Key Points
Eltrombopag plus diacerein is a potential salvage therapy for patients with ITP who were unresponsive to 14 days of eltrombopag at full dose.
Addition of diacerein to eltrombopag yields higher overall response rates and longer duration of response than eltrombopag alone.
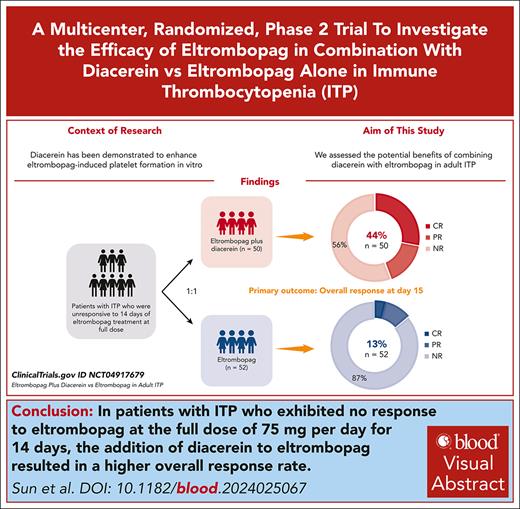
Visual Abstract
This study aimed to compare the efficacy and safety of eltrombopag plus diacerein vs eltrombopag alone in patients with primary immune thrombocytopenia (ITP) who were previously unresponsive to 14 days of eltrombopag treatment at the full dose. Recruited patients were randomly assigned 1:1 to receive either eltrombopag plus diacerein (n = 50) or eltrombopag monotherapy (n = 52). Overall response rate, defined as a platelet count of ≥30 × 109/L, at least doubling of the baseline platelet count, and no bleeding, was reached in 44% of patients in the eltrombopag plus diacerein group compared with 13% in the eltrombopag group at day 15 (P = .0009), and reached in 42% of patients in the combination group compared with 12% in the monotherapy group at day 28 (P = .0006). The addition of diacerein to eltrombopag also led to a longer duration of response (P = .0004). The 2 most common treatment-emergent adverse events were respiratory infection and gastrointestinal reactions in the combination group, and fatigue and respiratory infection in the eltrombopag group. In conclusion, eltrombopag plus diacerein was well tolerated, and induced higher overall response rates and longer duration of response than eltrombopag alone, offering a rejuvenating salvage therapy for patients with ITP unresponsive to 14 days of full dosage eltrombopag. Our work has the potential to enhance the care of patients treated with thrombopoietin receptor agonists, reducing the need for rapid transitions to less-preferable therapies. This study is registered at ClinicalTrials.gov as #NCT04917679.
Introduction
Primary immune thrombocytopenia (ITP) is an autoimmune disorder characterized by reduced platelet counts and bleeding, with underlying issues in platelet production and destruction.1,2 Thrombopoietin receptor agonists (TPO-RAs) are pivotal in the long-term management of ITP, which embraces top satisfaction among patient feedback.3-5 In response to the COVID-19 pandemic, TPO-RAs have been used earlier in off-label use.
However, the response rates of eltrombopag range from 45% to 94% in clinical and real-world settings, with nonnegligible failure or relapse, even at maximum dosages.6-8 Additionally, multiple realistic factors (eg, convenience, availability, potential adverse effects, and costs) restrict switching between TPO-RAs and transitioning to other subsequent treatments.9 Hence, combination therapies might be a more promising approach for reviving the effectiveness of eltrombopag.
Diacerein, an anthraquinone derivative used effectively and safely in osteoarthritis for 2 decades, exhibits anti-inflammatory properties.10 It is metabolized to rhein before systemic circulation and primarily acts by inhibiting inflammatory cytokines and nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasomes in autoimmune diseases.11 Notably, diacerein and rhein can sensitize various combination therapies, modulating cell cycle, apoptosis, and immunity.10-14 We incidentally noted that 3 patients with chronic ITP, who were unresponsive to eltrombopag at maximal dose, showed significant platelet increase after taking diacerein due to ostealgia without an underlying condition (supplemental Table 1; supplemental Figure 1 [available on the Blood website]). Our subsequent laboratory investigations unveiled that diacerein metabolite rhein significantly enhanced the effects of TPO-RAs on megakaryocyte polyploidization and platelet formation by amplifying phosphatidylinositol 3-kinase (PI3K) phosphorylation in human megakaryocyte cultures or in a murine model of ITP.15 These findings underscore diacerein's sensitizing role to TPO-RAs, which warrants further prospective studies to thoroughly evaluate the efficacy and safety of this combination as a salvage strategy for immune thrombocytopenia.
We hypothesized that the novel combination of eltrombopag and diacerein might extend eltrombopag efficacy and avoid rapid alteration to less-preferable therapies, especially in the management of the patients who were previously unresponsive to 14 days of full dosage eltrombopag treatment. Thus, this study aimed to evaluate the efficacy and safety of eltrombopag plus diacerein vs eltrombopag alone in patients with ITP.
Materials and methods
Study design
We conducted an open-label, investigator-initiated, randomized clinical trial in 5 tertiary medical centers in China (supplemental Figure 1). The study protocol was approved by the ethics committee of the Qilu Hospital of Shandong University (number 202008157) and by the ethics committees or institutional review boards of all other participating sites. All patients provided written informed consent, following the Declaration of Helsinki, before participating in the study.
Patients
Eligible patients were at least 18 years old, had a diagnosis of ITP according to the 2019 International Working Group consensus,3 and did not respond after receiving eltrombopag at the full dose of 75 mg per day for 14 days (platelet count <30 × 109/L or a value less than a 2-fold increase from their baseline platelet count, or bleeding). For patients aged >60 years, a bone marrow biopsy was required to exclude hematological malignancies. Patients with secondary causes of immune thrombocytopenia, drug-induced thrombocytopenia, or multiple immune cytopenia were excluded. Patients with the following conditions were also excluded: severe dysfunction of the heart, kidney, liver, or lung; severe immunodeficiency; malignancy; HIV; hepatitis B or C virus infection; and pregnancy or lactation.
Procedures
Statisticians randomly assigned (1:1) eligible participants to receive either eltrombopag plus diacerein or eltrombopag monotherapy. To guarantee the concealment of allocation, the statisticians did not participate in the remainder of the trial. Block randomization was performed centrally at the Qilu Hospital of Shandong University using SPSS version 23.0 to generate a randomization sequence with a block size of 4, which were then sealed in opaque envelopes. The other researchers were not informed of the block sizes. Competitive enrollment was conducted among the centers in a screening order. In this open-label study, physicians and participants were not masked to the treatment interventions; however, the experts responsible for the outcome assessment and data analysis were masked.
Both groups received eltrombopag orally at an initial daily dose of 75 mg for 14 days (days 1-14). Diacerein was given orally at an initial dose of 50 mg twice daily concomitantly for 14 days (days 1-14) in the combination group. Physicians were allowed to make individualized dose adjustments based on participants' platelet counts to ensure safety: (1) if platelet count was <150 × 109/L, original treatment was continued; (2) if the platelet count ranged from 150 × 109/L to 250 × 109/L, the treatment was modified to eltrombopag, 50 mg daily, plus diacerein, 50 mg twice daily, in the combination group, or eltrombopag, 50 mg daily, in the monotherapy group; (3) if the platelet count was >250 × 109/L, the original treatment was interrupted, the platelet count was reexamined every other day, and the treatment (eltrombopag, 50 mg daily, plus diacerein, 50 mg twice daily, in the combination group, or eltrombopag, 50 mg daily, in the monotherapy group) was not restarted until the platelet count was <100 × 109/L. If an initial response was achieved by day 15, eltrombopag with or without diacerein could continue. Patients who did not respond would stop the allocated treatment after the 15th day and were continuously followed up. During the screening and treatment period, participants were required to strictly follow the instructions for taking eltrombopag (remain fasted at least 4 hours after dinner before taking eltrombopag). The dose of diacerein was inferred from previous in vivo and in vitro results, and the regimen was chosen on the basis of available evidence on osteoarthritis and ITP.10,15 The treatment was administered mainly in an outpatient setting. During treatment and follow-up, receipt of additional interventions specific to ITP, such as corticosteroids, intravenous immunoglobulin, platelet transfusion, rituximab, and splenectomy, was considered treatment failure.
Routine visits were scheduled at baseline, once a week during the first 4 weeks, and at 4-week intervals thereafter. At each visit, we performed a clinical examination and recorded data on platelet counts, bleeding scores, and adverse events. Bleeding was assessed according to a standardized bleeding scale specific to ITP based on the site and severity of bleeding.16 Age was excluded from the original scale so that only bleeding symptoms were described. The bleeding score was calculated by adding points relevant to various clinical bleeding symptoms. Adverse events were graded according to the Common Terminology Criteria for Adverse Events version 4.0. For patients with severe adverse events, the allocated treatment was discontinued, and they were closely monitored or received rescue treatment until the adverse events disappeared. For patients who responded to the allocated treatment, the follow-up period lasted for at least 12 months or until relapse. For patients who did not respond and who relapsed, the follow-up period continued until other therapies specific to ITP were administered and was extended for 1 additional month after administration.
We also evaluated the patients’ health-related quality of life using the Immune Thrombocytopenia Patient Assessment Questionnaire (ITP-PAQ) at baseline and week 12. The ITP-PAQ is a 44-item questionnaire that includes 10 subscales. ITP-PAQ was translated into Chinese to make it suitable for the patients in this study. Scale scores were standardized from 0 to 100, with higher values indicating a better health-related quality of life. To illustrate the clinical significance of the changes in ITP-PAQ scores, we calculated the minimally important differences (MIDs). For the scales of ITP-related symptoms, bother, social activity, psychological health, overall quality of life, and women’s reproductive health, MID values of 8 to 12 were considered clinically significant. For the activity and fatigue scales, MID values of 10 to 15 were considered clinically significant. MIDs were not estimable for the fear and work scales.
Outcomes
The primary end point was the overall response on day 15 without any concomitant or rescue therapy. A complete response was defined as a platelet count of at least 100 × 109/L and absence of bleeding. A partial response was defined as a platelet count of at least 30 × 109/L but <100 × 109/L, a doubling of the baseline count, and no bleeding. No response was defined as a platelet count of <30 × 109/L, less than twice the baseline count, or the presence of bleeding.
The secondary end points included the overall response on day 28, time to response, duration of response, bleeding scores, health-related quality of life, and adverse events. Time to response was defined as the time from treatment initiation to a complete or partial response. The duration of the response was defined as the time from a complete or partial response to the loss of response (platelet count of <30 × 109/L, as measured on 2 occasions >1 day apart, or the presence of bleeding).
Statistical analysis
On the basis of preliminary findings,7,15,17 we hypothesized that the proportion of patients achieving an overall response at day 15 would be 40% with eltrombopag plus diacerein and 10% with eltrombopag monotherapy. To detect a difference in the incidence of an initial response with 90% power using the Pearson χ2 test and at a 2-sided significance level of .05, 39 participants were needed for each group. Assuming a dropout rate of 20%, the final sample size was 49 participants per group.
We included all participants who were randomly assigned (intention-to-treat population) in the description of baseline characteristics and the analysis of primary and secondary end points (excluding health-related quality of life). Health-related quality of life was assessed in participants who were randomly assigned, received allocated treatment, and completed the ITP-PAQ. Safety was evaluated in all participants who were randomly assigned and received the allocated intervention. We also described the incidence of the primary end point and 1 of the secondary end points (response at day 28) in the per-protocol population, which comprised participants who were randomly assigned and completed treatment and follow-up. The incidences of overall response on days 15 and 28 were compared between groups using the Fisher exact test. Odds ratios were calculated with 95% confidence intervals via the Baptista-Pike method. Time to response and bleeding scores were evaluated with the Mann-Whitney U test. Duration of response was assessed using the Kaplan-Meier method and log-rank test. Changes in ITP-PAQ subscale scores from baseline to week 12 were compared between groups with Mann-Whitney U tests. Adverse events were analyzed using the Fisher exact test. All tests were 2 sided, and we considered differences between the 2 groups to be statistically significant if P < .05. SPSS version 23.0 and GraphPad Prism version 9 were used for statistical analyses.
Results
Characteristics
Between September 1, 2020, and June 1, 2022, 140 individuals underwent screening. Among them, 38 were ineligible and excluded, leaving 102 patients enrolled and randomly assigned to either the eltrombopag plus diacerein group (n = 50) or the eltrombopag group (n = 52) for intention-to-treat analysis (Figure 1). Four patients did not receive their allocated intervention because of consent withdrawal (1 in the eltrombopag plus diacerein group vs 2 in the eltrombopag group) or diagnostic amendment (1 vs 0) and were therefore excluded from safety analysis. Three patients did not complete the treatment because they required other interventions (1 vs 2), 2 patients were lost to follow-up (1 vs 1) for unknown reasons, and the remaining 93 patients completed the treatment and follow-up (46 vs 47).
The baseline characteristics were well balanced between the groups (Table 1). The overall study population had a median age of 38.5 years (interquartile range [IQR], 27-57 years), and approximately half were women. Sixty-six (65%) of 102 patients had bleeding symptoms (generally mild to moderate), mainly including skin, mucosal, and visceral bleeding, with a median score of 2 (IQR, 0-4). The median platelet count of the total population at enrollment was 9.5 × 109/L (IQR, 3 × 109/L–13 × 109/L). The median duration of ITP was 11 months (IQR, 6-24 months) in the eltrombopag plus diacerein group and 12 months (IQR, 6-36 months) in the eltrombopag group. The median number of previous therapies was 3 (IQR, 2-4) in the eltrombopag plus diacerein group and 3 (IQR, 2-4) in the eltrombopag group. More than 65% of the patients in both groups received ≥3 therapies before enrollment.
Baseline characteristics
| Characteristic . | Eltrombopag plus diacerein (n = 50) . | Eltrombopag (n = 52) . | P value . |
|---|---|---|---|
| Age, y | 34 (27-57) | 44 (29-57) | .294 |
| Sex | |||
| Female | 28 (56) | 26 (50) | .559 |
| Male | 22 (44) | 26 (50) | .559 |
| Duration of ITP, mo | 11 (6-24) | 12 (6-36) | .578 |
| Baseline bleeding score | 2 (0-4) | 3 (0-5) | .557 |
| Baseline bleeding event | 31 (62) | 35 (67) | .679 |
| Baseline platelet count, ×109 cells/L | 8 (2-12) | 10 (4-15) | .230 |
| Platelets 0-10 × 109 cells/L | 31 (62) | 27 (52) | .325 |
| Platelets 11 × 109 cells/L-20 × 109 cells/L | 14 (28) | 20 (38) | .298 |
| Platelets 21 × 109 cells/L-29 × 109 cells/L | 5 (10) | 5 (10) | .999 |
| Previous therapy | |||
| Dexamethasone | 38 (76) | 43 (83) | .468 |
| Prednisone | 19 (38) | 21 (40) | .841 |
| Methylprednisolone | 8 (16) | 4 (8) | .230 |
| Intravenous immunoglobulin | 8 (16) | 7 (13) | .785 |
| Recombinant human thrombopoietin | 21 (42) | 16 (31) | .304 |
| Eltrombopag | 50 (100) | 52 (100) | .999 |
| Rituximab | 2 (4) | 3 (6) | .999 |
| Chinese medicine decoctions | 7 (14) | 6 (12) | .773 |
| Characteristic . | Eltrombopag plus diacerein (n = 50) . | Eltrombopag (n = 52) . | P value . |
|---|---|---|---|
| Age, y | 34 (27-57) | 44 (29-57) | .294 |
| Sex | |||
| Female | 28 (56) | 26 (50) | .559 |
| Male | 22 (44) | 26 (50) | .559 |
| Duration of ITP, mo | 11 (6-24) | 12 (6-36) | .578 |
| Baseline bleeding score | 2 (0-4) | 3 (0-5) | .557 |
| Baseline bleeding event | 31 (62) | 35 (67) | .679 |
| Baseline platelet count, ×109 cells/L | 8 (2-12) | 10 (4-15) | .230 |
| Platelets 0-10 × 109 cells/L | 31 (62) | 27 (52) | .325 |
| Platelets 11 × 109 cells/L-20 × 109 cells/L | 14 (28) | 20 (38) | .298 |
| Platelets 21 × 109 cells/L-29 × 109 cells/L | 5 (10) | 5 (10) | .999 |
| Previous therapy | |||
| Dexamethasone | 38 (76) | 43 (83) | .468 |
| Prednisone | 19 (38) | 21 (40) | .841 |
| Methylprednisolone | 8 (16) | 4 (8) | .230 |
| Intravenous immunoglobulin | 8 (16) | 7 (13) | .785 |
| Recombinant human thrombopoietin | 21 (42) | 16 (31) | .304 |
| Eltrombopag | 50 (100) | 52 (100) | .999 |
| Rituximab | 2 (4) | 3 (6) | .999 |
| Chinese medicine decoctions | 7 (14) | 6 (12) | .773 |
Data are number (percentage) or median (interquartile range).
Responses
As shown in Table 2, patients in the eltrombopag plus diacerein group demonstrated a significantly higher overall response rate than patients in the eltrombopag group at day 15 (44% vs 13%; P = .0009). Similar results were obtained in the per-protocol population (48% vs 15%; P = .0008) (supplemental Table 3). The eltrombopag plus diacerein therapy also induced a significantly higher complete response rate than eltrombopag monotherapy at day 15 (28% vs 4%; P = .0008) (Table 2), and similar results were seen in the per-protocol analysis (30% vs 4%; P = .0008). There was no significant difference in the partial response rate between the 2 groups on day 15 (Table 2).
Responses and outcomes in the eltrombopag plus diacerein vs eltrombopag groups
| Variable . | Eltrombopag plus diacerein (n = 50) . | Eltrombopag (n = 52) . | P value . | Odds ratio (95% CI) . |
|---|---|---|---|---|
| Overall response at day 15 | 22 (44) | 7 (13) | .0009 | 5.05 (1.89-12.52) |
| Complete response at day 15 | 14 (28) | 2 (4) | .0008 | 9.72 (2.25-44.38) |
| Partial response at day 15 | 8 (16) | 5 (10) | .39 | 1.79 (0.59-5.24) |
| Overall response at day 28 | 21 (42) | 6 (12) | .0006 | 5.55 (1.94-15.27) |
| Complete response at day 28 | 14 (28) | 2 (4) | .0008 | 9.72 (2.25-44.38) |
| Patial response at day 28 | 7 (14) | 4 (8) | .35 | 1.95 (0.56-6.26) |
| Patients who had a response | ||||
| Time to response, d | 6.5 (5-8) | 5 (5-7) | .45 | |
| Peak platelet count, ×109/L | 175.5 (115-272) | 90 (59-156) | .013 | |
| Additional therapies∗ | 15 (30) | 30 (58) | .0057 | |
| Corticosteroids | 2 (4) | 4 (8) | ||
| Intravenous immunoglobulin | 2 (4) | 2 (4) | ||
| Platelet transfusion | 1 (2) | 2 (4) | ||
| Avatrombopag | 5 (10) | 9 (17) | ||
| Hetrombopag | 2 (4) | 3 (6) | ||
| Rituximab | 1 (2) | 2 (4) | ||
| Danazol | 1 (2) | 3 (6) | ||
| Chinese medicine decoctions | 1 (2) | 5 (10) |
| Variable . | Eltrombopag plus diacerein (n = 50) . | Eltrombopag (n = 52) . | P value . | Odds ratio (95% CI) . |
|---|---|---|---|---|
| Overall response at day 15 | 22 (44) | 7 (13) | .0009 | 5.05 (1.89-12.52) |
| Complete response at day 15 | 14 (28) | 2 (4) | .0008 | 9.72 (2.25-44.38) |
| Partial response at day 15 | 8 (16) | 5 (10) | .39 | 1.79 (0.59-5.24) |
| Overall response at day 28 | 21 (42) | 6 (12) | .0006 | 5.55 (1.94-15.27) |
| Complete response at day 28 | 14 (28) | 2 (4) | .0008 | 9.72 (2.25-44.38) |
| Patial response at day 28 | 7 (14) | 4 (8) | .35 | 1.95 (0.56-6.26) |
| Patients who had a response | ||||
| Time to response, d | 6.5 (5-8) | 5 (5-7) | .45 | |
| Peak platelet count, ×109/L | 175.5 (115-272) | 90 (59-156) | .013 | |
| Additional therapies∗ | 15 (30) | 30 (58) | .0057 | |
| Corticosteroids | 2 (4) | 4 (8) | ||
| Intravenous immunoglobulin | 2 (4) | 2 (4) | ||
| Platelet transfusion | 1 (2) | 2 (4) | ||
| Avatrombopag | 5 (10) | 9 (17) | ||
| Hetrombopag | 2 (4) | 3 (6) | ||
| Rituximab | 1 (2) | 2 (4) | ||
| Danazol | 1 (2) | 3 (6) | ||
| Chinese medicine decoctions | 1 (2) | 5 (10) |
Data are number (percentage) or median (interquartile range).
CI, confidence interval.
The number refers to the number of additional therapies administered to nonresponders or relapsed patients.
By day 28, a significantly higher proportion of participants in the eltrombopag plus diacerein group (42%) achieved an overall response when compared with the eltrombopag monotherapy group (12%; P = .0006) (Table 2), which was consistent with the per-protocol analysis (46% vs 13%; P = .0006). Additionally, 28% of patients in the eltrombopag plus diacerein group and 4% of patients in the eltrombopag group achieved complete response by day 28 (P = .0008) (Table 2). In patients who achieved overall responses by day 15, the median time to response was 6.5 days in the eltrombopag plus diacerein group, with a peak platelet count of 175.5 × 109/L, in contrast to 5 days with a peak of 90 × 109/L in the eltrombopag group (Table 2). In the exploratory analysis, the 6-month overall response rate in the eltrombopag plus diacerein group was higher than that in the eltrombopag group (20% vs 4%; P = .014).
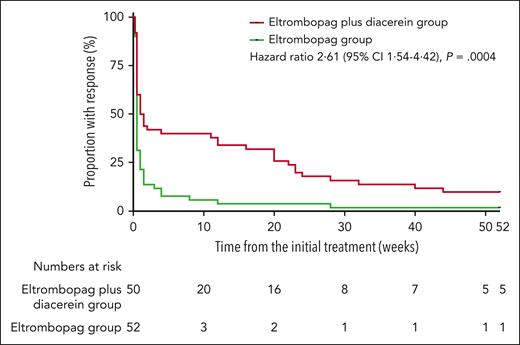
During the 12-month observational period, the duration of response was significantly longer in the eltrombopag plus diacerein group than in the eltrombopag group, according to the Kaplan-Meier analysis (hazard ratio, 2.61; 95% confidence interval, 1.54–4.42; P = .0004) (Figure 2). The median duration of response was 23 weeks in the combination group and 8 weeks in the eltrombopag monotherapy group. The median platelet counts were 114 × 109/L (IQR, 88.5 × 109/L –185 × 109/L) on day 28 and 74 × 109/L (49 × 109/L–136 × 109/L) at month 6 in patients who responded to the eltrombopag plus diacerein by day 15, whereas these were 78 × 109/L (56 × 109/L–108 × 109/L) on day 28 and 43.5 × 109/L (38 × 109/L–49 × 109/L) at month 6 in patients who responded to the eltrombopag monotherapy by day 15. At most time points, patients who responded by day 15 in the eltrombopag plus diacerein group had higher median platelet counts than those in the eltrombopag group (supplemental Figure 2).
Kaplan-Meier analysis of the duration of response. The Kaplan-Meier curve demonstrated longer duration of response in the eltrombopag plus diacerein group than in the eltrombopag group (P = .0004). CI, confidence interval.
Kaplan-Meier analysis of the duration of response. The Kaplan-Meier curve demonstrated longer duration of response in the eltrombopag plus diacerein group than in the eltrombopag group (P = .0004). CI, confidence interval.
To maintain patients’ platelet counts within a safe range, the drug dosages of 24% of patients in the eltrombopag plus diacerein group and 4% of patients in the eltrombopag group were individually reduced (P = .0036). Seven patients (14%) receiving eltrombopag plus diacerein achieved platelet counts of >250 × 109/L and discontinued the original treatment until their platelet count fell below 100 × 109/L. Subsequently, they received eltrombopag, 50 mg daily, plus diacerein. Five patients (10%) receiving eltrombopag plus diacerein achieved platelet counts between 150 × 109/L and 250 × 109/L and subsequently had their eltrombopag dosage reduced to 50 mg daily. In contrast, 2 patients (4%) receiving eltrombopag monotherapy with platelet counts between 150 × 109/L and 250 × 109/L also had their eltrombopag dosage reduced to 50 mg daily.
For patients who were unresponsive to the allocated treatment or who relapsed after the initial response, 20% of patients in the eltrombopag plus diacerein group and 40% of patients in the eltrombopag group received additional therapies specific to ITP and withdrew from the study after completing the additional 1-month follow-up (P = .032). The number of additional therapies administered in the eltrombopag plus diacerein group was significantly fewer compared with that in the eltrombopag monotherapy group (P = .0057) (Table 2).
The posttreatment bleeding scores and bleeding events are presented in supplemental Table 4. On day 15, eltrombopag plus diacerein induced similar bleeding scores and bleeding events as eltrombopag monotherapy. On day 28, eltrombopag plus diacerein resulted in significantly lower median bleeding scores (P = .0081) and significantly fewer bleeding events (11 vs 26; P = .0040) than eltrombopag alone.
Baseline ITP-PAQ scores were similar between the 2 groups (supplemental Table 5). As shown in supplemental Figure 3, eltrombopag plus diacerein induced significantly greater improvements in the scores of ITP-related symptoms (P = .030; above MID), fatigue (P = .0004), psychological health (P = .034; above MID), and overall quality of life (P = .0037) compared with eltrombopag monotherapy; the changes in scores for fatigue and overall quality of life were higher than the MID in the combination group but lower than the MID in the monotherapy group.
Adverse events
Overall, the occurrence of adverse events was comparable between the 2 groups (Table 3). Most adverse events were grade 1 or 2 and resolved spontaneously after treatment completion. Only 2 grade 3 adverse events were reported: 1 patient in the eltrombopag plus diacerein group with respiratory infection and fever, and 1 patient in the eltrombopag group with insomnia. Respiratory tract infection occurred in 19% of patients treated with eltrombopag plus diacerein and 14% of patients treated with eltrombopag. Additionally, 8% of patients in the eltrombopag plus diacerein group and 18% of patients in the eltrombopag group complained of fatigue. Gastrointestinal reactions were recorded in 15% of patients in the eltrombopag plus diacerein group (4% was loss of appetite, 4% was nausea, 4% was diarrhea, and 2% was abnormal pain), and 4% of patients in the eltrombopag group (2% was diarrhea and 2% was vomiting). Four patients (8%) in the eltrombopag plus diacerein group presented with dark urine. Fever was recorded in >5% of the patients in both groups. All adverse events were consistent with the product summaries of eltrombopag and diacerein. No grade 4 or 5 adverse events or deaths occurred during the study period.
Adverse events recorded in the safety analysis set
| Variable . | Eltrombopag plus diacerein (n = 48) . | Eltrombopag (n = 50) . | P value . | ||
|---|---|---|---|---|---|
| Grade 1-2 . | Grade 3 . | Grade 1-2 . | Grade 3 . | ||
| Patients with any adverse events | 17 (35) | 1 (2) | 13 (26) | 1 (2) | .390 |
| Total no. of adverse events∗ | 32 (67) | 1 (2) | 31 (62) | 1 (2) | .673 |
| Headache | 2 (4) | 0 | 3 (6) | 0 | .999 |
| Respiratory tract infection | 9 (19) | 0 | 7 (14) | 0 | .592 |
| Gastrointestinal reactions† | 7 (15) | 0 | 2 (4) | 0 | .088 |
| Elevated liver enzymes | 2 (4) | 0 | 3 (6) | 0 | .999 |
| Urinary tract infection | 0 | 0 | 1 (2) | 0 | .999 |
| Dark urine | 4 (8) | 0 | 0 | 0 | .054 |
| Fatigue | 4 (8) | 0 | 9 (18) | 0 | .234 |
| Fever | 2 (4) | 1 (2) | 3 (6) | 0 | .999 |
| Insomnia | 1 (2) | 0 | 1 (2) | 1 (2) | .999 |
| Rash | 1 (2) | 0 | 1 (2) | 0 | .999 |
| Arthralgia | 0 | 0 | 1 (2) | 0 | .999 |
| Variable . | Eltrombopag plus diacerein (n = 48) . | Eltrombopag (n = 50) . | P value . | ||
|---|---|---|---|---|---|
| Grade 1-2 . | Grade 3 . | Grade 1-2 . | Grade 3 . | ||
| Patients with any adverse events | 17 (35) | 1 (2) | 13 (26) | 1 (2) | .390 |
| Total no. of adverse events∗ | 32 (67) | 1 (2) | 31 (62) | 1 (2) | .673 |
| Headache | 2 (4) | 0 | 3 (6) | 0 | .999 |
| Respiratory tract infection | 9 (19) | 0 | 7 (14) | 0 | .592 |
| Gastrointestinal reactions† | 7 (15) | 0 | 2 (4) | 0 | .088 |
| Elevated liver enzymes | 2 (4) | 0 | 3 (6) | 0 | .999 |
| Urinary tract infection | 0 | 0 | 1 (2) | 0 | .999 |
| Dark urine | 4 (8) | 0 | 0 | 0 | .054 |
| Fatigue | 4 (8) | 0 | 9 (18) | 0 | .234 |
| Fever | 2 (4) | 1 (2) | 3 (6) | 0 | .999 |
| Insomnia | 1 (2) | 0 | 1 (2) | 1 (2) | .999 |
| Rash | 1 (2) | 0 | 1 (2) | 0 | .999 |
| Arthralgia | 0 | 0 | 1 (2) | 0 | .999 |
Data are number (percentage). There were no grade 4 or 5 adverse events.
Each patient might have >1 adverse event in the summary of total adverse events.
Gastrointestinal reactions included 2 (4%) loss of appetite, 2 (4%) nausea, 2 (4%) diarrhea, and 1 (2%) abdominal pain in the eltrombopag plus diacerein group, vs 1 (2%) diarrhea and 1 (2%) vomiting in the eltrombopag group.
Discussion
To our knowledge, this is the first randomized controlled trial to evaluate the efficacy and safety of eltrombopag plus diacerein in adult patients with ITP who had not responded to 14 days of eltrombopag management at the full dose. Significant improvements were observed in overall response rates and duration of response with the treatment of eltrombopag plus diacerein vs eltrombopag alone.
Our study showed an increase of 31% in the overall response at day 15 when comparing eltrombopag plus diacerein with eltrombopag monotherapy, providing evidence for the reviving effect of this oral combination therapy. The overall response rate on day 15 in the eltrombopag group (7 [13%] of 52) was slightly higher than that reported by Matthieu et al (0 [0%] of 3),18 possibly because of the multirefractoriness of the disease in their research (defined as no response to splenectomy, rituximab, and TPO-RAs) and their limited sample size. Several studies have reported that the addition of corticosteroids and/or immunosuppressive agents to TPO-RAs improves the response rate in patients refractory to romiplostim or eltrombopag.18-20 A retrospective study showed that 7 (70%) of 10 patients who were previously unresponsive to TPO-RAs achieved an overall response to the original romiplostim or eltrombopag therapy after receiving the addition of immunosuppressive agents with or without corticosteroids.18 However, the use of unknown and nonstandardized doses of corticosteroids, additional interventions specific to ITP, and different types of allocated agents in case reports and pilot studies has made it challenging to obtain high-quality evidence. In a single-center, single-arm study by Hong et al,20 8 (38%) of 21 patients who had not responded to previous eltrombopag therapy (75 mg daily for at least 30 days) responded to a combination of cyclosporine and eltrombopag with no other concomitant medications specific to ITP, similar to the overall response rate on day 15 in our combination group (44%). Notably, the impairment of health-related quality of life owing to chronic exposure to corticosteroids, or renal and neurologic toxicity of cyclosporin, cannot be ignored.
Our results also provided preliminary evidence of the long-term advantages of combining eltrombopag and diacerein over eltrombopag monotherapy. However, the reviving and extended efficacy induced by the combination is still gradually lost, explaining why relatively few patients remain on the combination beyond 24 weeks. The 6-month sustained response in the eltrombopag plus diacerein group (20%) was similar to the results of Hong et al, in which 3 (14%) of 21 patients achieved 6-month sustained responses after receiving treatment with eltrombopag and cyclosporine.20 However, the sustained response rate of eltrombopag plus prednisone was unclear.19 Moreover, the median duration of response was 23 weeks with the addition of diacerein to eltrombopag and 13 weeks with the combination of eltrombopag and cyclosporine.20
The mechanisms behind favorable eltrombopag plus diacerein treatment outcomes are worth exploring. This might be attributed to the amplification effects of diacerein on TPO-RAs by enhancing PI3K phosphorylation during megakaryocyte maturation and platelet formation.15 Similar to thrombopoietin, eltrombopag boosts megakaryocyte production in immune thrombocytopenia but has a limited impact on the final step of platelet formation and release.21 In 1 study, Barsam et al found that nonresponders to eltrombopag experienced increased megakaryocyte production but no increased platelet formation in the bone marrow, possibly because of platelet formation being blocked by antibodies.22 Further research evaluating the status of platelet antibodies before and after treatment is needed to consolidate the acting mechanism of diacerein.
The sustained remission achieved with eltrombopag plus diacerein cannot be solely explained by the enhancement of megakaryopoiesis and thrombopoiesis. A retrospective next-generation sequencing study revealed multiple potential molecular markers of refractoriness to eltrombopag using peripheral blood from patients with ITP, mainly involving PI3K signaling, transforming growth factor-β, other cytokine-related immune responses, and T helper cell 17 (Th17)/regulatory T cell (Treg) balance.23 Eltrombopag improved Tregs in ITP,24 but not enough to correct the immune imbalance of Th1 and Th17 cytokines in ITP.25 Therefore, combination therapy may overcome refractoriness and prolong the efficacy of eltrombopag. Diacerein and rhein have proven effective in inhibiting multiple proinflammatory cytokines, such as interleukin 1 (IL-1) and IL-17, in autoimmune diseases.10,11 In vivo research showed that rhein treatment ameliorated murine colitis by restoring Th17/Treg balance and decreasing Th17 and Th1 cells.26,27 Our data also showed that the addition of diacerein to eltrombopag significantly elevated transforming growth factor-β1 and decreased IL-17 in eltrombopag-refractory patients with ITP (supplemental Figure 4). These studies implicated the immune modulatory mechanisms of diacerein were uncovered by eltrombopag, thereby providing a theoretical basis for the feasibility of combination therapy. Furthermore, considering that other TPO-RAs (eg, romiplostim and avatrombopag) function by increasing platelet production, exploring the possibility of combining diacerein with these receptor agonists for potential sensitizing and synergistic effects in future condition management is warranted.
In this study, 15% of patients presented with grade 1 to 2 gastrointestinal reactions in the eltrombopag plus diacerein group vs 4% in the eltrombopag group. Diacerein has been reported to darken the urine color of some patients because of pigments in drug metabolism,28 and we observed that 8% of patients had dark urine in the combination group. Additionally, 8% of patients complained of fatigue in the combination group vs 18% in the monotherapy group, which aligns with our findings in health-related quality of life that the addition of diacerein to eltrombopag significantly improved fatigue, with statistical and clinical significance. There were no new reported adverse events and no adverse event–related study withdrawals. Thus, this multicenter study underscores the well-tolerated use of eltrombopag and diacerein in the Chinese population.
This study had several limitations. First, because of its open-label nature, participants in the combination group may have gained some psychological advantages by knowing that they had received the experimental treatment, thereby benefiting their health-related quality of life. To minimize evaluation bias, data collection and analyses were done by independent experts. Second, several TPO-RAs are currently available for ITP, and our study did not choose other receptor agonists as a control because only eltrombopag was approved in China during the study design. Third, diacerein is licensed in some European Union and Asian countries, but not available in countries such as the United States and Canada. To minimize the risk of severe diarrhea, diacerein is no longer recommended by the European Medicine Agency in patients aged ≥65 years. Finally, it is important to objectively evaluate the therapeutic effects by excluding better compliance with dietary restrictions. Additional studies with diverse population backgrounds and longer follow-up periods are warranted to confirm the reviving effect of diacerein on eltrombopag and to expand on other TPO-RAs.
In conclusion, our findings suggest that eltrombopag plus diacerein is a safe and effective salvage therapy for patients with ITP who were unresponsive to 14 days of eltrombopag treatment at the full dose. This oral combination induced higher early overall response rates and longer response durations than eltrombopag alone. Further studies are required to identify the optimal dose and duration of diacerein and to explore different combinations of diacerein with other TPO-RAs in patients with ITP.
Acknowledgments
The authors thank Xiaorong Yang (Clinical Epidemiology Unit, Qilu Hospital, Shandong University) for statistical consultation. They thank all patients who volunteered to participate in this trial, their families, and the trial staff members who care for them.
This work was funded by grants from the National Natural Science Foundation of China (82322003 [Y.H.], 82370131 [M.H.], and 82270131[Y.H.]), the Natural Science Foundation of Distinguished Young Scholars of Shandong Province (ZR2021JQ28 [Y.H.]), the Key Research and Development program of the Shandong Province (2021LCZX05 [M.H.]), the Young Taishan Scholar Foundation of Shandong Province (tsqn201909175 [Y.H.]), the Outstanding Young and Middle-Aged Scholar Foundation of Shandong University, the Emergency and Critical Care Medicine program of the Clinical Research Center of Shandong University (2021SDUCRCB009 [M.H.]), the Natural Science Foundation of Shandong Province (ZR2023QH222 [L.S.]), and the China Postdoctoral Science Foundation (2022M721962 [L.S.]).
Authorship
Contribution: L.S., M.H., and Y.H. designed the clinical trial; C.Y., H.Z., D.L., R.X., Y.W., and P.Q. enrolled and scheduled participants; X.H., J.W., Y.S., and J.P. analyzed the data; and L.S., M.H., and Y.H. prepared the original manuscript; all authors had full access to the primary data and contributed to interpretation of the data, critically revised and approved the final draft of the manuscript, and made the decision to submit it for publication.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yu Hou, Department of Hematology, Qilu Hospital of Shandong University, Shandong University, 107 Wenhuaxi Rd, 250012 Jinan, China; email: houyu2009@sina.com.
References
Author notes
Presented in abstract form in part at the 64th annual meeting of the American Society of Hematology, 10-13 December 2022, New Orleans, LA.
The study protocol and deidentified individual participant data that underlie the results reported in this article will be available to researchers who provide a reasonable method proposal. For meta-analysis purposes, individual participant data will be available beginning 9 months and ending 36 months after publication. To gain access, data requestors will need to send a description of the research proposals to the corresponding author (houyu@email.sdu.edu.cn) and sign a data access agreement.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal