Xanth- (derived from the word xanthos: “blond” or “yellow” in Greek) is a colorful prefix used in several cutaneous histiocytoses predominantly involved by lipid-laden macrophages: xanthoma, xanthogranuloma (XG), and xanthelasma. In this issue of Blood, Kemps et al1 describe the clinicopathologic as well as molecular findings of 16 Dutch children with juvenile XG (JXG) involving extracutaneous soft tissues.
They found variable clinical courses and treatment requirements ranging from the frequently indolent cases managed with observation (some spontaneously regressed) or local resection to the rarely aggressive phenotypes requiring chemotherapy or targeted treatment. This was despite the uniform findings of genetic alterations implicated in histiocytoses including recurrent clathrin heavy chain (CLTC)–spleen tyrosine kinase (SYK) fusions or colony stimulating factor-1 receptor (CSF1R) mutations in most of their patients. Interestingly, in the 5 patients with adult XG (AXG) that they used for comparison, only 1 was found to have a mutation. Although the natural history and the molecular findings of JXG have been previously described, the novelty of this study lies in the knowledge we gained from this meticulous case series, which combined the 2 pieces of information. In JXG, the genotype does not predict the phenotype.
The XG family includes JXG, AXG, and Erdheim-Chester disease (ECD), all characterized on tissue biopsy by abnormal accumulation of histiocytes with macrophage lineage differentiation. As these entities may present identically under the microscope, they have historically been distinguished by age and anatomic distribution. When frequent BRAFV600E mutation was identified in ECD, there was hope that genetic alterations would allow distinction between the systemic form of XG (ECD) and localized, self-limited forms (JXG/AXG). Indeed, the BRAFV600E mutation present in about half of ECD cases is nearly absent in JXG/AXG, found only rarely in central nervous system lesions. However, subsequent identification of other MAPK pathway and receptor tyrosine kinase alterations revealed significant genetic overlap between the members of the XG family.
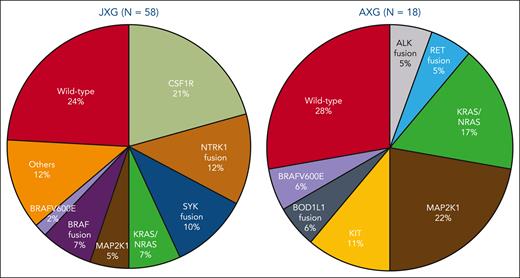
In this study, Kemps et al confirm previously reported genetic findings in JXG that fall into the overlap between JXG, AXG, and BRAF-wild-type ECD. The finding of driver mutations in all 16 JXG tumors differs from a prior study by Durham et al,2 which found mutations in only 26 of 55 JXG/AXG cases. This may reflect the exclusion of cutaneous JXG from this study, which has not been sequenced extensively and may be less likely to represent clonal neoplasms. In support of this possibility, a study by the French Histiocytosis Study Group identified mutations in only 4 of 21 JXG cases.3 However, unlike the Kemps and Durham series, the French study did not perform RNA sequencing and therefore gene fusions would have been missed. The figure combines the reported molecular findings in JXG and AXG from the studies by Kemps et al and Durham et al, showing similar genetic variants in pediatric and adult patients with XG, including KRAS, NRAS, and MAP2K1. Although only identified in JXG in these 2 studies, NTRK fusions have also been reported in AXG.4CLTC-SYK fusion has thus far only been identified in children with histiocytic neoplasms.5 Interestingly, CSF1R mutations have been reported in both pediatric and adult patients, manifesting as JXG in children but as ECD in adults.2,6 It is unclear why similar genetic mutations may cause progressive systemic disease in adults but tumefactive lesions that spontaneously regress in some children. The reasons behind this youthful advantage may include the host immune system or features inherent to the tumor. This is an intriguing question to be explored by future research in this field.
In bone marrow–based myeloid neoplasms, classification systems have begun to include molecular-based diagnostic categories. Although it may be tempting to similarly apply a molecular classification to histiocytic neoplasms, aside from BRAFV600E the genetics do not seem to independently predict disease behavior. Based on pathological and clinical features, Kemps et al advocate to include soft tissue XG tumors with CSF1R mutations and CLTC::SYK fusions as genetic variants of JXG. This approach differs from their prior multi-institutional publication that proposed the molecularly defined category of ALK+ histiocytosis, which includes subsets of cases that otherwise could be classified as genetic variants of JXG or ECD.7 ALK+ histiocytosis was adopted as a distinct diagnostic category by the recent World Health Organization and International Consensus Classification classification systems. The authors therefore excluded ALK+ histiocytosis from the current JXG cohort.
From the clinical perspective, there are more similarities than differences between JXG and AXG. Both conditions have a slightly male predilection, are nearly indistinguishable by physical and radiographic examinations, and can involve almost any organ even though there is predominant cutaneous involvement.8 Although spontaneous regression is common in JXG,9 a similar phenomenon can be observed in adults, albeit less described.10 In the latter, reporting bias may be a contributory factor since adults, compared with children, generally have less frequent wellness visits. Moreover, in adults, asymptomatic cutaneous lesions may not be brought to medical attention, especially if kinetically stable or spontaneously regressing. Finally, the management paradigm of observe carefully first, resect narrowly (wide excision unnecessary) second, and treat systemically last applies to both conditions. Larger case series of AXG with long-term follow-up are needed to provide a more systematic description of its natural history and allow proper comparison with JXG.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal