Numerous antibody-drug conjugates (ADCs) are being developed for cancer immunotherapy. Although several of these agents have demonstrated considerable clinical efficacy and have won Food and Drug Administration (FDA) approval, in many instances, they have been characterized by adverse side effects (ASEs), which can be quite severe in a fraction of treated patients. The key hypothesis in this perspective is that many of the most serious ASEs associated with the use of ADCs in the treatment of cancer can be most readily explained and understood due to the inappropriate processing of these ADCs via pathways normally followed for immune complex clearance, which include phagocytosis and trogocytosis. We review the key published basic science experiments and clinical observations that support this idea. We propose that it is the interaction of the ADC with Fcγ receptors expressed on off-target cells and tissues that can most readily explain ADC-mediated pathologies, which therefore provides a rationale for the design of protocols to minimize ASEs. We describe measurements that should help identify those patients most likely to experience ASE due to ADC, and we propose readily available treatments as well as therapies under development for other indications that should substantially reduce ASE associated with ADC. Our focus will be on the following FDA-approved ADC for which there are substantial literatures: gemtuzumab ozogamicin and inotuzumab ozogamicin; and trastuzumab emtansine and trastuzumab deruxtecan.
Introduction
The development and application of antibody-drug conjugates (ADCs) in the treatment of cancer is characterized by a voluminous literature based on the implementation of these agents in numerous malignancies.1-7 There have been substantial successes, and the ADCs generally have greater efficacy and specificity with fewer adverse side effects (ASEs) than traditional cancer chemotherapy, based on targeting by smaller amounts of these cytotoxic agents to antigens principally expressed on cancer cells.
However, with few exceptions, almost all ADCs have been reported to induce unexplained ASEs in a fraction of treated patients. The ASE-associated pathologies, which in some cases have been fatal, include reduction of normal blood cell counts (thrombocytopenia and neutropenia), liver damage (sinusoidal obstruction syndrome [SOS]) and increased levels of liver enzymes in the circulation, and lung damage (interstitial lung disease [ILD], also called pneumonitis).7-27 The mechanisms that mediate these ASEs are not likely to be due to nonspecific off-target effects.22 Our key hypothesis is that many of the ASEs associated with the use of ADCs in the treatment of cancer can be most readily explained and understood due to inappropriate processing of ADCs via pathways normally followed for immune complex (IC) clearance.28-32
We will review key published in vitro experiments, nonhuman primate (NHP) studies and clinical observations, some of which appear unrelated to the action of ADCs that lead us to this hypothesis. Based on these findings, we describe measurements that should help identify patients most likely to experience ASEs due to ADCs. We propose readily available treatments as well as modalities under development for other indications that should substantially reduce ASEs associated with ADCs. Our focus will be on the Food and Drug Administration (FDA)-approved ADCs for which there are substantial literatures: gemtuzumab ozogamicin (Mylotarg) and inotuzumab ozogamicin; and trastuzumab emtansine (T-DM1) and trastuzumab deruxtecan (T-DXd).12,18,23,24,33-38
Mechanisms of IC clearance
We hypothesize that mechanisms underlying ASEs associated with ADCs are the natural processes of Fcγ receptor–mediated elimination of IC containing immunoglobulin G (IgG).28-32 In these reactions, Fcγ receptor–expressing acceptor cells bind to and acquire IgG-containing IC, via phagocytosis or trogocytosis, that are either in solution (soluble IC), particulate IC, or are bound to donor cells. Particulate IC can include platelets with bound anti-platelet IgG or bound IC.
When the IC is bound to a donor cell, it can be transferred to an acceptor cell through a process called trogocytosis. This reaction does not involve phagocytosis; after formation of an immunological synapse between the donor and acceptor cell, a portion of donor cell membrane, along with the IC, is pinched off and internalized by the acceptor cell in a process requiring actin polymerization.32,39-42 We and others have demonstrated that moieties covalently and noncovalently bound to IgG in these ICs (eg, fluorescent or 125I labels; and dsDNA, respectively) are taken up and internalized by acceptor cells. In most but not all cases, the donor cell “escapes” with modest but no fatal damage to its plasma membrane.32,39-42 Trogocytosis can occur in the bloodstream and within tissues. We first reported on downregulation of CD20 (by trogocytosis) in circulating malignant B cells in patients with chronic lymphocytic leukemia treated with rituximab.43 Rapid clearance, initially, of B cells with the highest levels of CD20 may be in part responsible for these observations, but we note that 24 hours after rituximab infusion, the remaining circulating B cells had CD20 levels lower than the levels observed before rituximab infusion.43 Seliem et al and Laurent et al44,45 found that after rituximab therapy, B cells located in bone marrow, spleen, and lymph nodes suffer substantial loss of CD20. These decreases can also be due to epitope masking, downregulation and/or internalization of CD20, as well as trogocytosis, as reviewed by Roesser et al and Zhou et al.46,47
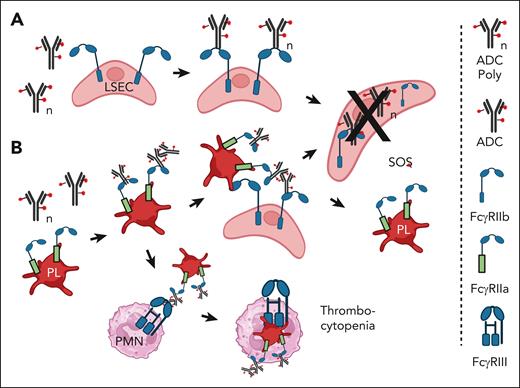
There is abundant evidence that all Fcγ receptors, including the “inhibitory” Fcγ receptor IIb, can mediate trogocytosis.48,49 This is particularly important with respect to the action of liver sinusoidal endothelial cells (LSECs), which express Fcγ receptor IIb. These cells have been demonstrated to be quite efficient at clearing particles, small IC, and therapeutic antibodies from the circulation.50-53 On this basis, following the report of James et al,51 SOS mediated by certain ADC is likely a consequence of internalization, via Fcγ receptor IIb, of ADC by LSECs, which are subsequently poisoned by the cytotoxic payload,51 as illustrated in Figure 1A. It should be possible to use intravenous immunoglobulin G (IVIG) or mAbs that specifically block Fcγ receptor IIb (such as 2B6 or Bl-1206) to inhibit trogocytosis. 56,57 This may require F(ab)2 forms of the mAbs to prevent the killing of B cells.
Proposed mechanism of tissue injury mediated by Mylotarg. (A) SOS is induced in the liver when FcγRIIb on LSECs mediates internalization of monomers or aggregates of ADC such as Mylotarg. The LSECs are killed by the ADC toxic payload.48-53 (B) Platelets bind ADC aggregates via FcγRIIa and are localized to the liver. LSEC take up and internalize the ADCs via FcγRIIb and are killed by the cytotoxic payload. It is not clear whether the platelets return to the circulation. Alternatively, monocytes and/or neutrophils (PMN) phagocytose the opsonized platelets via FcγRIII.54,55 Figure created with BioRender.com.
Proposed mechanism of tissue injury mediated by Mylotarg. (A) SOS is induced in the liver when FcγRIIb on LSECs mediates internalization of monomers or aggregates of ADC such as Mylotarg. The LSECs are killed by the ADC toxic payload.48-53 (B) Platelets bind ADC aggregates via FcγRIIa and are localized to the liver. LSEC take up and internalize the ADCs via FcγRIIb and are killed by the cytotoxic payload. It is not clear whether the platelets return to the circulation. Alternatively, monocytes and/or neutrophils (PMN) phagocytose the opsonized platelets via FcγRIII.54,55 Figure created with BioRender.com.
Another important and perplexing problem must focus on understanding why only certain cells appear to suffer damage mediated by toxic payloads of a particular ADC. For example, lung macrophages are most susceptible to damage mediated by T-DM1 and T-DXd, whereas LSECs are most likely to suffer damage mediated by ADC containing ozogamicin (Mylotarg and inotuzumab ozogamicin).12,18,23,24,33-38 We will review reports that provide evidence for the proposed “trojan horse” mechanisms that lead to ADC-mediated cytotoxicity of Fcγ receptor–expressing acceptor cells. There are differences in the details of the processes, but available evidence strongly suggests that the underlying reactions are all mediated by Fcγ receptors.
Supporting evidence: in vitro and NHP models
Macrophages can be killed
In 2007, Wang et al59 reported that a covalent methotrexate-human serum albumin-IgG complex (IVIG-HSA-MTX) is internalized by macrophages that are then killed. It was not determined whether the conjugate was monomeric or aggregated. Proof for the reaction being mediated by Fcγ receptors was provided by inhibition experiments: naïve unconjugated IVIG served as a competitive inhibitor that blocked the cytotoxic action of the IVIG-HSA-MTX. This experiment provides proof of principle that internalization of an IgG-containing cytotoxic agent can kill an Fcγ receptor–expressing cell. It also augurs for a reasonable approach for inhibiting ASEs due to ADCs: block Fcγ receptors on acceptor cells with IVIG. We have demonstrated that small amounts of IgG are quite effective at blocking trogocytosis in vitro.40,41
ADC-induced thrombocytopenia can lead to killing of macrophages
Huang et al reported that human platelets, which express Fcγ receptor IIa, can bind ICs containing human IgG, and these “IgG-opsonized” platelets are then subject to phagocytosis mediated by neutrophils and monocytes.54 Based on this mechanism, if the platelets were to bind an ADC, this would ultimately lead to destruction of the platelets due to phagocytosis as well as downstream poisoning and destruction of acceptor cells that internalized the ADC along with the platelets, as illustrated in Figure 1B.
This process is indeed demonstrable in an NHP model of liver injury based on infusion of an antibody-calicheamicin conjugate.55 Guffroy et al examined the processing of a “non-binding” mAb-calicheamicin conjugate (PF-0259) as a model for processing of the CD33-specific ADC gemtuzumab ozogamicin (Mylotarg) and the CD22-specific ADC inotuzumab ozogamicin. These ADCs can induce severe liver damage in a fraction of treated patients.19,33-36,38,60,61 The NHP model was designed to preclude any specific targeting effects (binding of the agent to CD33- or CD22-expressing cells) that might be responsible for cell damage mediated by these ADCs. The investigators found that infusion of PF-0259 in NHP resulted in rapid clearance of platelets from the circulation (ADC-induced thrombocytopenia) followed by “acute liver endothelial cell toxicity associated with platelet sequestration in liver sinusoids,” which resembled the early stages of SOS. This process is also illustrated schematically in Figure 1B. Modest aggregation of the ADC would be sufficient for it to bind to Fcγ receptor IIa on platelets and thus initiate the cell-killing pathway. This study also provides strong evidence that ADC can mediate tissue damage that is not related to the designated targeting site of the ADC.
Aggregated ADCs can be processed as ICs
The report by Aoyama et al is particularly relevant with respect to the postulated mechanisms involving Fcγ receptors.62 They found that aggregated ADCs are processed as ICs and could therefore be readily taken up by cells expressing Fcγ receptors, thus leading to the killing of the ingesting acceptor cells. This is an important concept; there is evidence that, due to a high level of drug conjugated to the carrier antibody, ADCs are subject to aggregation.63 We note that if the ADC were to bind to their cognate target cells, the ADC bound to the cells could therefore be processed as cell-bound IC and would then be subject to trogocytosis or phagocytosis by acceptor cells expressing Fcγ receptors, thus leading to killing of acceptor cells. Aoyama found that this “off-target cytotoxicity” could be reduced by using an FcγR-blocking antibody, which may provide effective and specific therapy to prevent ADC-induced cytotoxicity of susceptible nontarget cells expressing Fcγ receptors. They also found that engineering the Fc region of the ADC to “silence” its Fc function suppressed its cytotoxic potential, thereby providing additional evidence for the proposed cytotoxic mechanism.
Supporting evidence, in vivo: clinical studies/observations
Findings with ado-T-DM1
A clinical-basic science investigation with T-DM1, reported by Ansary et al,10 reinforces the NHP study of Guffroy.55 Ansary et al found that infusion of T-DM1 led to a rapid 50% decrease in levels of circulating 111indium-labeled autologous platelets in patients with breast cancer. They concluded that this observation points to “a direct toxic effect of T-DM1 on patients’ autologous circulating platelets.” Previous investigations by others had demonstrated that T-DM1 could also negatively influence maturation of platelet precursors, megakaryocytes.64,65 However, the more recent reports provide more direct and compelling evidence. We suggest that prophylactic “treatment” with IVIG would likely prevent the acute decrease in platelet counts mediated by T-DM1.
Results with Mylotarg
Evidence suggesting the importance of Fcγ receptors in mediating ASEs of an ADC was reported in 2005 by Leung and Liang.66 They found, in a study on a single patient, that IVIG could rapidly reverse thrombocytopenia induced by Mylotarg. This prescient study did not identify a particular mechanism mediated by Mylotarg. However, it is consistent with the hypothesis that binding of the ADC (likely aggregated) to platelets is responsible for the initial step that leads to their removal from the circulation, and by blocking Fcγ receptors with IVIG, platelet clearance can be prevented as well.
ASEs associated with the use of Mylotarg were largely mitigated by decreasing therapeutic doses by approximately twofold to threefold.37,67 This is a relatively small change but could be quite reasonable based on examining IVIG-based therapies. Mechanisms of action of IVIG involve several independent pathways,68 but our focus is on its blockade of Fcγ receptors, which led to its approval for the treatment of immune thrombocytopenia.69 IVIG is delivered IV at high doses (∼1 g per kg), inducing an increase in circulating IgG of ∼10 mg per mL. Under these conditions, it should increase the IgG bloodstream concentration twofold to threefold, thus blocking the action of Fcγ receptors, which would otherwise promote clearance and destruction of IgG-opsonized platelets. We suggest that the 2 fold to 3 fold decreased doses of Mylotarg present less of a “challenge” and can be more readily blocked by endogenous IgG even in individuals with lower levels of plasma IgG.
Can IgG prevent ASEs associated with ADCs?
Nagelkerke et al70 demonstrated that very small amounts of IgG (∼10 μg per mL) are adequate to block in vitro phagocytosis of IgG-opsonized erythrocytes. This leads to an unresolved conundrum as to why much more IgG appears to be required to block Fcγ receptors in vivo. We note that a specific role for sialylated IgG has been precluded.70 The clinical findings from IVIG treatment of patients with immune thrombocytopenia therefore indicate that the absolute concentration of IgG in the bloodstream (∼5-18 mg per mL) may have a major impact on whether an ADC exerts an ASE and is able to engage with Fcγ receptors.69,71 We suggest that in individuals with higher plasma concentrations of IgG (>10 mg per mL), IVIG would not be needed, because they would already be protected against the adverse effects of ADC due to adequate natural blockade of Fcγ receptors. On the contrary, if the plasma concentration of IgG is lower (eg, less than ∼7 mg per mL), interaction of infused ADC with Fcγ receptors would be considerably more pronounced (less inhibition), and infusion of IVIG would be required to increase the concentration of blocking IgG and thereby preclude the interaction of ADC with Fcγ receptors. On this basis, measures of the bloodstream concentration of IgG may provide diagnostic information indicating that patients with lower levels of plasma IgG (<7 mg per mL) are much more likely to experience ASEs due to ADCs than patients with levels >10 mg per mL. Gonzalez-Quintela et al71 have reported that ∼10% of normal individuals have plasma IgG concentrations <7 mg per mL.
The Kurlander effect72
The mechanisms we are examining do not include direct binding of ADC to antigenic sites on macrophages, but if this were to occur, then subsequent synergistic binding of the ADC to Fcγ receptors on macrophages could play a role in ADC-induced macrophage damage.5
ADC targeting of HER2
Trogocytosis can lead to killing of cells in vitro
We and others have demonstrated that cell lines and isolated human monocytes promote trogocytosis of human epidermal growth factor receptor 2 (HER2) cells opsonized with trastuzumab (TRA).41,73,74 The in vitro reaction is complete in ∼30 minutes, during which time HER2 has transferred to acceptor cells, and the donor cells are released alive and well. However, Matlung et al reported that under similar experimental conditions, neutrophils execute a fatal trogocytosis of TRA-opsonized cells, defined as “trogoptosis.”75,76 Velmurugan et al77 reported that long-term incubation (24 hours) of macrophages with TRA-opsonized cells also leads to donor cell killing. In these reactions, the acceptor cells take up and internalize both TRA and its ligand, HER2, and the opsonized donor cells suffer many injuries to their plasma membranes and may be killed due to membrane damage and/or reductions in critical signaling mechanisms. Velmurugan et al77 demonstrated that IVIG blocked trogocytosis, which emphasizes a possible approach to inhibit the killing of acceptor cells that would otherwise take up the ADC. Based on previous investigations of trogocytosis, ligands attached to TRA in ADC (eg, cytotoxic agents, such as the microtubule inhibitor DM1 in T-DM1, or deruxtecan, the topoisomerase inhibitor, in T-DXd) should also be taken up and internalized by acceptor neutrophils and macrophages. This “trojan horse” mechanism can lead to the poisoning and killing of Fcγ receptor–expressing acceptor cells.
Evidence for TRA-mediated trogocytosis in vivo
It has been reported that TRA therapy leads to substantial reductions in the levels of HER2 in cancer cells in patients after treatment for breast, gastric, or gastroesophageal cancer.78 It is quite likely that the loss of HER2 was due to trogocytosis. Indeed, Saeki et al79 and Suzuki et al74 reported that TRA treatment leads to the loss of HER2 from targeted cells. They suggested that this could compromise the efficacy of TRA-based ADCs if the ADCs were to be administered to patients recently treated with TRA. The previous treatment would lead to decreases in levels of the target HER2 on malignant cells.78 We note that other mAbs specific for HER2 also mediate trogocytosis.80
T-DXd in an NHP model
Additional support for the role of Fcγ receptors in ADC-induced pathologies is found in a 2020 study of the action of T-DXd in NHP.81 Kumagai et al81 reported that high doses of the ADC (likely to lead to some bloodstream aggregation) can induce ILD and liver damage based on its interaction with macrophages. Although the detailed pathway by which this ADC exerted its effects was not specifically elaborated, the investigators recognized that pathways associated with Fcγ receptors were likely, and they suggested that engineering/silencing the Fc region of the ADC to “abolish binding to FcγR” should be investigated. This strategy addresses the same question as to the mechanism as does the use of IVIG but may be more complex because it might also substantially reduce the efficacy as well as the lifetime of the ADC in the bloodstream. In the report by Kumagai et al,81 relatively high doses of ADC were required to induce pathologies, and few of the lower-dosed animals experienced ILD. We again suggest that endogenous IgG in the bloodstream should play a protective role. Therefore, quantitation of IgG levels in these studies may be quite revealing if it can be demonstrated that animals with higher levels of plasma IgG are less susceptible to ADC-induced lung damage. We note that Hallowell et al discussed the application of IVIG for a variety of conditions associated with ILD, but ADC-induced ILD was not considered.82
Although ∼10% of individuals who receive ADC derived from TRA experience ILD, sporatic reports indicate that ∼1% of patients who receive unconjugated TRA therapy also experience ILD.21,83,84 We suggest that, in these later patients, ICs containing TRA are deposited in the lungs, but their modest inflammatory effects would be amplified substantially if deruxtecan is coupled to the TRA (in an ADC) and then the ADC is deposited in the lungs.
An important and unresolved question centers on identifying which Fcγ receptors in the lung are most responsible for uptake of the ADC associated with TRA. Based on the report of Bruggeman et al, which analyzed Fc expression in macrophages in a variety of human tissues, it appears that FcγRI and IIa are most likely responsible for taking up the ADC in the lung.85 The inhibitory receptor, FcγRIIb, which is expressed at high levels in the liver, can be ruled out due to its very low expression levels in the lungs.
Targets of TRA in the circulation
Use of TRA-based ADC in treatment of solid tumors expressing HER2 is subject to additional complications. There is considerable evidence that in several HER2+ malignancies, circulating tumor cells express HER2.86-91 Administered ADC (eg, T-DM1 or T-DXd) could bind to these cells, and then the circulating cell-bound ADC could be subject to trogocytosis by acceptor cells (monocytes, neutrophils, or fixed tissue macrophages), ultimately leading to the killing of these acceptor cells. Figure 2A illustrates the proposed role of lung macrophages in inducing ILD.
ILD can occur when lung macrophages ingest ADCs containing trastuzumab. (A) The ADCs are bound to circulating cancer cells (CCs) and are removed from the cells by LMs via trogocytosis. The LMs are killed by the ADC payload. This reaction can also lead to the killing of neutrophils, which can mediate trogocytosis of trastuzumab-opsonized cells.75-81,85 (B) Soluble ICs composed of ADCs containing trastuzumab and the extracellular domain of HER2 (Antigen [Ag]) can be taken up by LM. Again, the LMs are killed by the ADC payload.92-96 LM, lung macrophage. Figure created with BioRender.com.
ILD can occur when lung macrophages ingest ADCs containing trastuzumab. (A) The ADCs are bound to circulating cancer cells (CCs) and are removed from the cells by LMs via trogocytosis. The LMs are killed by the ADC payload. This reaction can also lead to the killing of neutrophils, which can mediate trogocytosis of trastuzumab-opsonized cells.75-81,85 (B) Soluble ICs composed of ADCs containing trastuzumab and the extracellular domain of HER2 (Antigen [Ag]) can be taken up by LM. Again, the LMs are killed by the ADC payload.92-96 LM, lung macrophage. Figure created with BioRender.com.
Alternatively, the extracellular domain of HER2 can be released from tumor cells, and this soluble fragment of HER2 has been demonstrated in the circulation of patients with several forms of cancer.67,92-94 HER2 can form dimers,95 and it is reasonable to anticipate that soluble IC containing the extracellular domain of HER2 and either T-DM1 or T-DXd could be taken up by acceptor cells that express Fcγ receptors (Figure 2B). Circulating ICs deposit in the liver, spleen, or lungs, depending upon their stoichiometries.29,31,32,37,85,96 ILD associated with the treatment of HER2+ cancers could also be initiated due to internalization by lung macrophages of ICs containing these TRA-based ADCs.
On the basis of these observations, it is not surprising that ASEs are also associated with the treatment of HER2+ gastric cancer with the ADC T-DXd.9,36,61 Decreased neutrophil counts as well as ILD were observed in a fraction of treated patients. Based on the mechanisms we have discussed, it is quite likely the decrease in neutrophil counts was due to trogocytosis of the ADC by neutrophils, thus leading to their poisoning and elimination. ILD was previously reported for patients with HER2+ breast cancer who received this ADC, and in this case, it again appears that lung macrophages take up the ADC.97
In 2020, Shitara36 reported positive results for use of T-DXd compared with chemotherapy for the treatment of HER2+ gastric cancer. Once again, adverse effects associated with T-DXd included decreased neutrophil counts as well as ILD in a fraction of the treated patients. These findings can also be due to trogocytosis. Clinical evidence for the action of TRA in promoting loss of HER2 from targeted cancer cells in gastric cancer was also reported by Pietranonio et al.78
Recently Abulhelwa et al9 exhaustively reviewed 14 studies in which T-DXd was used in the treatment of a variety of HER2+ solid cancers (>1100 patients) and found that ILD was induced in ∼11% of the treated patients. They did not identify a likely mechanism but recognized that the toxic payload, deruxtecan, a topoisomerase I inhibitor, was likely responsible for the adverse effects. We suggest that in patients who experience ILD, T-DXd is taken up by lung macrophages due to IC processing via trogocytosis or phagocytosis. Moreover, as mentioned earlier, those individuals with the lowest titers of circulating IgG are likely to be the most susceptible to this reaction.
Proposed studies and potential therapies
Blockade of Fcγ receptors to abrogate acceptor cell killing by ADC can readily be tested in vitro: react Fcγ receptor–expressing acceptor cells with donor cells preopsonized with their cognate ADC. This procedure should lead to trogocytic uptake and internalization of the ADC by the acceptor cells, followed by their killing. This approach could also be used to screen ADC under development for potential off-target ASEs.
We have reported that trogocytosis of substrates from donor cells to acceptor cells is quite rapid (half-life of ∼5 minutes), and this reaction can be blocked in vitro by addition of small amounts of IgG.41,73 Therefore, use of IgG/IVIG or mAbs specific for Fcγ receptors should prevent the predicted killing of Fcγ-expressing acceptor cells in this reaction. Cytochalasin D blocks actin polymerization and trogocytosis,40 but it is likely to also block ADC-mediated killing of acceptor cells in vitro. We note that new reagents, IgG Fc trimers, are under development.98 These trimers should be more cost-effective, be easier to produce and administer, and have the same blocking mechanism of action as IVIG.98 We emphasize that these orthogonal “therapies” that focus on blocking Fcγ receptors should not inhibit the positive therapeutic effects of ADC. In brief, individuals treated with IVIG should have the same level of protection against the ASEs of the ADC as “naturally protected” subjects who have high levels of circulating IgG in the absence of IVIG therapy.
We suggest that a retrospective examination of patient records from clinical trials and studies may reveal a correlation between serum IgG levels and the frequency/severity of ASEs associated with treatment with ADC. Alternatively, future measurements of serum IgG levels of patients before and during treatment with ADC may be informative and are certainly readily accomplished.
Trogocytosis has also been reported for other FDA-approved mAbs such as daratumumab.99 It is likely that the ASEs we have discussed here will be observed with ADCs constructed in the future. Moreover, the approaches we have delineated for assessing and reducing ASEs may prove to be useful in addressing possible future problems associated with newly developed ADCs and/or with possible adverse effects associated with the future use of radioconjugates of mAbs.
Summary
In summary, we have cited numerous lines of evidence that point to Fcγ receptor–mediated reactions leading to substantial ASEs associated with the use of ADCs in immunotherapy of cancer. These ADCs have been demonstrated to have considerable levels of therapeutic efficacy, and the severe ASEs are associated with a minority of treated patients (usually ∼10%). Lower levels of IgG in the bloodstream are most likely to presage problems with ADCs, because these levels will not be adequate to block interaction and internalization of the “immune complexed ADC” by acceptor cells. On this basis, use of agents that block Fcγ receptors (IVIG or Fc multimers) should provide effective therapies to eliminate this problem. Alternatively, if specific Fcγ receptors on certain acceptor cells can be demonstrated to be responsible for the internalization of a particular ADC, then mAbs that block the action of these receptors may also be effective. Both in vitro experiments and tests in animal models and retrospective examination of existing clinical study data have the potential to validate these concepts.
Authorship
Contribution: R.P.T. and M.A.L. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ronald P. Taylor, Department of Biochemistry and Molecular Genetics, University of Virginia Health Sciences Center, Box 800733, Charlottesville, VA 22908-0733; email: rpt@virginia.edu.

![ILD can occur when lung macrophages ingest ADCs containing trastuzumab. (A) The ADCs are bound to circulating cancer cells (CCs) and are removed from the cells by LMs via trogocytosis. The LMs are killed by the ADC payload. This reaction can also lead to the killing of neutrophils, which can mediate trogocytosis of trastuzumab-opsonized cells.75-81,85 (B) Soluble ICs composed of ADCs containing trastuzumab and the extracellular domain of HER2 (Antigen [Ag]) can be taken up by LM. Again, the LMs are killed by the ADC payload.92-96 LM, lung macrophage. Figure created with BioRender.com.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/144/2/10.1182_blood.2024024442/2/m_blood_bld-2024-024442-gr2.jpeg?Expires=1769082921&Signature=0gWSOFpbtv2w4jsSMDcqvvR1yZWTE9k-EbKkCgCY1genQJ1gX~4NmUerbQ2Mr6jGrHK9ViPYBWGEzUt0uSqxnirgCZQCZEBZ~Gfcu6FUo9GBGZam4UN2sUPYUQqLTCH5RW64nIGLDzizU-c0Vep-HuqEhjwIIg5OAc9kV333Er1t3ds07b11bNXwlOPQRXq1RT633T21rDQ-ikz9AHjs2uDlNWvl9folBLq2rsKtTdQtMM5DozYua3pzlMaTT1ddBMQgfsyQ59G0g0i7hknOSOMqfBKrsuxynceiEeJz5M3XOri97MHcWooBmyWVY6jK1Piz1JyOZozFcpNXBUMSHg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal