In this issue of Blood, Gutierrez-Rodrigues et al1 characterize the spectrum of clonal hematopoiesis (CH) in a large international cohort of patients with telomere biology disorder (TBD) and identify distinct pathways of CH associated with germ line genotypes, clinical phenotypes, and risk of progression to myeloid malignancies.
All somatic tissues steadily acquire DNA mutations and undergo telomere attrition throughout life.2 CH is defined by acquired mutations in hematopoietic stem cells (HSCs) that confer a relative fitness advantage leading to expansion over time, of which a small minority of clones progress to hematologic malignancy. Patients with TBD have germ line defects in telomere maintenance associated with multiorgan dysfunction and a predisposition to developing myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). An enhanced understanding of the molecular events that mediate malignant progression in patients with TBD is critical to develop effective surveillance strategies and treatments that improve survival for patients with TBD. Moreover, elucidating the pathways of CH in patients with TBD holds promise to reveal the broader mechanisms linking telomere maintenance and risk of myeloid malignancies in the general population.
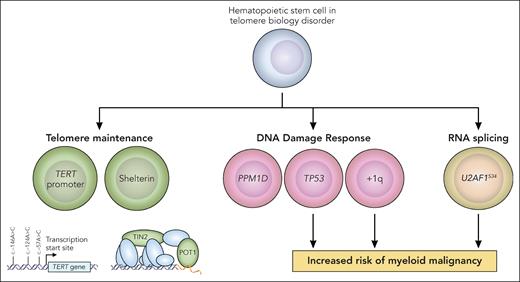
In this study, error-corrected next-generation sequencing (NGS) with a targeted panel comprising myeloid driver and telomere maintenance genes was performed on blood samples from a cohort of 207 patients with TBD (median age 27 years) with a diversity of germ line TBD genotypes. The overall prevalence of CH among patients with TBD (46%) was significantly higher across the age spectrum compared with 140 age-matched controls. Although typical age-associated CH is dominated by mutations in DNA methylation genes (DNMT3A and TET2),3 the most recurrent somatic mutations in patients with TBD occurred in genes related to DNA damage (TP53 and the phosphatase PPM1D), telomere maintenance (TERT promoter, POT1, TINF2), and RNA splicing (particularly U2AF1S34). Gain of chromosome 1q (+1q), encompassing the p53 regulator MDM4, was the most recurrent cytogenetic abnormality in patients with TBD. Additionally, the authors observed associations between specific CH alterations and TBD germ line genotypes: somatic POT1 and TINF2 loss-of-function mutations were associated with germ line TINF2 mutations, whereas TERT promoter mutations were more common among patients with germ line TERT mutations. Single-cell analyses confirmed that CH mutations occurred at the HSC–multipotent progenitor level and propagated to myeloid and lymphoid lineages. Additionally, RNA expression analysis of CD34+ cells from patients with TBD raised the possibility that U2AF1S34 mutations alter p53 and interferon signaling pathways. Overall, the results of this study significantly enhance our understanding of the spectrum and frequency of CH among patients with TBD, building upon and corroborating prior analyses.4-8 Collectively, these observations underscore a central theme: CH in the context of impaired telomere maintenance is dominated by somatic mutations in genes involved in (1) telomere maintenance, (2) DNA damage response (DDR), and (3) RNA splicing (see figure).
Pathways of CH in TBD. In the study by Gutierrez-Rodrigues et al, CH in patients with TBD was characterized by recurrent somatic mutations in genes related to telomere maintenance (green), DDR (pink), and RNA splicing (yellow). The authors found that mutations in TP53, gain of chromosome 1q, and U2AF1S34 were associated with an increased risk of progression to MDS and AML. Professional illustration by Patrick Lane, ScEYEnce Studios.
Pathways of CH in TBD. In the study by Gutierrez-Rodrigues et al, CH in patients with TBD was characterized by recurrent somatic mutations in genes related to telomere maintenance (green), DDR (pink), and RNA splicing (yellow). The authors found that mutations in TP53, gain of chromosome 1q, and U2AF1S34 were associated with an increased risk of progression to MDS and AML. Professional illustration by Patrick Lane, ScEYEnce Studios.
The authors found that the risk of myeloid malignancies in patients with TBD was influenced by gene-specific CH alterations. The cumulative incidence of MDS and AML in the total cohort was 8.6% and 20% at age 40 and 60, respectively. In a multivariable model, the presence of mutations in TP53, RNA splicing factors, and +1q were independently associated with the risk of MDS and AML. In contrast, gain-of-function PPM1D mutations and TERT promoter mutations were not associated with progression to myeloid malignancy. PPM1D is a p53 transcriptional target and negatively regulates multiple nodes of the DDR pathway.9 These observed differences in myeloid malignancy risk among somatic mutations in different DDR pathway genes suggest distinct cellular effects that drive clonal expansion and malignant transformation.
The results of this study also highlight important remaining questions related to the clinical impact of CH and the biological mechanisms that mediate clonal expansion in patients with TBD. From the clinical perspective, how should CH assessment be implemented into the hematologic monitoring for patients with TBD? Are there specific CH mutations that should trigger a clinical intervention? To address these questions, collaborative efforts at the consortium level are needed to augment statistical power, cross-validate molecular and clinical associations, and develop prognostic models to accurately identify patients with TBD at highest risk of MDS and AML. From the biological perspective, what additional somatic alterations influence HSC fitness and clinical outcomes in patients with TBD? The content of NGS panels used in analyses of CH in TBD varies substantially across studies,4,5,7,8 and these collectively have limited ability to identify novel CH drivers. Future analyses incorporating unbiased somatic genomic discovery may reveal the full spectrum of CH alterations under positive selection in the context of telomere dysfunction. Such a comprehensive understanding holds potential for the discovery of novel therapeutic approaches to augment normal hematopoiesis and reduce the risk of myeloid malignancies in patients with TBD.
Lastly, the work by Gutierrez-Rodrigues et al provides yet another line of evidence linking germ line variation, telomere maintenance, and CH. At the population level, germ line common and rare variants in telomere maintenance genes influence telomere length and patterns of CH.10 Collectively, these observations and those from studies of patients with TBD support a unifying biological model that telomere dysfunction throughout life, representing a composite of germ line–encoded telomere maintenance capacity and cellular replicative history, is a selection pressure favoring somatic mutations that alleviate this fitness constraint. If conclusively proven mechanistically, this paradigm would have far-reaching implications for our current understanding of the causal relationship between telomere maintenance and the emergence of myeloid malignancies with increasing chronological and, perhaps more accurately, biological age.
Conflict-of-interest disclosure: M.M. has received research funding from Gilead Sciences and honoraria from Celgene and Sanofi. C.R.R. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal