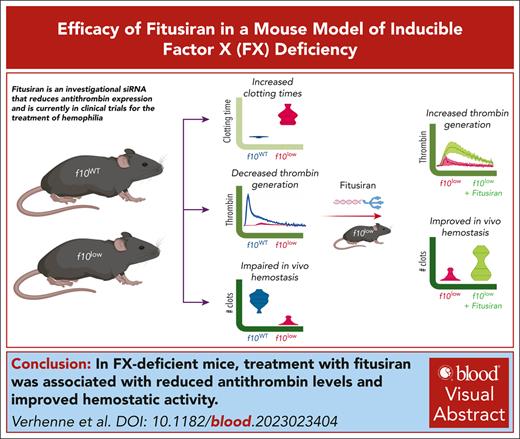
Key Points
Fitusiran is an investigational siRNA reducing antithrombin expression that is under clinical evaluation for the treatment of hemophilia.
Using inducible FX–deficient mice, we demonstrate that fitusiran has the potential to improve hemostasis in FX deficiency.
Visual Abstract
Factor X (FX) deficiency is a rare bleeding disorder manifesting a bleeding tendency caused by low FX activity levels. We aim to explore the use of fitusiran (an investigational small interfering RNA that silences antithrombin expression) to increase thrombin generation and the in vivo hemostatic potential under conditions of FX deficiency. We therefore developed a novel model of inducible FX deficiency, generating mice expressing <1% FX activity and antigen (f10low mice). Compared with control f10WT mice, f10low mice had sixfold and fourfold prolonged clotting times in prothrombin time and activated partial prothrombin time assays, respectively (P < .001). Thrombin generation was severely reduced, irrespective of whether tissue factor or factor XIa was used as an initiator. In vivo analysis revealed near-absent thrombus formation in a laser-induced vessel injury model. Furthermore, in 2 distinct bleeding models, f10low mice displayed an increased bleeding tendency compared with f10WT mice. In the tail-clip assay, blood loss was increased from 12 ± 16 μL to 590 ± 335 μL (P < .0001). In the saphenous vein puncture (SVP) model, the number of clots generated was reduced from 19 ± 5 clots every 30 minutes for f10WT mice to 2 ± 2 clots every 30 minutes (P < .0001) for f10low mice. In both models, bleeding was corrected upon infusion of purified FX. Treatment of f10low mice with fitusiran (2 × 10 mg/kg at 1 week interval) resulted in 17 ± 6% residual antithrombin activity and increased thrombin generation (fourfold and twofold to threefold increase in endogenous thrombin potential and thrombin peak, respectively). In the SVP model, the number of clots was increased to 8 ± 6 clots every 30 minutes (P = .0029). Altogether, we demonstrate that reduction in antithrombin levels is associated with improved hemostatic activity under conditions of FX deficiency.
Introduction
Coagulation factor X (FX) is a vitamin K–dependent protein that is mainly produced by hepatocytes and circulates in plasma at a concentration of about 10 μg/mL.1 Upon activation by the tissue factor/factor VIIa (TF/FVIIa) complex or the factor IXa/factor VIIIa (FIXa/FVIIIa) complex, FXa constitutes the enzymatic component of the prothrombinase complex that converts prothrombin into thrombin. Thrombin, in turn, is responsible for the formation of a stable, fibrin-rich blood clot.
Hereditary FX deficiency is a rare autosomal recessive disorder that occurs in about 1 in every 1 000 000 individuals.2,3 FX deficiency can be classified into type I (low FX antigen and low FX activity) or type II (near-normal FX antigen, low FX activity). Clinically, FX deficiency produces a variable bleeding tendency, with an inverse association between plasma activity levels and the severity of the bleeding phenotype. While patients with residual FX activity between 10% and 40% usually suffer minor spontaneous bleeding or bleeding after trauma, patients with activity levels below 10% are at high risk for major spontaneous bleeding.4 Patients with <1% FX activity are the most severely affected, with the occurrence of intracranial hemorrhages, gastrointestinal bleeding, and hemophilia-like bleeding episodes.5,6
Current treatment options have long been restricted to nonspecific FX-replacement therapy (eg, fresh-frozen plasma, prothrombin complex concentrates, and FIX concentrates with a specified FX content), and only 1 high-purity human plasma–derived FX concentrate (Coagadex; Bio Products Laboratory, Elstree, United Kingdom) is available for routine prophylaxis and on-demand treatment of bleeding episodes.3,7
Whereas treatment options for hemophilia A and B have been revolutionized by the arrival of so-called nonfactor therapies, such as emicizumab, antibodies against tissue factor pathway inhibitors, and fitusiran (small interfering RNA–mediated silencing of antithrombin), this is unfortunately not the case for FX deficiency. From a theoretical point of view, fitusiran could potentially be applicable to patients with reduced FX activity, as it targets the main natural FXa inhibitor, antithrombin. Fitusiran treatment in patients with hemophilia A and B aims to decrease antithrombin levels to 15% to 35% of normal.8-12 This reduction in antithrombin levels is associated with a near normalization of thrombin generation and significantly reduced bleeding in these patients.8-12
Here, we explored the possibility that fitusiran could potentially be used to ameliorate the bleeding tendency in FX deficiency. To this end, a mouse model applying the MX1-Cre/loxP recombination system to induce targeted disruption of the murine f10 gene was developed.13,14 This approach allowed us to generate mice with a severe deficiency of FX antigen and activity (<1% of normal, referred to as f10low mice), which was associated with a dramatic decrease in in vitro clotting activity and thrombin generation. These mice were viable, exhibited impaired thrombus formation in a laser-induced vessel wall injury model, and displayed a bleeding tendency in 2 different bleeding models. Bleeding could be reversed by the infusion of FX. Moreover, treatment with fitusiran reduced bleeding, suggesting that this agent could be explored for its use in patients with a FX deficiency.
Materials and methods
Materials
Human plasma–derived FX and factor XIa were from Cryopep (Montpellier, France). Fitusiran was kindly provided by Sanofi-Aventis (Gentilly, France). Tissue factor was from Stago (Asnières, France).
Generation of MX1Cre+f10flox/flox mice
The generation of MX1Cre+f10flox/flox mice was outsourced to Genoway (Lyon, France). Mice expressing a floxed f10 gene (F10tm1a(EUCOMM)Hmgu) were obtained from the European Mouse Mutant Archive (06365; HEPD0602_2_G07)14 and crossed with flippase-expressing C57Bl/6 mice to generate a conditional f10 allele (f10flox/flox). Subsequently, these mice were crossed with the MX1-Cre transgenic line (B6.Cg-Tg(Mx1-Cre)1CgnJ; Jackson Laboratories, Bar Harbor, ME). As control, MX1Cre–f10flox/flox littermates were used. FX deficiency was induced by 3 subsequent intraperitoneal injections of polyinosinic:polycytidylic acid (pI:pC 10 mg/kg; Invivogen, San Diego, CA) at a 48-hour interval. Mice were between 6 to 8 weeks of age at the time of pI:pC treatment, and both male and female mice were used in this study. FX levels were measured 3 weeks after the last injection in all mice used in the study.
Protein assays
Assays for FXa amidolytic activity, FVII, FVIII, FIX, and FX clotting activities, FX and von Willebrand factor antigen, as well as antithrombin activity, are described in the supplemental Methods, available on the Blood website.
Fitusiran treatment
Fitusiran (Sanofi-Aventis, Gentilly, France) was given subcutaneously twice to f10low mice at a dose of 10 mg/kg at a 1-week interval.15 Antithrombin levels were measured in plasma samples obtained 7 days after the second injection.
Blood sampling
Blood samples were taken via retro-orbital puncture from isoflurane-anesthetized mice. Blood was collected in EDTA (ethylenediamine tetraacetic acid) (1 volume [vol] 0.5 M EDTA + 9 vol blood) for blood counts or in citrate (1 vol 0.138 M citrate + 9 vol blood) for activity and antigen assays.
Thrombin generation assay
Thrombin generation in platelet-poor murine plasma was measured in a microtiter plate fluorometer according to the method described by Hemker et al (for detail refer to supplemental Material).16
Laser-induced vessel injury
Intravital video microscopy of the cremaster muscle microcirculation was performed as described (for details refer to supplemental Material).17
Tail-clip assay and SVP model
Statistical analysis
All data are presented as mean ± standard deviation unless indicated otherwise. Number (n) refers to the number of independent experiments or animals. The statistical analysis was performed using GraphPad Prism 9 software for Mac (La Jolla, CA). One-way analysis of variance followed by a Tukey or Dunnett multiple comparison test was performed when comparing multiple groups. Pairwise analysis was performed using the unpaired Student t test, with Welch correction where appropriate. Multiple χ2 test was performed online at the website: https://www.socscistatistics.com/tests/chisquare2/default2.aspx (last visited 27 October 2023). P < .05 was considered as statistically significant.
Animal housing and experiments were performed in accordance with French regulations (decree #2013-118; https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000027037840) and the experimental guidelines of the European Community (Directive 2010/63/EU; https://faolex.fao.org/docs/pdf/eur98296.pdf). Mice were kept in individually ventilated cages (2-5 mice/cage), with enrichment provided by bedding, nesting material, and aspen bricks.
This project was approved by the local ethical committee of Université Paris-Saclay (Comité d’Ethique en Expérimentation Animale n°26, protocol APAFIS#39643-2022120117122791-v4) and by the local ethical committee of Marseille (Comité d’Ethique en Expérimentation Animale n°14, protocol APAFIS#18710-201901301109218-v2).
Results
Induction of FX deficiency
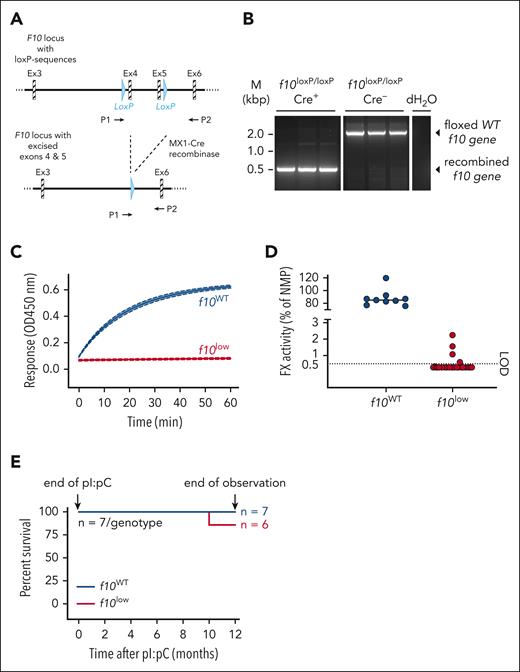
Transgenic mice homogenous for a loxP-flanked f10 gene and expressing Cre-recombinase under the control of the interferon-inducible MX1 promotor (ie, MX1Cre+f10flox/flox) were used to inactivate the f10 gene by administration of pI:pC (Figure 1A). Age and sex-matched MX1Cre–f10flox/flox littermates that were treated with pI:pC served as controls and are referred to as f10WT mice. Polymerase chain reaction analysis of genomic DNA isolated from the liver indicated that Cre-recombinase activation efficiently induces the elimination of exons 4 and 5 of the f10 gene in MX1Cre+f10flox/flox mice but not in f10WT mice (Figure 1B). Consequently, f10WT mice displayed efficient FX-activating enzyme from Russell's viper venom (RVV-X)–induced FX amidolytic activity, whereas residual FX amidolytic activity was virtually undetectable in plasma from pI:pC–treated MX1Cre+f10flox/flox mice (Figure 1C). In terms of residual activity, most of the latter group displayed activity levels below the detection limit of 0.5% (Figure 1D). Moreover, no FX antigen could be detected in the plasma of these mice. In the remainder of the study, only mice exhibiting <1% FX activity were used, and we refer to these mice as f10low mice. Importantly, besides strongly reduced FX activity, no reduction in red cell, white blood cell, or platelet counts were detected, nor were levels of FVII, FVIII, or FIX affected (supplemental Table 1). Expression of platelet receptors was unaltered, as was platelet function (supplemental Figures 1-2). We further observed no differences in survival between unchallenged f10low mice and f10WT control mice over a 1-year period (6/7 and 7/7; P = .32; Figure 1E). Mice displayed normal behavior during the observation period. No blood was found in the cages, and monthly analysis revealed stable hemoglobin levels over time. Inspection of mice sacrificed at 3 months (n = 5) or at 12 months (n = 6) revealed no signs of overt bleeding in the organs that were examined (brain, liver, lung, heart, or spleen), with FX activity levels remaining undetectable over this period. These data indicate that a severe FX deficiency can be induced, resulting in mice with less than 1% FX activity, which are viable for at least 1 year.
Engineering and analysis of inducible FX–deficient mice. (A) Mice containing an engineered f10 gene with loxP sequences surrounding exons 4 and 5. Upon activation of Cre-recombinase, both exons are excised. P1 and P2 indicate the locations of primers used for postinduction analysis. (B) Genomic DNA was isolated from liver of pI:pC–treated Cre+ and Cre–f10flox/flox mice. A virtually complete recombined F10 locus was observed in Cre+ mice. (C) A representative curve of RVV-X–induced FXa amidolytic activity over time in plasma from f10WT mice (n = 3) and f10low mice (n = 3). (D) Residual FX activity measured in RVV-X–induced activity assay. LOD of this assay is 0.5% FX. All mice having <1% FX activity are designated as f10low mice. (E) Survival curve of of pI:pC–treated f10WT mice and f10low mice (both n = 7). No differences in survival after 12 months were observed. LOD, limit of detection.
Engineering and analysis of inducible FX–deficient mice. (A) Mice containing an engineered f10 gene with loxP sequences surrounding exons 4 and 5. Upon activation of Cre-recombinase, both exons are excised. P1 and P2 indicate the locations of primers used for postinduction analysis. (B) Genomic DNA was isolated from liver of pI:pC–treated Cre+ and Cre–f10flox/flox mice. A virtually complete recombined F10 locus was observed in Cre+ mice. (C) A representative curve of RVV-X–induced FXa amidolytic activity over time in plasma from f10WT mice (n = 3) and f10low mice (n = 3). (D) Residual FX activity measured in RVV-X–induced activity assay. LOD of this assay is 0.5% FX. All mice having <1% FX activity are designated as f10low mice. (E) Survival curve of of pI:pC–treated f10WT mice and f10low mice (both n = 7). No differences in survival after 12 months were observed. LOD, limit of detection.
Loss of circulatory FX results in severely impaired in vitro coagulation
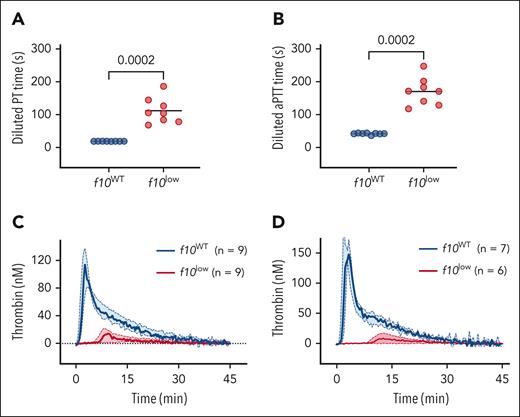
Plasma obtained from f10WT mice and f10low mice was then analyzed in a number of in vitro hemostasis assays. First, in both the prothrombin time (PT) assay (19 ± 1 seconds vs 112 ± 39 seconds; P = .0002; Figure 2A) and the activated partial prothrombin time (aPTT) assay (42 ± 3 seconds vs 170 ± 42 seconds; P = .0002; Figure 2B) the clotting time was significantly prolonged in plasma from f10low mice compared with f10WT littermates. We next analyzed the plasma of these mice for thrombin generation, triggered by TF or FXIa. Regardless of the trigger used, thrombin generation was strikingly reduced in the plasma of f10low mice (Figure 2C-D; Table 1). All of the relevant parameters (lag time, endogenous thrombin potential (ETP), thrombin peak, and time to peak) were significantly different between both groups. Together, these data are compatible with FX activity being severely reduced to levels below 1%.
Residual hemostatic activity in platelet-poor plasma of f10low mice. (A-B) One-stage clotting assays, PT (A) and activated partial prothrombin time (aPTT) (B) were performed using plasma of pI:pC–treated f10WT mice (n = 8) and f10low mice (n = 8). Horizontal lines represent mean values. Statistical analysis was performed using unpaired Student t test. (C-D) Thrombin generation was performed using TF (C) or FXIa (D) as initiator. Solid lines represent median values, and colored areas indicate interquartile range. Blue: f10WT mice and red: f10low mice. Number of measurements are indicated for each condition. Detailed values of thrombin generation parameters (lag time, ETP, thrombin peak, and time to peak) are summarized in Table 1.
Residual hemostatic activity in platelet-poor plasma of f10low mice. (A-B) One-stage clotting assays, PT (A) and activated partial prothrombin time (aPTT) (B) were performed using plasma of pI:pC–treated f10WT mice (n = 8) and f10low mice (n = 8). Horizontal lines represent mean values. Statistical analysis was performed using unpaired Student t test. (C-D) Thrombin generation was performed using TF (C) or FXIa (D) as initiator. Solid lines represent median values, and colored areas indicate interquartile range. Blue: f10WT mice and red: f10low mice. Number of measurements are indicated for each condition. Detailed values of thrombin generation parameters (lag time, ETP, thrombin peak, and time to peak) are summarized in Table 1.
Thrombin generation parameters in f10WT mice and f10low mice: effect of initiator (TF vs factor XIa)
| . | TF (1 pM) . | Factor XIa (0.15 nM) . | ||||
|---|---|---|---|---|---|---|
| f10WT (n = 9) . | f10low (n = 9) . | P value . | f10WT (n = 7) . | f10low (n = 6) . | P value . | |
| Lag time (min) | 1.0 ± 0.3 | 7.0 ± 2.8 | <.0001 | 1.4 ± 0.6 | 15 ± 8 | .0009 |
| ETP (nM·min) | 1020 ± 96 | 149 ± 126 | <.0001 | 1103 ± 174 | 109 ± 96 | <.0001 |
| Peak thrombin (nM) | 111 ± 11 | 14 ± 8 | <.0001 | 164 ± 16 | 10 ± 8 | <.0001 |
| Time to peak (min) | 3.0 ± 0.6 | 12 ± 6 | .0004 | 3.0 ± 0.6 | 18 ± 7 | .0001 |
| . | TF (1 pM) . | Factor XIa (0.15 nM) . | ||||
|---|---|---|---|---|---|---|
| f10WT (n = 9) . | f10low (n = 9) . | P value . | f10WT (n = 7) . | f10low (n = 6) . | P value . | |
| Lag time (min) | 1.0 ± 0.3 | 7.0 ± 2.8 | <.0001 | 1.4 ± 0.6 | 15 ± 8 | .0009 |
| ETP (nM·min) | 1020 ± 96 | 149 ± 126 | <.0001 | 1103 ± 174 | 109 ± 96 | <.0001 |
| Peak thrombin (nM) | 111 ± 11 | 14 ± 8 | <.0001 | 164 ± 16 | 10 ± 8 | <.0001 |
| Time to peak (min) | 3.0 ± 0.6 | 12 ± 6 | .0004 | 3.0 ± 0.6 | 18 ± 7 | .0001 |
Data represent mean ± SD. Statistical analysis was performed using unpaired Student t test, and Welch correction was applied where necessary.
Defective in vivo thrombus formation in f10low mice
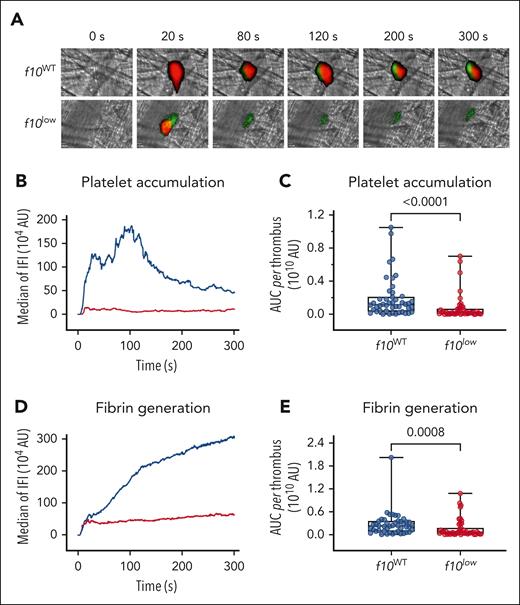
To study FX deficiency in vivo, we first evaluated the kinetics of arteriolar thrombus formation in the cremaster microcirculation after laser injury. Both f10WT mice and f10low mice were infused with fluorescently labeled antibodies against fibrin and the platelet-surface marker glycoprotein Ib (GpIb) to monitor thrombus formation in real time (Figure 3A; supplemental Videos 1-2). Compared with f10WT mice, f10low mice were characterized by a reduced accumulation of platelets, illustrated by a fivefold to sixfold lower median platelet accumulation (median fluorescence intensity: 0.2 × 109 (95% confidence interval [CI], 0.1 × 109 to 0.3 × 109) vs 1.1 × 109 (95% CI, 0.8 × 109 to 1.5 × 109); P < .0001; Figure 3B-C). In parallel, fibrin deposition was reduced by more than fourfold in the f10low mice than in the f10WT mice (median fluorescence intensity: 0.5 × 109 (95% CI, 0.5 × 109 to 0.8 × 109) vs 2.3 × 109 (95% CI, 1.3× 109 to 3.0 × 109); P = .0008; Figure 3D-E). These data show that the strongly reduced FX activity levels in plasma result in defective thrombus formation in vivo. Importantly, the notion that some fibrin is generated indicates that FX activity levels are not absolutely zero but that minor levels of FX (which are below our limit of detection) are still present to allow some thrombin-mediated fibrin formation.
Reduced thrombus formation in f10low mice in laser–induced vessel injury model. A localized laser injury was induced in the cremaster arterioles of f10WT (n = 4) or f10low (n = 4) mice. Clot formation was visualized by monitoring the accumulation of platelets and the deposition of fibrin. Platelets (red) and fibrin (green) were detected using AlexaFluor647-conjugated anti-GPIb and Alexa Fluor 488-conjugated antifibrin antibodies, respectively. (A) Representative digital composite fluorescence and bright-field images of platelet accumulation (red) and fibrin deposition (green) in f10WT (n = 46 clots) and f10low (n = 44 clots) mice before (0 s) and 20, 80, 120, 200, and 300 s after laser injury. (B,D) Kinetic curves displaying platelet accumulation and fibrin deposition in f10WT (blue; 46 clots) and f10low (red; 44 clots) mice. Plotted is median IFI for platelets (B) and fibrin (D) fluorescence vs time after injury. (C,E) Distribution of the integrated platelet (C) or fibrin (E) fluorescence intensity for each of the 46 clots in f10WT mice and 44 clots in f10low mice. Plotted is the AUC for each individual thrombus. Presented are Box-Whisker plots with minimum to maximum values, with all individual data points shown. Statistical analysis was performed using the Mann-Whitney test. AUC, area under the curve; IFI, integrated fluorescence intensity.
Reduced thrombus formation in f10low mice in laser–induced vessel injury model. A localized laser injury was induced in the cremaster arterioles of f10WT (n = 4) or f10low (n = 4) mice. Clot formation was visualized by monitoring the accumulation of platelets and the deposition of fibrin. Platelets (red) and fibrin (green) were detected using AlexaFluor647-conjugated anti-GPIb and Alexa Fluor 488-conjugated antifibrin antibodies, respectively. (A) Representative digital composite fluorescence and bright-field images of platelet accumulation (red) and fibrin deposition (green) in f10WT (n = 46 clots) and f10low (n = 44 clots) mice before (0 s) and 20, 80, 120, 200, and 300 s after laser injury. (B,D) Kinetic curves displaying platelet accumulation and fibrin deposition in f10WT (blue; 46 clots) and f10low (red; 44 clots) mice. Plotted is median IFI for platelets (B) and fibrin (D) fluorescence vs time after injury. (C,E) Distribution of the integrated platelet (C) or fibrin (E) fluorescence intensity for each of the 46 clots in f10WT mice and 44 clots in f10low mice. Plotted is the AUC for each individual thrombus. Presented are Box-Whisker plots with minimum to maximum values, with all individual data points shown. Statistical analysis was performed using the Mann-Whitney test. AUC, area under the curve; IFI, integrated fluorescence intensity.
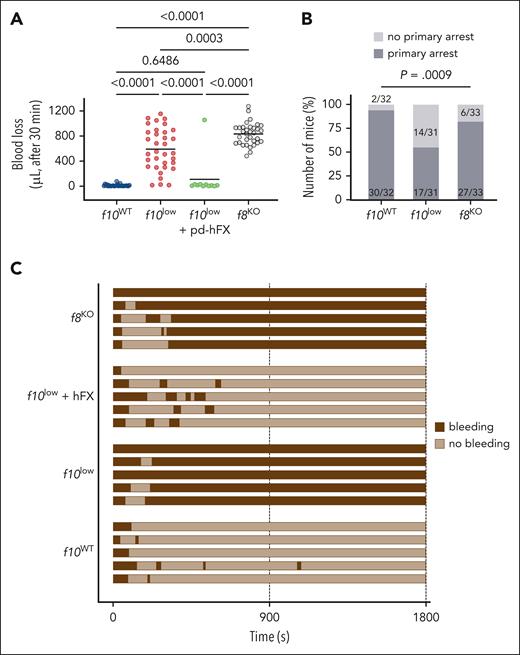
f10low mice display increased bleeding in a tail-clip bleeding model
To get insight into the bleeding phenotype, both f10WT and f10low mice were subjected to the tail-clip bleeding model, in which the distal tip of the tail (3 mm) is excised. In all cases, mice were monitored for 30 minutes after injury. Blood loss in f10WT mice was minimal and did not exceed 25 μL in most mice (12 ± 16 μL; n = 30; Figure 4A). In contrast, blood loss was more pronounced in f10low mice (590 ± 335 μL; n = 34; P < .0001; Figure 4A). We also tested for blood loss in f10low mice that received 50 U/kg human FX (which results in FX plasma levels of 70%-100%). Blood loss in these mice was 108 ± 315 μL (n = 11; P = .65 vs f10WT mice and P < .0001 vs f10low mice), demonstrating that the increased bleeding tendency originated from the FX deficiency (Figure 4A). Interestingly, blood loss in f10low mice was much more variable compared with f8KO mice, with 40% of the f10low mice having less blood loss than the f8KO mice (Figure 4A). Whereas most of the f8KO mice were characterized by the presence of a primary arrest, this happened in only 50% of the f10low mice (P = .0009; Figure 4B-C). This suggests that the presence of FX might be relevant in the primary hemostatic response.
Increased bleeding tendency for f10low mice in tail-clip model. (A) The terminal 3 mm of the tail were amputated from f10WT mice (blue), f10low mice (red), f10low mice that received FX (50 U/kg) 5 minutes before injury (green), and f8KO mice (f8KO; gray). Blood loss was monitored for 30 minutes, and the amount of shed blood was measured. Statistical analysis was performed using 1-way analysis of variance (ANOVA) with Tukey correction for multiple comparisons. (B) Number of mice that made (dark gray) or not (pale gray) a primary bleeding stop after tail amputation. The number of mice with a primary stop was significantly reduced in f10low mice than in f10WT and f8KO mice. Statistically significant difference in the number of mice with primary stop was assessed using a χ2 test for multiple groups. (C) Representative bleeding profiles of f10WT mice, f10low mice, f10low mice that received FX (50 U/kg) 5 minutes before injury, and FVIII-deficient mice.
Increased bleeding tendency for f10low mice in tail-clip model. (A) The terminal 3 mm of the tail were amputated from f10WT mice (blue), f10low mice (red), f10low mice that received FX (50 U/kg) 5 minutes before injury (green), and f8KO mice (f8KO; gray). Blood loss was monitored for 30 minutes, and the amount of shed blood was measured. Statistical analysis was performed using 1-way analysis of variance (ANOVA) with Tukey correction for multiple comparisons. (B) Number of mice that made (dark gray) or not (pale gray) a primary bleeding stop after tail amputation. The number of mice with a primary stop was significantly reduced in f10low mice than in f10WT and f8KO mice. Statistically significant difference in the number of mice with primary stop was assessed using a χ2 test for multiple groups. (C) Representative bleeding profiles of f10WT mice, f10low mice, f10low mice that received FX (50 U/kg) 5 minutes before injury, and FVIII-deficient mice.
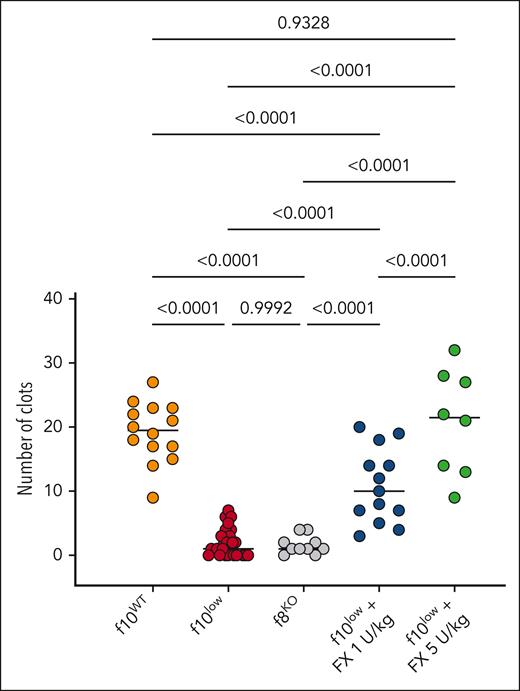
f10low mice have a reduced hemostatic activity in the SVP model
From studies with f8KO mice, we noticed that the SVP model is more severe compared with the tail-clip model, as they require 50% and 20% FVIII for full correction, respectively. We therefore also examined the bleeding phenotype of the f10low mice in this particular model. A severe bleeding phenotype in the f10low mice was observed, with 2 ± 2 clots being formed over a 30 minute period (n = 28; Figure 5). f10WT mice generated 19 ± 5 clots over a 30 minute period (n = 14; P < .0001). Interestingly, the f10low mice displayed a similar impeded hemostasis as the f8KO mice, which also generated 2 ± 2 clots over a 30 minute period (Figure 5). Treatment of f10low mice with a low-dose FX (1 U/kg; which results in FX plasma levels of 1.5%-2%) partially corrected bleeding to an intermediate phenotype (11 ± 6 clots over a 30 minute period; n = 13; P < .0001 compared with f10WT mice and f10low mice; Figure 5). A fivefold higher dose (5 U/kg; which results in FX plasma levels of 7%-10%) resulted in complete normalization of the bleeding phenotype (21 ± 8 clots over a 30 minute period; n = 8; P = .933 compared with f10WT mice). These data show that the SVP model is an attractive method to evaluate whether procoagulant agents are able to rescue the bleeding phenotype in FX deficiency.
Reduced hemostatic activity in SVP model for f10low mice. The number of clots over a 30-minute period observed for f10WT mice (orange), f10low mice (red), FVIII-deficient mice (f8KO; gray), f10low mice receiving purified FX (1 U/kg; blue or 5 U/kg; green). Each symbol represents an individual mouse. Horizontal bars represent median values. Statistical analysis was performed via 1-way ANOVA with Tukey correction for multiple comparisons.
Reduced hemostatic activity in SVP model for f10low mice. The number of clots over a 30-minute period observed for f10WT mice (orange), f10low mice (red), FVIII-deficient mice (f8KO; gray), f10low mice receiving purified FX (1 U/kg; blue or 5 U/kg; green). Each symbol represents an individual mouse. Horizontal bars represent median values. Statistical analysis was performed via 1-way ANOVA with Tukey correction for multiple comparisons.
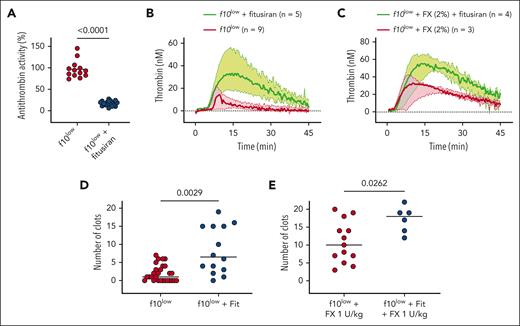
Fitusiran increases thrombin generation in FX deficiency
Knowing that antithrombin is the main inhibitor of FXa and thrombin, we hypothesized that by reducing antithrombin expression, thrombin generation would be improved. We first tested this in thrombin generation assays using plasma from fitusiran-treated f10low mice. Once FX deficiency was induced, f10low mice (having <1% FX activity) received 2 weekly doses of fitusiran (10 mg/kg). Seven days after the last fitusiran injection, plasma samples were obtained and analyzed for residual antithrombin activity levels. Antithrombin levels were 96 ± 20% (n = 13) in f10low mice that received vehicle injections, whereas antithrombin levels were reduced to 17 ± 6% (n = 21) in fitusiran-treated f10low mice (P < .0001; Figure 6A). Reduced antithrombin levels had no effect on PT or aPTT clotting times (supplemental Figure 3). With regard to thrombin generation, however, the plasma of f10low mice treated with fitusiran displayed a markedly increased thrombin generation compared with that of untreated f10low mice (Table 2; Figure 6B). Lag time was shortened twofold (P = .0332), whereas ETP and peak thrombin were increased sixfold (P = .0002) and 2.6-fold (P = .0136), respectively. Because FX levels below 1% are very rare in patients, we also compared the effect of fitusiran in plasma spiked with 2% FX activity to mimic a moderate phenotype. Despite thrombin generation being more efficient in the presence of 2% FX compared with <1%, the reduced levels of antithrombin due to fitusiran treatment still had a beneficial effect on thrombin generation under these conditions (Table 2; Figure 6C). Both ETP (783 ± 463 nM·min vs 1724 ± 260 nM·min; P = .0179) and peak thrombin (31 ± 11 nM vs 59 ± 10 nM; P = .0193) were increased in the plasma of fitusiran-treated mice. Thus, reduction in antithrombin activity levels is associated with increased thrombin generation at low or virtually absent FX levels.
Fitusiran improves hemostasis in FX deficiency. (A) f10low mice received 2 doses of fitusiran at a 1-week interval. Seven days after the last dose, plasma was analyzed for antithrombin activity in nontreated (red symbols) and fitusiran-treated f10low mice (blue). Each symbol represents an individual mouse, and horizontal bars represent mean values. Statistical analysis was performed using an unpaired Student t test. (B-C) TF-induced thrombin generation in nontreated (red curves) and fitusiran-treated (green curves) f10low mice. Plasma levels of FX were <1% (B) or plasmas were spiked with FX (2% of normal plasma) before measuring thrombin generation. Solid lines represent median values, and colored areas represent interquartile range. Detailed values of thrombin generation parameters (lag time, ETP, thrombin peak, and time to peak) are summarized in Table 2. (D-E) The number of clots over a 30-minute period observed in f10low mice (red) and fitusiran-treated f10low mice (blue) that did (E) or did not (D) receive low-dose FX (1 U/kg) 5 minutes before injury. Each symbol represents an individual mouse. Horizontal bars represent median values. Statistical analysis was performed using an unpaired Student t test.
Fitusiran improves hemostasis in FX deficiency. (A) f10low mice received 2 doses of fitusiran at a 1-week interval. Seven days after the last dose, plasma was analyzed for antithrombin activity in nontreated (red symbols) and fitusiran-treated f10low mice (blue). Each symbol represents an individual mouse, and horizontal bars represent mean values. Statistical analysis was performed using an unpaired Student t test. (B-C) TF-induced thrombin generation in nontreated (red curves) and fitusiran-treated (green curves) f10low mice. Plasma levels of FX were <1% (B) or plasmas were spiked with FX (2% of normal plasma) before measuring thrombin generation. Solid lines represent median values, and colored areas represent interquartile range. Detailed values of thrombin generation parameters (lag time, ETP, thrombin peak, and time to peak) are summarized in Table 2. (D-E) The number of clots over a 30-minute period observed in f10low mice (red) and fitusiran-treated f10low mice (blue) that did (E) or did not (D) receive low-dose FX (1 U/kg) 5 minutes before injury. Each symbol represents an individual mouse. Horizontal bars represent median values. Statistical analysis was performed using an unpaired Student t test.
Thrombin generation parameters in f10WT mice and f10low mice: effect of fitusiran
| . | No extra FX . | With 2% FX . | ||||
|---|---|---|---|---|---|---|
| f10low (n = 9) . | f10low + fitusiran (n = 4) . | P value . | f10low (n = 3) . | f10low + fitusiran (n = 4) . | P value . | |
| Lag time (min) | 7.0 ± 2.8 | 3.3 ± 1.6 | .033 | 2.3 ± 0.6 | 3.8 ± 2.5 | .365 |
| ETP (nM·min) | 149 ± 126 | 904 ± 404 | .0002 | 783 ± 463 | 1724 ± 260 | .018 |
| Peak thrombin (nM) | 14 ± 8 | 36 ± 20 | .014 | 32 ± 11 | 59 ± 10 | .019 |
| Time to peak (min) | 12 ± 6 | 13 ± 1 | .752 | 13 ± 6 | 17 ± 5 | .379 |
| . | No extra FX . | With 2% FX . | ||||
|---|---|---|---|---|---|---|
| f10low (n = 9) . | f10low + fitusiran (n = 4) . | P value . | f10low (n = 3) . | f10low + fitusiran (n = 4) . | P value . | |
| Lag time (min) | 7.0 ± 2.8 | 3.3 ± 1.6 | .033 | 2.3 ± 0.6 | 3.8 ± 2.5 | .365 |
| ETP (nM·min) | 149 ± 126 | 904 ± 404 | .0002 | 783 ± 463 | 1724 ± 260 | .018 |
| Peak thrombin (nM) | 14 ± 8 | 36 ± 20 | .014 | 32 ± 11 | 59 ± 10 | .019 |
| Time to peak (min) | 12 ± 6 | 13 ± 1 | .752 | 13 ± 6 | 17 ± 5 | .379 |
Fitusiran ameliorates the bleeding tendency in FX deficiency
We next evaluated the effect of fitusiran treatment in f10low mice on their in vivo hemostatic activity. Compared with untreated f10low mice, fitusiran-treated f10low mice had a significantly increased number of clots in the SVP model: 8 ± 6 clots over a 30 minute period (n = 14) vs 2 ± 2 clots over a 30 minute period (n = 28; P = .0029; Figure 6D). A similar prohemostatic effect was observed in the tail-clip assay. While vehicle-treated f10low mice displayed increased blood loss compared with f10WT mice (P = .0068), fitusiran treatment reduced blood loss similar to that of f10WT mice (P = .319 compared with f10WT mice; supplemental Figure 4).
We also performed an analysis in which fitusiran-treated and untreated f10low mice received a low dose of FX (1 U/kg) 5 minutes before injury. As expected, infusion of 1 U/kg FX resulted in increased clot formation (11 ± 6 clots over a 30 minute period; n = 13; Figure 6E). However, combined with fitusiran treatment, a significant further increase in the number of clots was obtained (17 ± 4 clots over a 30 minute period; n = 6; P = .0262; Figure 6E). Interestingly, this number of clots was no longer different from that observed in f10WT mice (P = .35). These data show that fitusiran has a substantial procoagulant effect in a mouse model for FX deficiency.
Discussion
FX deficiency produces a variable bleeding tendency, the frequency and severity of which are dependent on residual FX activity levels. Its treatment is currently mainly dependent on replacement therapy using concentrates enriched in FX. By using a novel mouse model for FX deficiency, we show here that small interfering RNA–mediated transcriptional silencing of antithrombin is associated with reduced bleeding. It seems possible, therefore, that this approach could potentially be used for the prophylactic treatment of patients with an FX deficiency and bleeding complications.
To test new therapeutic options for the management of FX deficiency, we developed a novel mouse model. At present, 2 other mouse models for FX deficiency have been reported. First, full FX deficiency induced by targeted disruption of the f10 gene (f10KO) resulted in partial embryonic lethality and lethal perinatal bleeding.20 Their model suggests that a total FX deficiency is incompatible with life, at least at the early stage of embryonic development. This is in line with the notion that deletion of both F10 alleles in humans has not yet been reported. Nevertheless, the early perinatal mortality of these mice limits preclinical evaluation. To overcome premature death in f10KO mice, Tai and colleagues elegantly restored low-level FX expression by reconstituting the f10 gene with a variant exon 8 that contains the human FX Friuli mutation (Pro343Ser missense mutation).21 Homozygous FX Friuli mice were viable and characterized by normal antigen levels but reduced plasma activity levels (6% of normal level).21 Consequently, this knockin mouse model represents a mild-to-moderate type II FX deficiency. Unfortunately, no data on the bleeding phenotype of these mice were reported. In this respect, our mice represent the first model for type I FX deficiency, with both activity and antigen levels being below the limit of detection upon recombination of the f10 gene.
Regarding the overall phenotype of these mice, it was surprising that, when unchallenged, f10low mice have a similar 1-year survival as their wild-type littermates, and no overt spontaneous bleeds were observed. Additional studies are ongoing, in which a more detailed analysis of organs with regard to fibrosis, vascular leakage, and microbleeds is being included.
The reduced FX activity in plasma of f10low mice correlated with strongly reduced procoagulant activity in a diversity of activity assays, including PT, aPTT, and both TF- and FXIa-induced thrombin generation. It should be noted that in the thrombin generation assays, thrombin generation was not zero but that some minor, residual thrombin generation was observed. Residual thrombin generation was also present in vivo, as can be derived from our different models. First, some minimal fibrin formation was detected in the laser-injury model (ie, the green spots present in Figure 3A), which conceivably originates from thrombin being generated during the procedure. Second, the bleeding phenotype in the tail-clip assay was quite variable and less severe than that of f8KO mice (Figure 4). We feel that the most reasonable explanation for this is that residual levels of FX were available. At this point, it is unclear whether this relates to residual FX production by some untargeted hepatocytes and/or other cells, like fibroblasts, macrophages, or endothelial cells.14,22 Such residual FX production is not fully unexpected because there is no whole-body knockout of the f10 gene. Instead, the f10 gene is only inactivated in cells that are exposed to interferon, generated by the pI:pC–induced inflammatory response. These cells include hepatocytes and hematopoietic cells, with hepatocytes being the main source of circulating FX. It has been shown that this approach results in the virtual complete deletion of the floxed gene in the liver and spleen, whereas DNA recombination reaches only 20% to 70% in other tissues.23 Because small amounts of FX can be found in the extravascular space,24 it is also possible that there is still some FX retained in the extravascular space, which may become available locally upon vascular injury. However, we have not been able to detect any extravascular FX in the f10low mice yet when performing histological analysis of vessels and organs, which could be related to a lack of proper antibodies that are sensitive enough to detect low levels of extravascular FX. It is further relevant to mention that human plasma also contains minor amounts of fibrinogen-like protein 2, a serine protease that has the potential to convert prothrombin into thrombin.25,26 It cannot be excluded that traces of this protein are also present in murine plasma, which could contribute to minor thrombin generation.
Despite the presence of any residual, albeit undetectable, levels of FX, f10low mice exhibit a true bleeding phenotype. In both bleeding models applied (tail-clip and SVP models), increased bleeding was observed compared with the wild-type littermates. One intriguing observation was that in the tail-clip model, f10low mice displayed impaired primary hemostasis, with about 50% of the mice having no primary bleeding arrest after injury. Such impaired hemostasis is not observed in f8KO mice, nor, to the best of our knowledge, in f9KO mice. The underlying mechanism remains unclear. We considered that f10low mice could potentially manifest reduced platelet function. However, all relevant platelet receptors (GpIbα, GpIbβ, GpVI, αIIbβ3, integrin α2, and PAR4) were expressed normally (supplemental Figure 1). Furthermore, platelet aggregation induced by adenosine diphosphate, collagen, or thrombin was similar to that of platelets from f10WT mice (supplemental Figure 2). We suspect that levels of FXa or thrombin are too low to facilitate sufficient platelet activation or aggregation. This may also be compatible with the observation that after some initial platelet adhesion in the laser-injury model, the platelet aggregates are unstable and rapidly dissolve (Figure 3). The absence of sufficient thrombin generation also causes a lack of a firm fibrin network, further contributing to the disappearance of platelets from the site of injury.
The hemostatic characterization having confirmed that we have a unique model to test therapeutic approaches for FX deficiency, we set out to correct the bleeding in f10low mice, and initially, we treated mice by infusing purified FX before injury. Unsurprisingly, such an infusion immediately improved the hemostatic capacity of these mice, and bleeding was corrected in a dose-dependent fashion. We then decided to explore the possibility of applying a treatment option that has originally been designed for the treatment of hemophilia A and B, that is, fitusiran. Fitusiran treatment is associated with the transcriptional silencing of antithrombin expression. Antithrombin inhibits several serine proteases associated with coagulation, including thrombin, FXa, and FIXa. As such, our hypothesis was that a reduction in antithrombin would improve the hemostatic potential of these f10low mice. By using a similar dosage as reported previously,15 a strong reduction in antithrombin activity levels was indeed obtained (17 ± 6%). These residual levels of antithrombin are similar to those aimed for in the treatment of patients with hemophilia with fitusiran. In the initial stages of its clinical evaluation, with patients being dosed 50 or 80 mg fitusiran, residual antithrombin levels were between 10% and 20% of baseline.11 To reduce the risk of thrombosis, the final dosing regimen aims for residual antithrombin levels between 15% and 35%.11 Fitusiran treatment had no effect on clotting times in the PT or aPTT (supplemental Figure 3), which is not unexpected with PT and aPTT being relatively insensitive to antithrombin levels.27 This is in agreement with the observation that no relevant changes in PT and aPTT clotting times were observed in a phase 1 clinical trial evaluating fitusiran in patients with hemophilia A and B with inhibitors.28 Whereas no changes in PT or aPTT were detected, reduced antithrombin levels in f10low mice were associated with increased in vitro thrombin generation in f10low mice. Thrombin peak levels were increased twofold to threefold, and ETP was increased sixfold. In this regard, the effect of fitusiran on thrombin generation resembles that of what is observed in patients with hemophilia, who are characterized by a thrombin peak being increased threefold to fourfold.8-10,28
Because only a few patients with FX deficiency have levels below 1%, we also performed experiments in which we spiked the plasma of f10low mice with 2% FX, conditions of a moderate FX deficiency. Even under these conditions, thrombin generation was significantly increased in the plasma of fitusiran-treated f10low mice: thrombin peak and ETP were increased another twofold, with the ETP being in the range of f10WT mice.
Decreasing antithrombin levels in patients with hemophilia to <35% is associated with a significant reduction in bleeding events, with median annual bleeding rates approaching 0.8-10,12 A similar beneficial effect on hemostasis was observed in the SVP model, with a significantly increased number of clots that were produced over a 30-minute period. Again, we were able to detect this in mice having <1% residual FX as well as in mice that received a low dose of FX (1 U/kg; resulting in FX levels between 1.5 and 2%). Moreover, the blood loss of fitusiran-treated f10low mice was similar to that of f10WT mice in the tail-clip model (supplemental Figure 4).
These data demonstrate that fitusiran might be a potential candidate for the treatment of patients with FX deficiency. At this point, it would be difficult to predict how efficient its treatment for patients with FX deficiency would be. We know from our experience with other nonfactor approaches that there is no direct extrapolation possible from data obtained in animal models to the human situation. Nevertheless, fitusiran could be an attractive alternative to the current treatment options based on FX-replacement therapy.
Acknowledgment
The authors thank Eric Bun for technical assistance during the study.
Authorship
Contribution: S.V., G.M., H.M., F.A., L.C., L.P.-D., and C.D. performed experiments; S.V., G.M., H.M., L.P.-D., C.D., P.J.L., and O.D.C. analyzed data; C.C., C.V.D., P.J.L., and O.D.C. designed and supervised the study; P.J.L. wrote the initial version of the manuscript; and all authors contributed to its final editing.
Conflict-of-interest disclosure: P.J.L. received research support to the institute from Sanofi for this study and from Roche, Sobi, and Pfizer for unrelated studies. The remaining authors declare no competing financial interests.
Correspondence: Peter J. Lenting, INSERM U1176, 80 rue du Général Leclerc, Le Kremlin-Bicetre 94276, France; email: peter.lenting@insrem.fr.
References
Author notes
S.V. and G.M. contributed equally to this study.
Data are available on request from the corresponding author, Peter J. Lenting (peter.lenting@insrem.fr).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal