. (C) Representative specimen of interventionally retrieved human pulmonary embolus (white line) that was retrieved using a transfemoral mechanical thrombectomy device. Scale bar = 5 cm. See supplemental Figure 2A and supplemental Video 1 for further imaging data on the same patient. (D) Representative IF staining of a human pulmonary embolus retrieved through interventional thrombectomy and stained for CD42b, fibrinogen, and phosphatidylserine (PS). Scale bar = 10 μm (left) and 3 μm (right panel). See supplemental Figure 2C-D for individual channels, further examples, and unstained controls. (E) Experimental scheme of inferior vena cava (IVC) stenosis model. (F) Quantification of circulating PPs in wild-type mice undergoing the stenosis-induced vena cava thrombosis model, comparing mice without vs those with evidence of caval thrombosis. Student t-test, 2-tailed, unpaired. (G) Linear regression analysis to correlate thrombus weight in mice with percentage circulating PPs or the indicated platelet surface markers. (H) Representative IF staining of a murine IVC thrombus stained for CD42b, fibrinogen, and PS. The highlighted, framed area is shown in split channels on the right-hand side. Scale bars = 25 μm (left panel), 15 μm (other panels). See supplemental Figure 2G for unstained controls. (I) Scheme of flow reduction–induced mesenteric vein thrombosis and live-imaging approach and representative confocal overview (generated through maximum intensity projection) of a ligated mesenteric vein; the white silk thread is visualized in the upper part of the image. Scale bar = 50 μm. (J) Representative confocal image of a developing thrombus in a mesenteric vein 1 hour after flow reduction. Right: split channels showing a procoagulant platelet derived from the highlighted area (white dashed line) of the developing thrombus. Scale bars = left 50, right 5 μm. See supplemental Video 2 for correlating live microscopy. (K) Scheme of flow reduction–induced IVC thrombosis and live-imaging approach as well as representative confocal overview of a developing thrombus. Right: split channels showing a procoagulant platelet derived from the highlighted area (white dashed line) of the developing thrombus. Micrographs below the CD42b channel highlight the colocalization of CD42b and PS along with local binding of fibrin(ogen). Pseudocolored image depicts areas of colocalization between CD42b and PS. Scale bars = left 50, right 10 μm. White arrow indicates the direction of blood flow. Scale bar = 50 μm. (L) Representative confocal image of a developing thrombus in an IVC ≈3 hours after flow reduction. Scale bar = 50 μm. See supplemental Video 3 for correlating live microscopy. Graph to right: normalized intensity profiles of CD41, fibrin(ogen), and PS, corresponding to red line in the image to the left. (M) Explanatory scheme and quantification of triple-positive areas vs Fbg/PS-only positive areas in IVC thrombi, representing fibrin(ogen) recruitment of procoagulant platelets vs fibrin(ogen) recruitment by leukocytes and/or endothelial cells. Individual data points correspond to randomly acquired images of IVC thrombi (n = 30) derived from n = 4 mice. Unless otherwise stated, all statistical tests were Student t-test, 2-tailed, unpaired. P values corresponding to asterisks: ∗P < .05, ∗∗P < .01, ∗∗∗P < .005.
Patient characteristics
| Clinical characteristics . | No DVT/no PE (n = 20) . | Confirmed DVT/PE (n = 9) . |
|---|---|---|
| Age, y | 63.94 (24.87) | 67.33 (17.51) |
| % Female | 50 | 44.4 |
| Wells score (PE) | 0.50 (0.62) | 1.22 (0.44) |
| Wells score (DVT) | 0.44 (0.62) | 1.33 (1.12) |
| % on antiplatelet therapy (aspirin) | 15 | 33.3 |
| % on antiplatelet therapy (P2Y12 antagonist) | 0 | 0 |
| % with coronary artery disease | 15 | 33.3 |
| % with previous stroke | 10 | 22.2 |
| % on anticoagulation | 20 | 11.1 |
| Laboratory values | ||
| High-sensitivity troponin T, ng/ml | 0.067 (0.07) | 0.098 (0.19) |
| D-dimer, mg/ml | 2.15 (1.02) | 3.96 (2.89) |
| Leukocyte counts, G/l | 8.64 (3.64) | 7.61 (3.06) |
| Neutrophil counts, G/l | 6.77 (3.56) | 4.86 (1.77) |
| Platelet counts, G/l | 240 (72.56) | 233.38 (38.40) |
| C-reactive protein, mg/dl | 3.26 (5.32) | 1.27 (0.93) |
| Hemoglobin, g/dl | 12.71 (2.04) | 12.89 (1.20) |
| Clinical characteristics . | No DVT/no PE (n = 20) . | Confirmed DVT/PE (n = 9) . |
|---|---|---|
| Age, y | 63.94 (24.87) | 67.33 (17.51) |
| % Female | 50 | 44.4 |
| Wells score (PE) | 0.50 (0.62) | 1.22 (0.44) |
| Wells score (DVT) | 0.44 (0.62) | 1.33 (1.12) |
| % on antiplatelet therapy (aspirin) | 15 | 33.3 |
| % on antiplatelet therapy (P2Y12 antagonist) | 0 | 0 |
| % with coronary artery disease | 15 | 33.3 |
| % with previous stroke | 10 | 22.2 |
| % on anticoagulation | 20 | 11.1 |
| Laboratory values | ||
| High-sensitivity troponin T, ng/ml | 0.067 (0.07) | 0.098 (0.19) |
| D-dimer, mg/ml | 2.15 (1.02) | 3.96 (2.89) |
| Leukocyte counts, G/l | 8.64 (3.64) | 7.61 (3.06) |
| Neutrophil counts, G/l | 6.77 (3.56) | 4.86 (1.77) |
| Platelet counts, G/l | 240 (72.56) | 233.38 (38.40) |
| C-reactive protein, mg/dl | 3.26 (5.32) | 1.27 (0.93) |
| Hemoglobin, g/dl | 12.71 (2.04) | 12.89 (1.20) |
Data are given as mean (standard deviation) unless otherwise indicated.
Next, we assessed the phenotype of platelets in a murine model of venous thrombosis, the IVC stenosis model.3 IVC stenosis mice that formed thrombi had higher PP levels compared with those without thrombus development (Figure 1E-F). Circulating PP levels positively correlated with thrombus weight, a finding that was not apparent for other canonical platelet activation markers, like GPIIBIIIA (Figure 1G). Corresponding to our findings derived from human venous emboli, murine vena cava thrombi stained positive not only for CD41 and fibrinogen, but also showed accumulation of PS+ platelets (Figure 1H). These findings indicate a role for PPs in venous clot formation.
We next used a new model of flow reduction–induced mesenterial vein thrombosis as well as confocal live imaging of the IVC stenosis model to assess the early dynamics of venous thrombus formation and the contribution of PPs (Figure 1I-M; supplemental Videos 2 and 3). Early platelet recruitment was a hallmark of thrombus formation both in mesenteric veins and the IVC (Figure 1J-L). Furthermore, we detected the local accumulation of balloon-shaped, PPs that recruited fibrin(ogen) to their surface, as previously described for PPs in vivo13 (Figure 1J-K; supplemental Figure 3A-F). Consistent with previous findings,21 fibrin(ogen) was also deposited on the endothelium in the absence of platelets, whereas endothelial cells and leukocytes also partially stained positive for PS, the colocalization of platelets, PS, and fibrinogen surpassed that of PS and fibrinogen alone, indicating PPs as a substantial factor for the local recruitment of coagulation factors in DVT (Figure 1L-M; supplemental Figure 3E-F).
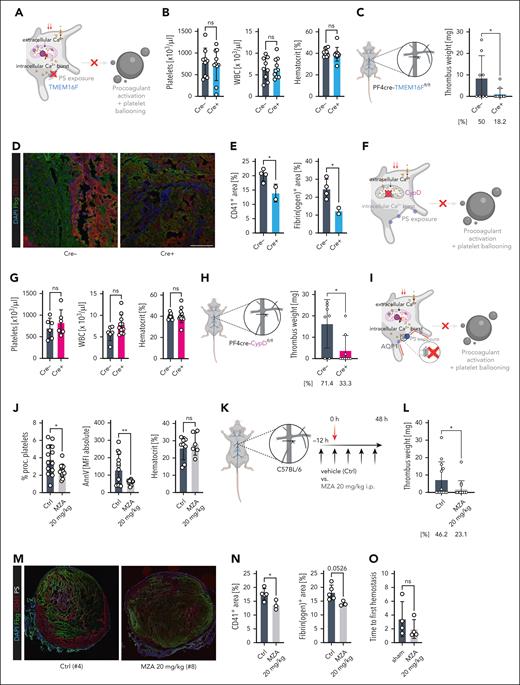
Given the positive correlation of platelet PA and venous thrombosis in both patients and mice, we reasoned that PA might contribute to thrombus development in DVT. Thus, we subjected mice with platelet- and megakaryocyte-specific genetic ablation of transmembrane protein 16F (PF4cre-TMEM16Ffl/fl) to our IVC stenosis model (Figure 2A-E; supplemental Figure 4A-B). TMEM16F is a multifunctional transmembrane scramblase necessary for the final step of PA (ie, flipping the negatively charged PS to the outer layer of the cell membrane),11-13 thus rendering TMEM16F-deficient platelets unable to form this procoagulant surface13 (Figure 2A; supplemental Figure 4A). We found no difference in platelet, neutrophil, or monocyte counts and no differences in hemoglobin levels between Cre-positive and negative animals (Figure 2B; supplemental Figure 5A-C). The weights of collected thrombi were significantly reduced in TMEM16F-deficient mice, suggesting a crucial contribution of PA in venous thrombosis (Figure 2C). Furthermore, the thrombus incidence in PF4cre-TMEM16Ffl/fl mice declined from 50% to 18.2% (Figure 2C). Thrombi collected from TMEM16F-deficient mice were not only characterized by reduced size and weight, but also reduced relative fibrin(ogen) content, suggesting that recruitment of the coagulation cascade and subsequent consolidation of the venous clot rely on procoagulant platelets (Figure 2D-E; supplemental Figure 5D-E). Neither leukocyte or neutrophil counts nor neutrophil extracellular trap formation differed between Cre-negative and Cre-positive animals, suggesting a limited impact of PP-driven leukocyte activation or recruitment on venous thrombus formation in this model (supplemental Figure 5F-G). We confirmed our findings in a second model of genetic PA deficiency, the platelet-specific cyclophilin D-deficient mouse line PF4cre-CypDfl/fl (supplemental Figure 4A-B): Although there was no effect on platelet, monocyte, or neutrophil counts, thrombus incidence declined in CypD-deficient mice from 71.4% to 33.3% (Figure 2F-H; supplemental Figure 5H). Furthermore, CypD deficiency resulted in a significant reduction of thrombus weights (Figure 2H).
Genetic and pharmacologic inhibition of platelet PA alleviates venous thrombus formation. (A) Schematic of affected signaling pathways of platelet PA in PF4cre-TMEM16Ffl/fl mice. (B) Cell counts of peripheral blood for the indicated cell types and hematocrit quantification. Student t-test, 2-tailed, unpaired. (C) Experimental scheme of DVT in PF4cre-TMEM16Ffl/fl mice as well as thrombus weight (plotted) and incidence (percentage below graph) for n = 11 Cre– and n = 14 Cre+ PF4cre-TMEM16F mice. (D) Representative confocal image of IVC thrombi retrieved from Cre– or Cre+ mice. Scale bar = 100 μm. See supplemental Figure 5D-E for unstained examples of the same thrombi. (E) Quantification of PS-, CD41-, and Fbg-positive areas relative to total thrombus area. (F). Schematic of affected signaling pathways of platelet PA in PF4cre-CypDfl/fl mice. (G) Cell counts of peripheral blood for the indicated cell types and hematocrit quantification. (H) Experimental scheme of DVT in PF4cre-CypDfl/fl mice as well as thrombus weight (plotted) and incidence (percentage below graph) for n = 8 Cre– and n = 13 Cre+ PF4cre-CypD mice. (I) Schematic of affected signaling pathways of platelet PA in wild-type mice treated with the CA inhibitor methazolamide (MZA), which blocks aquaporin-1 (AQP-1)–mediated water influx into the ballooning platelet. (J) Flow cytometry–based measurement of percentage of procoagulant platelets and platelet MFIs for surface PS (measured through annexin V) in peripheral blood samples. Right: hematocrit measurements for mice from both experimental groups. (K) Experimental scheme of DVT and pharmacologic ablation of platelet PA using repetitive intraperitoneal injections of MZA in wild-type mice. (L) Thrombus weight in n = 14 vehicle- and n = 15 MZA-treated Bl6 mice. (M) Representative confocal image of IVC thrombi retrieved from vehicle- or MZA-treated mice. Scale bar = 500 μm. See supplemental Figure 6C for unstained controls. (N) Quantification of CD41- and Fbg-positive areas relative to total thrombus area. (O) Quantification of bleeding times of Bl6 mice undergoing tail section after pretreatment with vehicle or 20 mg/kg BW MZA. Unless otherwise stated, all statistical tests were Student t-test, 2-tailed, unpaired. P values corresponding to asterisks: ∗P < .05, ∗∗P < .01, ∗∗∗P < .005. For the schemes shown in A, F, and I, supplemental Figure 4A-B depicts a more detailed representation of signaling cascades implicated in platelet PA.
Genetic and pharmacologic inhibition of platelet PA alleviates venous thrombus formation. (A) Schematic of affected signaling pathways of platelet PA in PF4cre-TMEM16Ffl/fl mice. (B) Cell counts of peripheral blood for the indicated cell types and hematocrit quantification. Student t-test, 2-tailed, unpaired. (C) Experimental scheme of DVT in PF4cre-TMEM16Ffl/fl mice as well as thrombus weight (plotted) and incidence (percentage below graph) for n = 11 Cre– and n = 14 Cre+ PF4cre-TMEM16F mice. (D) Representative confocal image of IVC thrombi retrieved from Cre– or Cre+ mice. Scale bar = 100 μm. See supplemental Figure 5D-E for unstained examples of the same thrombi. (E) Quantification of PS-, CD41-, and Fbg-positive areas relative to total thrombus area. (F). Schematic of affected signaling pathways of platelet PA in PF4cre-CypDfl/fl mice. (G) Cell counts of peripheral blood for the indicated cell types and hematocrit quantification. (H) Experimental scheme of DVT in PF4cre-CypDfl/fl mice as well as thrombus weight (plotted) and incidence (percentage below graph) for n = 8 Cre– and n = 13 Cre+ PF4cre-CypD mice. (I) Schematic of affected signaling pathways of platelet PA in wild-type mice treated with the CA inhibitor methazolamide (MZA), which blocks aquaporin-1 (AQP-1)–mediated water influx into the ballooning platelet. (J) Flow cytometry–based measurement of percentage of procoagulant platelets and platelet MFIs for surface PS (measured through annexin V) in peripheral blood samples. Right: hematocrit measurements for mice from both experimental groups. (K) Experimental scheme of DVT and pharmacologic ablation of platelet PA using repetitive intraperitoneal injections of MZA in wild-type mice. (L) Thrombus weight in n = 14 vehicle- and n = 15 MZA-treated Bl6 mice. (M) Representative confocal image of IVC thrombi retrieved from vehicle- or MZA-treated mice. Scale bar = 500 μm. See supplemental Figure 6C for unstained controls. (N) Quantification of CD41- and Fbg-positive areas relative to total thrombus area. (O) Quantification of bleeding times of Bl6 mice undergoing tail section after pretreatment with vehicle or 20 mg/kg BW MZA. Unless otherwise stated, all statistical tests were Student t-test, 2-tailed, unpaired. P values corresponding to asterisks: ∗P < .05, ∗∗P < .01, ∗∗∗P < .005. For the schemes shown in A, F, and I, supplemental Figure 4A-B depicts a more detailed representation of signaling cascades implicated in platelet PA.
Finally, translating our findings to a clinically applicable approach, we aimed to pharmacologically reduce platelet PA to alleviate venous thrombosis. We have previously shown that combined blockade of platelet glycoproteins GPIIBIIIA and GPVI can be used to attenuate PP formation13; however, both receptors are also known mediators of thrombus formation and, therefore, do not represent an appropriate pharmacologic strategy to selectively target platelet PA in thrombosis. Furthermore, GPIIBIIIA inhibition is associated with substantial bleeding complications, limiting its clinical utility in cardiovascular prevention.22 Ballooning of PPs is crucially governed by both ion and water influx, and inhibitors of carbonic anhydrases (CAs) that also interfere with aquaporin function have been shown to block this mechanism7,23 (supplemental Figure 4A-B). Indeed, a previous study provided evidence that in vivo application of a clinically used CA inhibitor, MZA, reduced platelet PA and attenuated arterial thrombosis.23 In vitro, we found that MZA effectively reduced platelet PA without affecting P-selectin exposure or integrin activation (supplemental Figure 6A). In line, we found that MZA-treated mice showed a remarkable reduction in platelet PS exposure without affecting the expression of other canonical platelet markers (Figure 2I-K; supplemental Figure 6B). This reduction in platelet PA also alleviated DVT in our model, as shown by a significant relative reduction in thrombus incidence by 50% (46.2% vs 23.1%) and lower thrombus weights in MZA- compared with vehicle-treated animals (Figure 2L). Furthermore, histologic assessment revealed reduced amounts of CD41 and a trend toward reduced fibrin(ogen) expression in thrombi from MZA-treated mice, whereas neutrophil infiltration and neutrophil extracellular trap formation were comparable across treatment groups (Figure 2M-N; supplemental Figure 6D-E). Treatment with MZA did not affect hemostasis following mechanical trauma (Figure 2O; supplemental Figure 6F).
Although previous studies have highlighted platelet activation in patients with PE,24 this is the first study, to our knowledge, to consistently reveal PP function in both patients with venous thrombosis or PE and translationally relevant murine DVT models. Our data thus provide translational evidence for an important role of PPs in promoting venous thrombus formation. Previous work suggests a binary distribution of platelets, with the majority of platelets recruited to thrombi taking on a proaggregatory, activated phenotype characterized by integrin activation and filopodia formation, and a separate fraction of highly activated, PPs that form in different parts of the thrombus and bind a multitude of plasmatic clotting factors.25,26 Selective inhibition of platelet PA may serve as a novel therapeutic approach in preventing venous thrombosis and concomitant thromboembolism. This is particularly intriguing given that systemic CA inhibitors are already part of clinically established therapeutic approaches (eg, in patients with heart failure).27 Moreover, defects in PA formation, as observed in platelet-specific CypD- or TMEM16F-knockout animals, are not associated with impaired hemostasis following traumatic vessel injury.11,13 In line, patients with Scott syndrome, a congenital TMEM16F defect, only have a comparably mild bleeding phenotype.28
We note that detection of PPs in the circulation of patients per se is not specific to venous thrombosis.9,10,13,14,29 However, both in this cohort of patients with suspected DVT or PE and in mice developing stenosis-induced venous thrombosis, higher levels of circulating PPs were indicative of incident venous thrombosis and could therefore be helpful for clinical decision-making when added to well-established diagnostic tools, such as D-dimer.
Several signaling cascades upstream of CypD and TMEM16F have been shown to be implicated in platelet PA in a context-dependent manner, including neutrophil-derived S100A8/910 and subsequent platelet pyroptosis30 as well as (auto)antibodies associated with heparin- or vaccine-induced thrombosis and thrombocytopenia.9 Some of these thromboinflammatory factors are also known to fuel DVT,17,31,32 warranting further investigations to determine (1) the upstream mechanisms leading to PA in DVT and (2) the sequence of events that promote platelet PA. Along these lines, assessing the impact of platelet PA on differential recruitment of leukocytes and concomitant thrombus resolution remains unclear.33 We also note that our findings cannot be extrapolated to other entities of venous clot formation (eg, following ferric chloride exposure or traumatic injury, both of which are driven by other pathophysiological mechanisms). Although the IVC stenosis model is comparable to human DVT in terms of thrombus composition and driving factors, like flow reduction and subsequent recruitment of platelets and leukocytes,3,34 it lacks key components of human anatomy and physiology, like vein valves and associated differences in blood flow and local hypoxia. Whether platelet PA also drives DVT in other models, like complete IVC ligation or electrolytic IVC models, remains to be investigated.35 Finally, more insights are needed to balance pharmacologic PA blockade with adverse effects of CA inhibition, such as increasing hematocrit levels, a risk factor for DVT itself.
In summary, we provide robust evidence of an essential contribution of PPs in venous thrombosis across species. Our study fills an important gap of evidence and provides the in vivo framework for the investigation of pharmacologic platelet inhibition as a novel therapeutic strategy in venous thrombosis.
Acknowledgments
The authors thank all laboratory members for technical support, especially Judith Arcifa for the preparation of human thrombi.
This study was supported by the Else Kröner-Fresenius-Stiftung (individual grant, R.K.); the Deutsche Forschungsgemeinschaft (DFG) individual grants (R.K. [KA 6324/1-1], L.N.), the SFB 1123 (L.N. [B06], K.S. [A07]), the SFB 1054 (T.B. [B03 and Z01]), and the DFG Clinician Scientist Program PRIME (R.K., K.P. [413635475]); the German Centre for Cardiovascular Research (Clinician Scientist Programme [L.N.], Postdoc Start-up Grant [R.K., L.N.]). This work was also supported by the European Research Council (ERC-StG-2020 “T-MEMORE” [K.S.], ERC-AdG-2019 “Immunothrombosis” [S.M.]), the Corona Foundation (L.N.), and the FöFoLe+ program at LMU Munich (R.K.).
Authorship
Contribution: R.K. and L.N. initiated the study; R.K. and L.N. conceptualized the study; B.K., K.S., L.N., R.K., and S.M. performed methods; B.K., R.D., M.M., L.D.F., D.R., J.S., L. Li, C.G., M.S., L. Kääb, L.E., S.A., K.Y., R.E., Q.L., L. Kammerer, T.T.M., J.G., A.K., K.P., M.T., and R.K. performed investigations; B.K., R.D., M.M., and R.K. performed formal analysis; R.K. and L.N. wrote the original draft; R.K. performed data curation and software; R.K. and M.M. performed visualization; R.K., B.K., L.N., and S.M. performed supervision and project administration; R.K., K.S., L.N., and S.M. acquired funding; and all authors edited the manuscript.
Conflict-of-interest disclosure: T.B. and J.K. have an exclusive licensing agreement with BioLegend, Inc, for the commercialization of mC1-multimer. The remaining authors declare no competing financial interests.
Correspondence: Rainer Kaiser, LMU University Clinic, Marchioninistr, 1581377 Munich, Germany; email: rainer.kaiser@med.uni-muenchen.de; Badr Kilani, LMU University Clinic, Marchioninistr, 1581377 Munich, Germany; email: badr.kilani@med.uni-muenchen.de; and Leo Nicolai, LMU University Clinic, Marchioninistr, 1581377 Munich, Germany; email: leo.nicolai@med.uni-muenchen.de.
References
Author notes
B.K. and L.N. contributed equally and are ordered alphabetically.
For original data, please contact rainer.kaiser@med.uni-muenchen.de, badr.kilani@med.uni-muenchen.de, or leo.nicolai@uni-muenchen.de.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal