In this issue of Blood, Zhou et al1 uncover a novel mechanism of resistance to menin inhibitors in KMT2A-rearranged (KMT2A-R) leukemias through Polycomb repressive complex 1.1 (PRC1.1) loss and epigenetic reactivation of noncanonical menin targets. They also demonstrate how BCL2 inhibitors can overcome this resistance in PRC1.1-deficient acute myeloid leukemia (AML).
The KMT2A gene on chromosome 11 is recurrently rearranged in a subset of childhood and adult AML. Despite recent advances in treatment, KMT2A-R AMLs are associated with dismal long-term survival outcomes.2 Small molecule inhibitors that block the interaction of the chromatin adapter menin with KMT2A are currently being evaluated in clinical trials for KMT2A-R patients and are showing great promise.3,4 By interrupting the menin-KMT2A interaction, which is crucial in leukemogenesis and preventing menin binding to chromatin, these inhibitors suppress leukemic progression and promote differentiation of myeloblasts.5,6 However, patients might develop acquired resistance after an initial response.3 One of the most common mechanisms of resistance described so far involves somatic MEN1 (menin) mutations, which reduce the affinity of the menin-KMT2A interaction.3 This mechanism accounts for nearly 40% of the menin inhibition resistance cases, and other mechanisms of resistance and sensitization have been identified using genetic screens.7,8
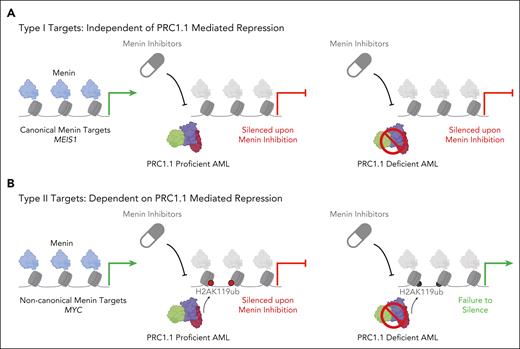
Zhou et al designed and constructed a CRISPR knockout library targeting chromatin regulators to identify potential epigenetic regulators of therapeutic response to menin inhibition. Their results revealed multiple independent PRC1.1 complex genes as top hits whose CRISPR inactivation conferred resistance to menin inhibitor treatment. Polycomb proteins are epigenetic modifiers involved in stem cell self-renewal and differentiation and are dysregulated in cancer. Prior studies have shown links between Polycomb complex genes and the menin-KMT2A complex in regulating gene transcription.9 By generating stable PRC1.1 knockout cell lines, the authors observed reduced myeloid differentiation upon menin inhibitor treatment and signs of drug resistance both in vitro and in vivo. They further investigated the PRC1.1 loss-associated resistance mechanism by performing RNA sequencing combined with chromatin occupancy studies. Strikingly, these studies identified 2 distinct types of genes. The first type (type I), such as the canonical menin target gene MEIS1, were silenced upon menin inhibitor treatment regardless of PRC1.1 activity. The second type (type II), exemplified by MYC, required PRC1.1-mediated deposition of the repressive histone 2 A lysine 119 ubiquitination (H2AK119ub) mark for their continued silencing following menin inhibitor treatment (see figure for schematic). Unlike the type I genes that remained repressed, these type II genes were epigenetically reactivated in cells deficient for PRC1.1 (see figure).
PRC1.1 plays a role in menin inhibitor target repression. (A) Canonical menin targets such as MEIS1 (left) are repressed upon menin inhibitor treatment irrespective of PRC1.1 activity (center and right). (B) Type II menin targets such as MYC show enrichment of both menin and PRC1.1-deposited H2AK119ub (left). In PRC1.1-proficient cells treated with menin inhibitor, these targets are silenced due to the suppression of menin and subsequent repression by H2AK119ub (center). However, in PRC1.1-deficient cells receiving menin inhibitor treatment, the loss of PRC1.1 reduces the H2AK119ub signal, leading to the epigenetic reactivation of these genes (right). Image created with BioRender.com.
PRC1.1 plays a role in menin inhibitor target repression. (A) Canonical menin targets such as MEIS1 (left) are repressed upon menin inhibitor treatment irrespective of PRC1.1 activity (center and right). (B) Type II menin targets such as MYC show enrichment of both menin and PRC1.1-deposited H2AK119ub (left). In PRC1.1-proficient cells treated with menin inhibitor, these targets are silenced due to the suppression of menin and subsequent repression by H2AK119ub (center). However, in PRC1.1-deficient cells receiving menin inhibitor treatment, the loss of PRC1.1 reduces the H2AK119ub signal, leading to the epigenetic reactivation of these genes (right). Image created with BioRender.com.
A closer look at the noncanonical (type II) menin targets revealed that these were enriched for MYC-associated genes, which accounted in large part to menin inhibitor resistance in PRC1.1-deficient cells. Therefore, Zhou et al conclude that dual targeting of menin and MYC may help overcome this resistance in PRC1.1-deficient AML cells. They demonstrate that combining BRD4 inhibitor ABBV075, or MYC inhibitor APTO-253 with menin inhibitors helped overcome resistance in PRC1.1-deficient AML cells.
Next, Zhou et al aimed to sensitize menin inhibitor-resistant AML cells to a more clinically relevant therapy than MYC inhibitors. Since AML cells with a diminished monocytic differentiation signature are more susceptible to BCL2 blockade,10 and PRC1.1-deficient cells exhibited an enrichment in primitive lineage-associated markers and a reduction in monocyte differentiation signature, the authors tested venetoclax, a BCL2 inhibitor, on PRC1.1-deficient cells. In these studies, they observed an increase in venetoclax-sensitive gene signatures and enhanced efficacy of venetoclax in PRC1.1-deficient KMT2A-R leukemias in vitro and in vivo. The authors thus describe the use of venetoclax as a strategy to counteract menin inhibitor resistance.
Finally, Zhou et al showed that AML cell lines adapted long term to resist menin inhibitor treatment show reduced expression levels of PRC1.1 complex genes and increased sensitivity to BCL2 inhibitors, showing that transcriptional adaptation to menin inhibition may involve PRC1.1 loss of expression. These data further support the association of PRC1.1 complex with menin inhibitor resistance and highlight the potential of venetoclax as a therapeutic strategy in such cases.
The Zhou et al study opens new avenues for further investigation. The next step will be to investigate this novel nongenetic resistance mechanism in patients with AML with acquired resistance to menin inhibitors, aiming to gain a more comprehensive understanding of whether this mechanism plays a role in humans with AML. Specifically, it remains to be seen whether epigenetic or genetic inactivation of PRC1.1 complex genes occurs in menin inhibitor-treated patients with AML, studies that will be enabled by the availability of larger cohorts of menin inhibitor-treated patient samples. Further exploration is needed to understand how menin and PRC1.1 complex regulate the expression of noncanonical menin gene targets, balancing their expression in physiological contexts, and how these targets are activated in the absence of PRC1.1 and under menin inhibition in the pathological context.
In summary, Zhou et al shed light on a novel set of noncanonical menin targets, such as MYC, that promote resistance in PRC1.1-deficient KMT2A-R AML cells and proposed a strategy to overcome this resistance.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal