In cases with clonal cytopenia of undetermined significance (CCUS) the challenge of identifying patients at higher risk for disease progression remains still an unmet need. The recent study by Xie et al in this issue of Blood offers an innovative approach to this clinical problem through the development of a clonal cytopenia risk score (CCRS).1 This novel model not only refines our predictive capacity but opens new pathways for targeted or even preventive therapeutic strategies that may transform the clinical management of patients with CCUS.
Over the past 5 decades, our approach to investigating myelodysplastic neoplasms (MDS) and acute myeloid leukemia (AML) has evolved significantly. We have transitioned from focusing on phenotypes to focusing on genotypes, we have developed successive prognostic scores, and we are gradually moving from generalized treatment protocols toward personalized therapy. Despite these advancements, we continue to rely on the percentage of blasts to distinguish MDS from AML, with a significant dependence on the subjective analysis in cytomorphology.2,3 These arbitrary thresholds fail to acknowledge the continuum that exists between these 2 entities. Even more, already 10 years ago it was recognized that this continuum from MDS to AML begins much earlier than previously thought, challenging us to rethink our diagnostic and treatment strategies.4,5 Increased sensitivity of molecular techniques and the broader application of exome and genome sequencing have unveiled the phenomenon of clonal hematopoiesis of indeterminate potential (CHIP), even in individuals without hematologic diseases.
By definition, individuals with CCUS exhibit detectable somatic mutations within their hematopoietic cells (often in genes such as DNMT3A, TET2, and ASXL1) with a variant allele frequency (VAF) of ≥2%, or they may have a clonal chromosomal abnormality alongside at least 1 unexplained cytopenia. Despite these genetic abnormalities, these patients do not fulfill the diagnostic criteria for MDS, as their bone marrow examinations do not show significant dysplasia or an elevated blast count. Consequently, CCUS was defined as a possible precursor lesion.2,3
Communicating the diagnosis, prognosis, and treatment options for MDS and AML has always been a complex challenge for both physicians and patients. Initiating discussions about a newly diagnosed case of CCUS or CHIP presents an even greater challenge. To address this issue, recent studies have begun to explore the risk of progression from CHIP to myeloid neoplasms (MNs).6,7 Both previously published individualized risk prediction models for patients with clonal hematopoiesis rely on molecular data (specific mutations and VAF) but also require various additional blood values (such as mean corpuscular volume or red cell distribution width) or biochemistry parameters (such as C-reactive protein or alkaline phosphatase). However, for CCUS, validated risk scores based on large cohorts remain scarce.
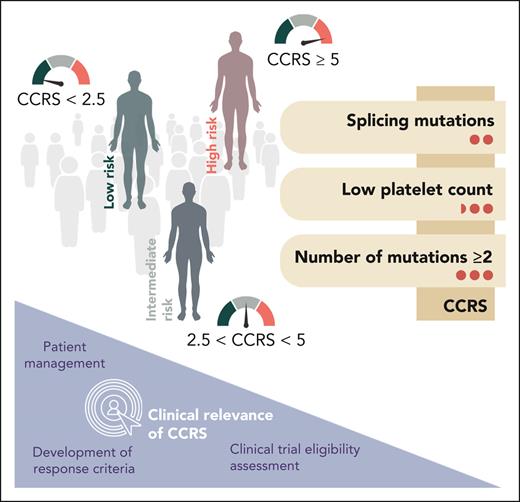
Xie et al now developed the CCRS by analyzing 357 patients with CCUS across 17 academic centers, identifying 3 key adverse prognostic factors: splicing mutations (score = 2 points), platelet count <100 × 109/L (score = 2.5 points), and having ≥2 mutations (score = 3 points). The easy and applicable score stratifies patients into low-risk (score <2.5), intermediate-risk (score 2.5-<5), and high-risk (score ≥5) groups for progressing to MN (see figure). The CCRS predicted the 2-year cumulative incidence of MN progression with remarkable accuracy across the different risk groups: low risk (6.4%), intermediate risk (14.1%), and high risk (37.2%) (P <.0001). The model was further validated on an independent cohort of 104 patients with CCUS, showing a c-index of 0.64 (P = .005) for stratifying the respective cumulative incidences of MN. Although the CCUS cohort was exclusively assembled from academic centers and may predominantly represent a high-risk population, reliability of the results is anticipated to be robust as all cases also underwent verification by bone marrow biopsy.
Risk stratification of clonal cytopenias: introducing a simplified score for a complex challenge. The CCRS is based on 3 parameters with different associated weights (right side; weights are indicated by red circles). Patients are categorized into low-, intermediate-, and high-risk groups (shown on the left). The clinical relevance of the CCRS is depicted in the bottom left corner.
Risk stratification of clonal cytopenias: introducing a simplified score for a complex challenge. The CCRS is based on 3 parameters with different associated weights (right side; weights are indicated by red circles). Patients are categorized into low-, intermediate-, and high-risk groups (shown on the left). The clinical relevance of the CCRS is depicted in the bottom left corner.
From a clinical perspective, assessing the risk of CCUS progression is expected to be straightforward, given that the respective clinical (blood count, ie, platelets) and molecular data (gene panel including genes of interest in splicing genes, epigenetic regulator genes, transcriptional and signaling pathways) are now readily accessible for most patients. The CCRS not only facilitates a more precise evaluation of progression risk but also promises to be invaluable in designing and comparing future clinical trials that are critically needed in this area.
The introduction of the CCRS marks a significant advancement, urging us to reflect on its wider impact in the field of hematology. The integration of genetic, clinical, and molecular data signifies a move toward precision medicine, envisioning a future where detailed genomic information drives personalized therapeutic regimens. This study not only sheds light on clonal cytopenia but also serves as a blueprint for future research in other hematologic (pre-)conditions, in which similar models could potentially predict and alter disease trajectories.
The work of Xie et al highlights this remarkable progress in medical research, underscoring the perpetual need for excellence in this field. Such achievements are only attainable through collective effort, sharing data, and fostering collaborations. This study exemplifies the power of combining scientific data with cross-institutional and international partnerships, challenging fortress mentality in favor of expanding our collective knowledge. The journey ahead is not solely about uncovering new knowledge; it is also laden with opportunities to redefine therapeutic options. With the daily influx of data and the existing advanced global infrastructure, including cloud computing, it is imperative to implement diverse approaches. This also encompasses the integration of artificial intelligence–based methods into medical workflows and routine diagnostics. Only the triad of reliable data, scientific cooperation, and sophisticated software tools will pave the way for better treatment, prolong patients’ lives, and enhance the quality of care.
Conflict-of-interest disclosure: T.H. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal