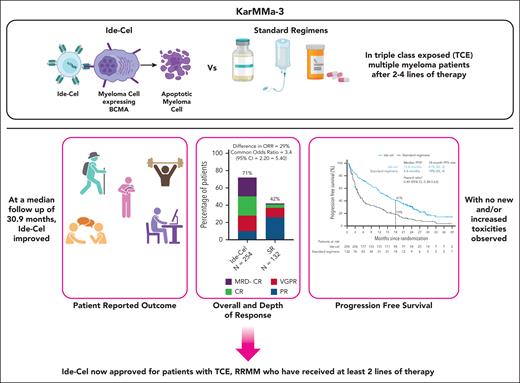
In this issue of Blood, Ailawadhi and colleagues report on the preplanned final analysis of progression-free survival (PFS) at a median follow-up of 30.9 months in patients with triple-class–exposed multiple myeloma (MM) enrolled in the KarMMa-3 study.1 With nearly 3 years of follow-up, this study is relevant in outlining no new, unexpected, and/or increased toxicities as compared with the initial report, and in confirming the efficacy of idecabtagene vicleucel (ide-cel) compared with standard regimens (SRs) in patients with relapsed and refractory MM (RRMM).2
MM is paradigmatic of effective and impactful research and development, leading to the approval of 19 novel agents over the past 2 decades, resulting in a two- to threefold improvement in overall survival for patients.3 Despite these advancements, MM remains incurable and rapidly fatal for those with biologically aggressive, triple-class–refractory disease. B-cell maturation antigen (BCMA) has been the undiscussed star molecular target in recent years, with the clinical development of antibody drug conjugate, bispecific T-cell engagers, and chimeric antigen receptor (CAR) T cells.4 In March 2021, ide-cel became the first CAR-T therapy product approved by the US Food and Drug Administration as a fifth line of treatment for patients with RRMM.5 A second BCMA-targeting CAR-T therapy, ciltacabtagene autoleucel (cilta-cel), was approved a year later.6 KarMMa-3 is a 2:1 randomized, open-label study evaluating ide-cel against 5 SRs in 386 patients with RRMM who had received 2 to 4 prior lines and were refractory to the previous regimen. KarMMa-3 provided the basis for Food and Drug Administration approval of ide-cel as third-line therapy in triple-class–exposed MM patients.
A significant proportion of the intent-to-treat patient population in this trial had biologically aggressive disease, as shown by high-risk cytogenetics (44%) and extramedullary disease (24%). Sixty-six percent of the patients had triple-class refractory disease and nearly all patients (95%) were daratumumab refractory. In this updated report, ide-cel maintained a significant risk reduction in progressive disease or death as compared with SRs (hazard ratio, 0.49; 95% confidence interval, 0.38-0.63), with a median PFS of 13 months vs 4.4 months, respectively. The PFS benefit was proportionally greater in patients who received ide-cel in earlier lines. Overall response rate and minimal residual disease–negative complete responses were 71% and 22% in the ide-cel arm and 42% and 1% in the SR arm, respectively.
Overall survival was not different across arms (41.4 months vs 37.9 months for patients who received ide-cel and SR, respectively; hazard ratio, 1.01); however, 56% of patients receiving an SR crossed over upon progressive disease (PD) and received ide-cel. Although median PFS was longer for patients on SRs who received ide-cel vs those who did not (not reached vs 10 months), the design of the study makes it impossible to determine whether this improved outcome was the result of selection bias, with patients on SRs with rapidly PD being unable to receive ide-cel.
An early (<6 months) mortality signal was noted in the ide-cel arm, mostly related to patients progressing prior to receiving CAR-T therapy. Eighty-three percent of patients received bridging therapy consisting of a maximum 1 cycle of treatment with one of the SRs allowed by the study. It is important to note that, due to study-related constraints, patients in the ide-cel arm were off anti-MM therapy for a median of 26 days out of the first 60 days since randomization, resulting in poor MM control and PD prior to ide-cel administration. Among the patients who received ide-cel, the mortality rate was similar to SR and largely related to PD in patients with high-risk features. Importantly, there were no new treatment-related adverse events with this extended follow-up and there were no delayed neurologic complications, including parkinsonism. There were no cases of malignancy arising from CAR-T therapy, and second primary malignancy incidence was comparable across arms.
A significantly higher dropout rate between leukapheresis and ide-cel infusion was seen in patients receiving more lines of therapy as compared with those receiving fewer lines (12.7% vs 5.3%, respectively). Four MM-related deaths were observed during the cell-manufacturing period. These data point to the importance of anti-MM regimens that are effective in controlling the disease during CAR-T manufacturing as well as the aggressive behavior of RRMM due to natural selection of more biologically aggressive MM subclones during each disease relapse.
Patient-reported outcome data confirm improvement in quality-of-life metrics in patients receiving ide-cel as compared with standard of care, emphasizing not just a quantitative improvement in PFS but also a qualitative one.
Although PFS curves clearly separated, there was no overt evidence of a plateau in patients receiving CAR T cells, consistent with the notion that ide-cel is not curative as a single agent in RRMM.
Although this trial is pivotal in allowing access to ide-cel in earlier lines of therapy, several questions remain unanswered. First, what determines the longevity of response? Potential candidates include the preexisting fitness of the T-cell compartment, characteristics of the CAR-T therapy product itself, and/or the composition of the bone marrow milieu.7,8 Correlative studies are warranted to identify these mechanisms. Second, the optimal selection and sequencing of BCMA-targeting agents with 2 approved CAR-T therapies and 2 approved BiTEs is not known.9 Several factors are in play here, such as social determinants of accessibility to care, personal preferences, frailty, pattern of disease relapse, availability of adequate bridging options, and, in the real-world setting, constraints around manufacturing capability and potential product failure. Third, optimal agents for bridging therapy and potential use of maintenance strategies post–CAR-T therapy need exploration. These points are of critical importance to guarantee feasibility, effectiveness, and safety of CAR-T therapy in patients with MM. Finally, long-term safety concerns, such as secondary primary malignancies and infectious complications, need to be monitored carefully, particularly as cellular therapies are moving in earlier lines of therapy with expected longer patient survival.
The report by Ailawadhi and colleagues contributes to the excitement surrounding therapeutic advancements in MM that have profoundly impacted the lives of many patients and their families (see figure). At the same time, it underscores the continuous need for more effective therapies and a deeper understanding of the mechanisms of disease relapse as we strive for a cure for patients with MM.
Summary of the main findings from the updated analysis of the KarMMa-3 study. Figure generated with BioRender.com.
Summary of the main findings from the updated analysis of the KarMMa-3 study. Figure generated with BioRender.com.
Conflict-of-interest disclosure: G.B. has received consulting fees from Prothena.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal