Visual Abstract
The microbiota, comprising bacteria, fungi, and viruses residing within our bodies, functions as a key modulator in host health and states, including immune responses. Studies have linked microbiota and microbiota-derived metabolites to immune cell functions. In this review, we probe the complex relationship between the human microbiota and clinical outcomes of cellular therapies that leverage immune cells to fight various cancers. With a particular emphasis on hematopoietic cell transplantation and chimeric antigen receptor T-cell therapy, we explore the potential mechanisms underpinning this interaction. We also highlight the interventional applications of the microbiota in cellular therapy while outlining future research directions in the field.
Introduction
Cellular therapy began with blood transfusion after the discovery of the ABO blood group system.1 Since that time, the use of cells for cancer treatment has expanded and evolved. Hematopoietic cell transplantation (HCT), which has been used to treat bone marrow failure since the 1950s, has become a standard of care for hematologic malignancies.2 With an advanced understanding of immune cell functions, cell manipulation, and gene editing, adoptive cell therapy has emerged. The use of tumor-infiltrating lymphocytes (TILs) along with interleukin-2 (IL-2) to treat patients with metastatic melanoma is a notable milestone.3,4 More recently, genetically modified cell products, including chimeric antigen receptor (CAR) T cells and T-cell–receptor engineered T cells (TCR-Ts), have greatly improved the clinical outcomes for patients with cancer. The US Food and Drug Administration has approved these therapies to be used for patients with lymphoma, some forms of leukemia, multiple myeloma (MM), and melanoma.5,6 Despite the promising outcomes after cellular therapy, clinical efficacies may vary and patients may experience adverse events, such as graft-versus-host disease (GVHD),7,8 CAR-mediated toxicity, and even disease relapse.9
Notably, findings establish a crucial connection between the efficacy and safety of cellular therapy and the intricate composition of the host's microbiota. The human microbiota, consisting of bacteria, fungi, and viruses, is primarily located in the gut, skin, and oral and nasal cavities.10 The majority of the microbiota resides in the gut,11 in which the bacteria are dominated by strict anaerobes.12 Patients undergoing cellular therapies may have a disrupted microbiota composition due to alterations in their diet and exposure to medications. Additionally, immune suppression heightens susceptibility to infections, exacerbating dysbiosis. In this review, we summarize the impact of microbiota on the antitumor effect of cellular therapies, with a focus on nongenetically and genetically modified cellular therapies, as well as possible mechanisms, gaps, and future directions in the field.
Microbiota and nongenetically modified cellular therapies
Nongenetically modified cellular therapies are those that do not undergo gene editing, including HCT, TIL, and natural killer (NK) cell therapies. Owing to its potent graft-versus-leukemia (GVL) effect, HCT is a potentially curative therapy for hematologic malignancies.13 HCT is classified as autologous HCT (auto-HCT) or allogeneic HCT (allo-HCT), depending on whether the source of the cells is the patient or a donor, respectively.
The fecal microbial α-diversity, indicating intestinal microbiota variety and abundance, declines in both recipients of auto-HCT and allo-HCT likely because of pre-HCT conditioning and antibiotics; α-diversity reaches a nadir at ∼2 weeks after HCT, followed by a partial recovery.14-18 Despite the decreased diversity in most recipients of auto-HCT and allo-HCT, those retaining high diversity generally show improved outcomes, including increased overall survival (OS),16-20 increased clinical response,21 decreased progression,16 decreased infection,22,23 decreased GVHD,18,24,25 and decreased transplant-related toxicity26,27 (Table 1). Specifically, for auto-HCT recipients, increased diversity is associated with improved clinical response,21 increased OS, increased progression-free survival (PFS),16 milder diarrhea,26 decreased bacteremia,23 and higher serum chemotherapy drug level,28 but also correlates with increased neutropenic fever.26 For allo-HCT recipients, increased diversity correlates with better survival,17-20 decreased infection,22,23 and less incidence and severity of acute GVHD (aGVHD)18,24 and chronic GVHD.25 Moreover, greater microbial diversity is associated with better T-cell recovery,17 especially innate-like mucosal-associated invariant T and Vδ2+ T cells, which further correlates with extended OS and decreased aGVHD.29 Interestingly, there seems to be no significant association between microbial diversity and relapse after allo-HCT.20,37
Association between microbial diversity changes and outcomes of cellular therapy
| Therapy . | Sampling sites∗ . | Technology and pipeline∗ . | Findings . | Reference . |
|---|---|---|---|---|
| Human | ||||
| Auto-HCT | US | 16S UPARSE | Increased α-diversity in perineutrophil engraftment fecal samples is associated with increased OS and PFS | 16 |
| US | 16S QIIME2 and DADA2 | Increased α-diversity in perineutrophil engraftment fecal samples is correlated with CR/VGPR vs PR | 21 | |
| US | 16S QIIME | Increased α-diversity in posttransplant samples is correlated with less severe diarrhea; increased diversity is associated with the development of neutropenic fever. | 26 | |
| US | 16S DADA2 | Increased oral microbial α-diversity is correlated with higher salivary and serum melphalan levels | 28 | |
| Allo-HCT | US | 16S Mothur | Low baseline fecal α-diversity is associated with pulmonary complications | 27 |
| France | 16S QIIME | Decreased α-diversity in pretreatment fecal samples is associated with subsequent bacteremia | 22 | |
| Italy and Poland† | 16S QIIME2 | Increased α-diversity in pretreatment fecal samples is associated with increased OS and lower grades of aGVHD | 18 | |
| US | 16S DADA2 | Increased α-diversity in periengraftment samples is associated with MAIT and Vδ2 T-cell frequency at day 30 in patients who received unmodified PBMC grafts | 29 | |
| US, Germany, and Japan | 16S vsearch, usearch, and QIIME | Increased α-diversity in periengraftment fecal samples is associated with increased OS and a lower transplantation-related mortality rate | 19 | |
| US | 16S DADA2 | Increased α-diversity in periengraftment fecal samples is associated with increased OS, decreased nonrelapse mortality, and better CD4 and CD8 T-cell recovery | 17 | |
| US | 16S DADA2 | Increased α-diversity in perineutrophil recovery fecal samples is associated with lower grades of aGVHD | 24 | |
| US | Shotgun metagenomics QIIME2 and vegan.R | Patients who developed cGVHD showed a change of β-diversity and a greater loss of α-diversity from baseline to after engraftment | 25 | |
| US | 16S Mothur | Increased α-diversity in postengraftment fecal samples is associated with increased OS and a lower transplantation-related mortality rate | 20 | |
| Auto-HCT/allo-HCT | China | 16S QIIME and UPARSE | Increased α-diversity in posttransplant samples is associated with decreased bacteremia | 23 |
| CAR T cell | China | 16S QIIME2 | α-Diversity is decreased in fecal samples from PR patients compared with fecal samples from CR patients at the CRS peak time point. β-Diversity is different between PR and CR samples at the same time point. | 30 |
| Mouse | ||||
| Syngeneic BMT | US | 16S Mothur | Authors defined intestinal microbiota score = Shannon index × fecal bacterial abundance. Higher intestinal microbiota score is associated with higher weight of periovarian fat, bone marrow, and thymic cellularity after transplantation. | 31 |
| Allo-HCT | US | 16S Mothur | Higher α-diversity in posttransplant samples is associated with better survival and lower GVHD score | 32 |
| US | 16S | Orally gavaging B fragilis increases posttransplant α-diversity in the ileum, and ameliorates GVHD | 33 | |
| US | 16S QIIME2 and DADA2 | High vitamin A diet is associated with decreased α-diversity in posttransplant fecal samples and more severe lung GVHD | 34 | |
| Australia | Metagenomics QIIME | Wild-type mice cohoused continuously with IL-17RA–deficient mice display decreased α-diversity and accelerated GVHD | 35 | |
| CAR T cell | US | 16S QIIME2 | Oral vancomycin treatment decreased the α-diversity of fecal samples from mice with human FMT. CAR T-cell therapy in combination with vancomycin slowed down tumor progression. | 36 |
| Therapy . | Sampling sites∗ . | Technology and pipeline∗ . | Findings . | Reference . |
|---|---|---|---|---|
| Human | ||||
| Auto-HCT | US | 16S UPARSE | Increased α-diversity in perineutrophil engraftment fecal samples is associated with increased OS and PFS | 16 |
| US | 16S QIIME2 and DADA2 | Increased α-diversity in perineutrophil engraftment fecal samples is correlated with CR/VGPR vs PR | 21 | |
| US | 16S QIIME | Increased α-diversity in posttransplant samples is correlated with less severe diarrhea; increased diversity is associated with the development of neutropenic fever. | 26 | |
| US | 16S DADA2 | Increased oral microbial α-diversity is correlated with higher salivary and serum melphalan levels | 28 | |
| Allo-HCT | US | 16S Mothur | Low baseline fecal α-diversity is associated with pulmonary complications | 27 |
| France | 16S QIIME | Decreased α-diversity in pretreatment fecal samples is associated with subsequent bacteremia | 22 | |
| Italy and Poland† | 16S QIIME2 | Increased α-diversity in pretreatment fecal samples is associated with increased OS and lower grades of aGVHD | 18 | |
| US | 16S DADA2 | Increased α-diversity in periengraftment samples is associated with MAIT and Vδ2 T-cell frequency at day 30 in patients who received unmodified PBMC grafts | 29 | |
| US, Germany, and Japan | 16S vsearch, usearch, and QIIME | Increased α-diversity in periengraftment fecal samples is associated with increased OS and a lower transplantation-related mortality rate | 19 | |
| US | 16S DADA2 | Increased α-diversity in periengraftment fecal samples is associated with increased OS, decreased nonrelapse mortality, and better CD4 and CD8 T-cell recovery | 17 | |
| US | 16S DADA2 | Increased α-diversity in perineutrophil recovery fecal samples is associated with lower grades of aGVHD | 24 | |
| US | Shotgun metagenomics QIIME2 and vegan.R | Patients who developed cGVHD showed a change of β-diversity and a greater loss of α-diversity from baseline to after engraftment | 25 | |
| US | 16S Mothur | Increased α-diversity in postengraftment fecal samples is associated with increased OS and a lower transplantation-related mortality rate | 20 | |
| Auto-HCT/allo-HCT | China | 16S QIIME and UPARSE | Increased α-diversity in posttransplant samples is associated with decreased bacteremia | 23 |
| CAR T cell | China | 16S QIIME2 | α-Diversity is decreased in fecal samples from PR patients compared with fecal samples from CR patients at the CRS peak time point. β-Diversity is different between PR and CR samples at the same time point. | 30 |
| Mouse | ||||
| Syngeneic BMT | US | 16S Mothur | Authors defined intestinal microbiota score = Shannon index × fecal bacterial abundance. Higher intestinal microbiota score is associated with higher weight of periovarian fat, bone marrow, and thymic cellularity after transplantation. | 31 |
| Allo-HCT | US | 16S Mothur | Higher α-diversity in posttransplant samples is associated with better survival and lower GVHD score | 32 |
| US | 16S | Orally gavaging B fragilis increases posttransplant α-diversity in the ileum, and ameliorates GVHD | 33 | |
| US | 16S QIIME2 and DADA2 | High vitamin A diet is associated with decreased α-diversity in posttransplant fecal samples and more severe lung GVHD | 34 | |
| Australia | Metagenomics QIIME | Wild-type mice cohoused continuously with IL-17RA–deficient mice display decreased α-diversity and accelerated GVHD | 35 | |
| CAR T cell | US | 16S QIIME2 | Oral vancomycin treatment decreased the α-diversity of fecal samples from mice with human FMT. CAR T-cell therapy in combination with vancomycin slowed down tumor progression. | 36 |
BMT, bone marrow transplantation; cGVHD, chronic GVHD; CRS, cytokine release syndrome; IL-17RA, interleukin 17 receptor A; PBMC, peripheral blood mononuclear cell; PR, partial response; US, United States; VGPR, very good partial response.
Conclusions may vary based on the sampling site and methodology.
All human studies were performed in adult cohorts except this one in a pediatric cohort.
The link between microbial α-diversity and HCT outcomes has also been shown in preclinical models. In a syngeneic mouse bone marrow transplantation model, a higher intestinal microbiota score (α-diversity × fecal bacterial abundance) is associated with increased bone marrow and thymic cellularity.31 In allo-HCT mouse models, higher α-diversity correlates with better survival and decreased GVHD32-35 (Table 1).
Beyond α-diversity, specific taxa composition and abundance also relate to HCT outcomes. For example, the abundance of Clostridia and butyrate producers, along with the ratios of strict-to-facultative anaerobes can serve as a predictive feature for the outcomes of allo-HCT recipients with GVHD.38,39 Elevated abundance of Enterococcus correlates with worse OS and higher GVHD-related mortality,40 whereas more Eubacterium limosum is associated with lower relapse and progression risk.37 The presence of Staphylococcus is positively linked to effects on lymphocyte recovery41; however, it is also linked to lower CD4+ T-cell counts on day 100 after allo-HCT.17 Additionally, Bacteroides fragilis correlates with increased circulating exhausted T cells after allo-HCT.42
Potential drivers of the microbiota in HCT recipients comprise host genetics, intestinal inflammation, diet, and medications, including conditioning regimens, immunosuppressive drugs, and antibiotics.43-45 Antibiotics are widely used in patients undergoing HCT for prophylaxis or management of bacteremia and febrile neutropenia.46 They are closely linked to the outcomes of HCT, mostly to worse outcomes, in line with decreased α-diversity, including shorter survival,27,47-50 increased relapse,51 increased infection,14,52-55 and higher grades of GVHD.47,49,56-60 In contrast, some cohorts showed that antibiotics such as piperacillin-tazobactam, vancomycin (IV), and metronidazole are associated with decreased neutropenic fever and bacteremia in auto-HCT,61,62 and decreased aGVHD in allo-HCT.63 Although studies on gut decontamination are limited in clinical cohorts, data from this setting have shown decreased aGVHD and decreased bacteremia56,64 (Table 2).
Role of antibiotics in modulating microbiota and outcomes of cellular therapy
| Antibiotics . | Targets . | Microbial effects . | Impact on cellular therapy . |
|---|---|---|---|
| Auto-HCT | |||
| Piperacillin-tazobactam | Cell wall synthesis | ↓ α-Diversity65 | ↓ Neutropenic fever61 |
| Vancomycin (IV) | Cell wall synthesis | ↓ α-Diversity65 Change in β-diversity65 | ↓ Neutropenic fever62 ↓ Bacteremia62 |
| Doxycycline | Protein synthesis (30S) | Change in β-diversity65 | ↓ Central venous catheter infections66 |
| Ciprofloxacin | Nucleic acid synthesis | ↓ Aerobes | ↓ Neutropenic fever62 ↓ Bacteremia62 |
| Allo-HCT | |||
| Any antibiotics | — | ↓ Clostridia50 | ↑ Transplant-related mortality50, ↑ aGVHD47 ↓ OS47 |
| Combination of antimicrobial medications | — | Total gastrointestinal decontamination | ↓ aGVHD56 ↓ Bacteremia64 |
| Anaerobic antibiotics | — | ↓ Anaerobes ↓ α-Diversity48 | ↑ Lower respiratory tract disease52 ↑ Respiratory viral infections52 |
| Penicillins with or without a β-lactamase inhibitor | Cell wall synthesis | ↓ Gram-positive bacteria | ↑ aGVHD57 |
| Piperacillin-tazobactam | Cell wall synthesis | ↓ Bacteroidetes49, ↓ Lactobacillus49, ↓ Bifidobacteriales48, ↓ Clostridiales48 ↓ Anaerobes15 | ↑ GVHD49 ↑ GVHD-related mortality48 |
| Third-generation or higher cephalosporins | Cell wall synthesis | Change in β-diversity65 | ↑ aGVHD57 |
| Aztreonam | Cell wall synthesis | ↓ Gram-negative aerobes | ↑ aGVHD57 |
| Carbapenems | Cell wall synthesis | ↑ Bacteroides59, ↓ Enterococcus and UBA181959, ↓ Anaerobes15, Change in β-diversity65, ↓ Bifidobacteriales48 ↓ Clostridiales48 | ↑ aGVHD57,58 ↑ Intestinal aGVHD59 ↑ GVHD-related mortality48,49 ↓ Fever episodes67 |
| Vancomycin (IV) | Cell wall synthesis | ↓ α-Diversity65 Change in β-diversity65 | ↑ aGVHD57 ↑ CMV reactivation53 |
| Doxycycline | Protein synthesis (30S) | Change in β-diversity65 | ↓ Central venous catheter infections66 |
| Azithromycin | Protein synthesis (50S) | ↓ Aerobes and anaerobes | ↑ Overall mortality27, ↑ T-cell exhaustion51, ↑ Relapse51 ↑ Viral lower respiratory tract infection54 |
| Clindamycin | Protein synthesis (50S) | ↓ Gut anti-inflammatory Clostridia60 | ↑ GVHD60 |
| Fluoroquinolones | Nucleic acid synthesis | ↑ Proteobacteria domination14 | ↑ aGVHD57, ↑ Bacteremia with aerobic gram-negative bacilli14, ↑ Pulmonary complications27 ↑ Overall mortality27 |
| Metronidazole | Nucleic acid synthesis | ↑ Enterococcal domination14 Change in β-diversity65 ↓ Butyrate-producing bacteria | ↑ Vancomycin-resistant Enterococcus bacteremia14, ↑ Overall mortality27 ↓ aGVHD63 |
| Trimethoprim-sulfamethoxazole | Metabolic pathway (antifolate) | Change in β-diversity65 ↑ Fluoroquinolone-resistant Enterobacterales colonization55 | ↑ aGVHD57 ↑ Gram-negative bloodstream infections55 |
| CAR T-cell therapy | |||
| Any antibiotics | — | — | ↓ OS68,69 ↑ Progression69 |
| PIM | Cell wall synthesis | ↓ Anaerobes | ↓ OS68, ↓ PFS68 ↑ ICANS68 |
| High-risk antibiotics (meropenem, cefepime, ceftazidime and piperacillin-tazobactam) | Cell wall synthesis | ↓ α-Diversity69, Change in β-diversity69, ↓ Roseburia, Bifidobacterium, Lactobacillus, and Eubacterium spp69 ↑ Prevotella, Veillonella or Enterococcus spp69 | ↓ OS69, ↓ PFS69, ↑ Tumor burden69, ↑ Serum LDH and CRP69, ↓ Peripheral T-cell counts69 ↑ ICANS69 |
| Antibiotics . | Targets . | Microbial effects . | Impact on cellular therapy . |
|---|---|---|---|
| Auto-HCT | |||
| Piperacillin-tazobactam | Cell wall synthesis | ↓ α-Diversity65 | ↓ Neutropenic fever61 |
| Vancomycin (IV) | Cell wall synthesis | ↓ α-Diversity65 Change in β-diversity65 | ↓ Neutropenic fever62 ↓ Bacteremia62 |
| Doxycycline | Protein synthesis (30S) | Change in β-diversity65 | ↓ Central venous catheter infections66 |
| Ciprofloxacin | Nucleic acid synthesis | ↓ Aerobes | ↓ Neutropenic fever62 ↓ Bacteremia62 |
| Allo-HCT | |||
| Any antibiotics | — | ↓ Clostridia50 | ↑ Transplant-related mortality50, ↑ aGVHD47 ↓ OS47 |
| Combination of antimicrobial medications | — | Total gastrointestinal decontamination | ↓ aGVHD56 ↓ Bacteremia64 |
| Anaerobic antibiotics | — | ↓ Anaerobes ↓ α-Diversity48 | ↑ Lower respiratory tract disease52 ↑ Respiratory viral infections52 |
| Penicillins with or without a β-lactamase inhibitor | Cell wall synthesis | ↓ Gram-positive bacteria | ↑ aGVHD57 |
| Piperacillin-tazobactam | Cell wall synthesis | ↓ Bacteroidetes49, ↓ Lactobacillus49, ↓ Bifidobacteriales48, ↓ Clostridiales48 ↓ Anaerobes15 | ↑ GVHD49 ↑ GVHD-related mortality48 |
| Third-generation or higher cephalosporins | Cell wall synthesis | Change in β-diversity65 | ↑ aGVHD57 |
| Aztreonam | Cell wall synthesis | ↓ Gram-negative aerobes | ↑ aGVHD57 |
| Carbapenems | Cell wall synthesis | ↑ Bacteroides59, ↓ Enterococcus and UBA181959, ↓ Anaerobes15, Change in β-diversity65, ↓ Bifidobacteriales48 ↓ Clostridiales48 | ↑ aGVHD57,58 ↑ Intestinal aGVHD59 ↑ GVHD-related mortality48,49 ↓ Fever episodes67 |
| Vancomycin (IV) | Cell wall synthesis | ↓ α-Diversity65 Change in β-diversity65 | ↑ aGVHD57 ↑ CMV reactivation53 |
| Doxycycline | Protein synthesis (30S) | Change in β-diversity65 | ↓ Central venous catheter infections66 |
| Azithromycin | Protein synthesis (50S) | ↓ Aerobes and anaerobes | ↑ Overall mortality27, ↑ T-cell exhaustion51, ↑ Relapse51 ↑ Viral lower respiratory tract infection54 |
| Clindamycin | Protein synthesis (50S) | ↓ Gut anti-inflammatory Clostridia60 | ↑ GVHD60 |
| Fluoroquinolones | Nucleic acid synthesis | ↑ Proteobacteria domination14 | ↑ aGVHD57, ↑ Bacteremia with aerobic gram-negative bacilli14, ↑ Pulmonary complications27 ↑ Overall mortality27 |
| Metronidazole | Nucleic acid synthesis | ↑ Enterococcal domination14 Change in β-diversity65 ↓ Butyrate-producing bacteria | ↑ Vancomycin-resistant Enterococcus bacteremia14, ↑ Overall mortality27 ↓ aGVHD63 |
| Trimethoprim-sulfamethoxazole | Metabolic pathway (antifolate) | Change in β-diversity65 ↑ Fluoroquinolone-resistant Enterobacterales colonization55 | ↑ aGVHD57 ↑ Gram-negative bloodstream infections55 |
| CAR T-cell therapy | |||
| Any antibiotics | — | — | ↓ OS68,69 ↑ Progression69 |
| PIM | Cell wall synthesis | ↓ Anaerobes | ↓ OS68, ↓ PFS68 ↑ ICANS68 |
| High-risk antibiotics (meropenem, cefepime, ceftazidime and piperacillin-tazobactam) | Cell wall synthesis | ↓ α-Diversity69, Change in β-diversity69, ↓ Roseburia, Bifidobacterium, Lactobacillus, and Eubacterium spp69 ↑ Prevotella, Veillonella or Enterococcus spp69 | ↓ OS69, ↓ PFS69, ↑ Tumor burden69, ↑ Serum LDH and CRP69, ↓ Peripheral T-cell counts69 ↑ ICANS69 |
The symbol ↑ represents an increase, and the symbol ↓ represents a decrease.
CMV, cytomegalovirus; CRP, C-reactive protein; LDH, lactate dehydrogenase; PIM, piperacillin/tazobactam, imipenem/cilastatin, and meropenem.
As a component of the microbiota, the abundance of fungi is also correlated with allo-HCT outcomes. Despite the overall density and biodiversity of gut fungi remaining stable during allo-HCT, notable changes occur in the composition of fungal microbiota in patients with or without candidemia.70 The presence of viable fungi in fecal samples is associated with decreased survival and elevated transplant-related mortality. Specifically, dominance of Candida parapsilosis is linked to worse survival.71
Although the link between microbiota and HCT is well-studied, less is understood about its relationship with TIL or NK cell therapies. A paucity of studies have identified a link between microbiota and the activity of TILs.72-74 Specifically, 1 preclinical study showed that commensal Barnesiella intestinihominis increases the proportion of interferon gamma–producing γδTILs while decreasing IL-17–producing γδTILs in a fibrosarcoma model.73 Another study identified a correlation between the presence of Fusobacterium nucleatum and increased TILs in patient colorectal carcinoma tissue. Interestingly, this association varies based on the tumor’s microsatellite instability (MSI) status. In MSI-high tumors, the presence of F nucleatum is inversely linked with TILs, whereas in non–MSI-high tumors a positive correlation is observed.74
For NK cell therapy, there are contradictory findings regarding the role of microbiota. For example, 1 study in a pancreatic tumor model showed that germ-free mice have increased intratumoral NK cell infiltration and activation compared with specific pathogen–free mice.75 In contrast, another study in lymphoma, colon adenocarcinoma, and mammary carcinoma models showed that germ-free mice have decreased tumor-infiltrating NK cells.76
Microbiota and genetically modified cellular therapies
Genetically modified cellular therapies use genetic engineering techniques to improve the specificity of autologous or allogeneic immune cells. TCR-Ts are engineered by cloning the TCR α and β chains that have a desired specificity for tumor antigens into T cells.77 This design enables TCR-Ts to recognize tumor antigens in a major histocompatibility complex-dependent manner. Another method for improving T-cell specificity is through the genetic integration of CARs. This design allows CAR T cells to recognize tumor cells in a major histocompatibility complex–independent manner.
Antibiotics are commonly used alongside TCR-T and CAR T-cell therapies. Although less is known about the impact of antibiotics on TCR-T therapy, clinical studies have shown that antibiotic use in the weeks before CAR T-cell therapy has a negative effect on clinical outcomes. One study found that >60% of patients receive antibiotics in the 4 weeks before CAR T-cell infusion.68 Two independent studies68,69 covering 4 US and 3 German medical centers showed that the most commonly used antibiotics before CAR T-cell infusion include trimethoprim/sulfamethoxazole, piperacillin/tazobactam, vancomycin (IV), cefepime, ciprofloxacin, levofloxacin, and meropenem. Both studies indicate that any antibiotic usage within the 369 or 468 weeks before CAR T-cell infusion correlates with reduced OS and PFS.
Smith et al68 focused on anaerobe-targeting antibiotics piperacillin/tazobactam, imipenem/cilastatin, and meropenem (PIM). They found that exposure to PIM in the 4 weeks before CD19-targeted CAR T-cell infusion is associated with reduced OS and PFS more significantly than exposure to any antibiotics. Furthermore, PIM usage is correlated with increased immune effector cell–associated neurotoxicity syndrome (ICANS) in patients with non-Hodgkin lymphoma (NHL). Stein-Thoeringer et al69 defined “high-risk antibiotics” as meropenem, cefepime, ceftazidime, and piperacillin-tazobactam, and they found that exposure to these antibiotics is correlated with a higher rate of disease progression as well as shorter PFS and OS. High-risk antibiotics also correlate with a higher incidence of ICANS (Table 2).
Antibiotics and chemotherapy drugs, such as cyclophosphamide,78 likely contribute to decreased microbial α-diversity in patients before CAR T-cell infusion compared with healthy donors68; ongoing exposure to antibiotics and dietary changes may be key drivers of further reductions in α-diversity after infusion.69 Exposure to high-risk antibiotics exacerbates this decreased α-diversity, compared with non–high-risk antibiotics.30,69 Diversity is correlated with outcomes, because it is lower in patients with a partial response compared with a complete response (CR)30 (Table 1). By 16S ribosomal RNA (rRNA) sequencing, Smith et al identified genera including Ruminococcus, Bacteroides, and Faecalibacterium that are positively associated with CAR T-cell response in patients with NHL and acute lymphocytic leukemia (ALL).68 They also identified genera including Blautia, Ruminococcus, Bacteroides, and Faecalibacterium that are associated with no cytokine release syndrome or ICANS. By shotgun metagenome sequencing, Stein-Thoeringer et al69 identified that species including Bacteroides eggerthii, Ruminococcus lactaris, Eubacterium sp.CAG 180, and Akkermansia muciniphila are positively associated with CR in patients with NHL. They also identified species including Bacteroides stercoris, B fragilis, and Roseburia faecis that are associated with no CR. By 16S rRNA sequencing, Hu et al30 identified that genera Bifidobacterium, Faecalibacterium, Ruminococcus, and Dialister are positively correlated with CR in patients with MM and NHL. Conversely, Bifidobacterium, Collinsella, and Roseburia are negatively correlated with CR in patients with ALL. Furthermore, Bifidobacterium and Leuconostoc are correlated with higher grades of cytokine release syndrome in patients with MM. Bifidobacterium's inconsistent link with outcomes across these CAR T-cell studies may stem from varying strain composition, warranting more extensive research for better understanding.
Mouse experiments have indicated that the impact of antibiotics on CAR T-cell therapy may vary with different antibiotics. Kuczma et al used a systemic A20 lymphoma and anti-CD19 CAR T-cell model in which the animals received gut decontaminating antibiotics, including ciprofloxacin, gentamicin, bacitracin, and streptomycin, in their drinking water. They found increased CAR T-cell percentage in long-term survivors in the antibiotic recipients, whereas OS remained unchanged.79 In contrast, in a solid tumor model using A20 lymphoma and B16-CD19 melanoma cell lines and anti-CD19 CAR T-cell treatment, Uribe-Herranz et al found that vancomycin treatment enhances CAR T-cell function, indicated by smaller tumors and increased T-cell infiltration36 (Table 1).
Possible mechanisms
Microbial competition
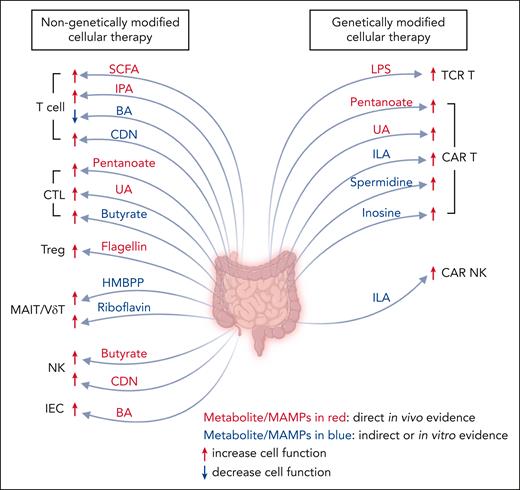
Microorganisms engage in robust competition for essential resources, including space and nutrients. This competition becomes particularly pronounced in patients with cancer because of distinct factors that modify resource availability and introduce stress to the microbiota. Such factors may include cancer initiation, disease progression and metastasis, dietary changes, medications, and surgical procedures.80 The resulting decreased diversity observed in patients undergoing HCT and CAR T-cell therapy could be an outcome of this intense competition. Notably, fecal samples from patients with NHL and B-cell ALL showed a dominance of commensal Clostridia and Bacteroidetes, as well as potential pathobionts, including Escherichia, Klebsiella and Enterococcus.68 The microbiota that survive in this competitive environment may further impact the immune system and cellular therapy outcomes via the immune signals and metabolites they produce (Figure 1; Table 3).
The role of microbe-associated molecular patterns and microbial metabolites on target cells for cellular therapy. The intestinal microbiota generates microbe-associated molecular patterns and metabolites, both of which play a pivotal role in modulating the functionality of immune cells as well as IECs, thereby influencing the outcome of cellular therapy. CDN, cyclic dinucleotides; ILA, indole-3-lactic acid; IPA, indole-3-propionic acid. Figure created with BioRender.com.
The role of microbe-associated molecular patterns and microbial metabolites on target cells for cellular therapy. The intestinal microbiota generates microbe-associated molecular patterns and metabolites, both of which play a pivotal role in modulating the functionality of immune cells as well as IECs, thereby influencing the outcome of cellular therapy. CDN, cyclic dinucleotides; ILA, indole-3-lactic acid; IPA, indole-3-propionic acid. Figure created with BioRender.com.
Microbe-associated molecular patterns, microbial metabolites, and their effect on antitumor activity of cellular therapy
| Microbial metabolites . | Antitumor effect on cellular therapies . | Reference . |
|---|---|---|
| Nongenetically modified cellular therapy | ||
| Cyclic dinucleotide | Activates the stimulator of interferon gene pathway in T cells and NK cells, inhibiting the proliferation of T cells81 but augmenting the antitumor function of T cells82 and NK cells83 | 81-83 |
| SCFAs | Pentanoate and butyrate treatments increase HDAC class I enzyme inhibition and mTOR activity in CTLs, enabling stronger antitumor function through enhanced CD25, TNF-α, and IFN-γ production | 84 |
| Butyrate increases the production of IFN-γ and granzyme B in CTLs | 85 | |
| SCFA receptor GPR109A on T cells increases T-cell activation and alloreactivity by enhancing mitochondrial oxidative phosphorylation while reducing T-cell apoptosis | 86 | |
| Butyrate supports liver-resident NK cells via IL18/IL-18 receptor signaling | 87 | |
| MTCs | Indole-3-propionic acid enhances H3K27ac at the Tcf7 superenhancer, enhances T-cell stemness, progenitor-exhausted CD8+ T-cell differentiation, and antitumor function | 88 |
| Secondary BAs | Microbial BAs attenuate FXR activation, reduce the proliferation of human T cells, and are associated with longer OS in allo-HCT | 89 |
| Protect IECs and maintain the GVL effect | 90 | |
| UA | It improves the persistence and effector functions of CTLs via ERK1/2 signaling | 91 |
| Riboflavin | Riboflavin and HMBPP improve MAIT and Vδ2+ T-cell activation and cytotoxicity | 29 |
| 4-HMBPP | ||
| Genetically modified cellular therapy | ||
| LPS | LPS administration increases adoptively transferred TCR-Ts and enhances their IFN-γ production and antitumor function via Toll-like receptor 4 signaling | 92 |
| SCFAs | Pentanoate and butyrate treatment boosts CD25, TNF-α, and IFN-γ in CAR T cells, enhancing their antitumor function | 84 |
| MTCs | Indole-3-lactic acid enhances the binding of H3K27ac in the enhancer regions of IL12a,93 which can potentiate the cytotoxicity of CAR T cells94 and CAR NK cells95 | 93-95 |
| PAs | Spermidine enhances CAR T-cell proliferation, elevates memory phenotype, and amplifies cytotoxicity in vitro | 96 |
| UA | Enhances CAR T-cell differentiation into TSCM and TCM, and bolsters cytotoxicity through Pink1-dependent mitophagy and the Wnt pathway | 97 |
| It improves the persistence and effector functions of CAR T cells via ERK1/2 signaling | 91 | |
| Inosine | It induces CAR T-cell epigenetic reprogramming and improves CAR T-cell stemness and cytotoxicity | 98 |
| Microbial metabolites . | Antitumor effect on cellular therapies . | Reference . |
|---|---|---|
| Nongenetically modified cellular therapy | ||
| Cyclic dinucleotide | Activates the stimulator of interferon gene pathway in T cells and NK cells, inhibiting the proliferation of T cells81 but augmenting the antitumor function of T cells82 and NK cells83 | 81-83 |
| SCFAs | Pentanoate and butyrate treatments increase HDAC class I enzyme inhibition and mTOR activity in CTLs, enabling stronger antitumor function through enhanced CD25, TNF-α, and IFN-γ production | 84 |
| Butyrate increases the production of IFN-γ and granzyme B in CTLs | 85 | |
| SCFA receptor GPR109A on T cells increases T-cell activation and alloreactivity by enhancing mitochondrial oxidative phosphorylation while reducing T-cell apoptosis | 86 | |
| Butyrate supports liver-resident NK cells via IL18/IL-18 receptor signaling | 87 | |
| MTCs | Indole-3-propionic acid enhances H3K27ac at the Tcf7 superenhancer, enhances T-cell stemness, progenitor-exhausted CD8+ T-cell differentiation, and antitumor function | 88 |
| Secondary BAs | Microbial BAs attenuate FXR activation, reduce the proliferation of human T cells, and are associated with longer OS in allo-HCT | 89 |
| Protect IECs and maintain the GVL effect | 90 | |
| UA | It improves the persistence and effector functions of CTLs via ERK1/2 signaling | 91 |
| Riboflavin | Riboflavin and HMBPP improve MAIT and Vδ2+ T-cell activation and cytotoxicity | 29 |
| 4-HMBPP | ||
| Genetically modified cellular therapy | ||
| LPS | LPS administration increases adoptively transferred TCR-Ts and enhances their IFN-γ production and antitumor function via Toll-like receptor 4 signaling | 92 |
| SCFAs | Pentanoate and butyrate treatment boosts CD25, TNF-α, and IFN-γ in CAR T cells, enhancing their antitumor function | 84 |
| MTCs | Indole-3-lactic acid enhances the binding of H3K27ac in the enhancer regions of IL12a,93 which can potentiate the cytotoxicity of CAR T cells94 and CAR NK cells95 | 93-95 |
| PAs | Spermidine enhances CAR T-cell proliferation, elevates memory phenotype, and amplifies cytotoxicity in vitro | 96 |
| UA | Enhances CAR T-cell differentiation into TSCM and TCM, and bolsters cytotoxicity through Pink1-dependent mitophagy and the Wnt pathway | 97 |
| It improves the persistence and effector functions of CAR T cells via ERK1/2 signaling | 91 | |
| Inosine | It induces CAR T-cell epigenetic reprogramming and improves CAR T-cell stemness and cytotoxicity | 98 |
ERK, extracellular signal–regulated kinase; FXR, farnesoid X receptor; HDAC, histone deacetylase; IFN-γ, interferon gamma; MAIT, mucosal-associated invariant T cells; TCM, central memory T cells; TNF-α, tumor necrosis factor α; TSCM, memory stem T cells.
Microbe-host direct signaling
Microbe-associated molecular patterns, including lipopolysaccharide, peptidoglycan, flagellin, and cyclic dinucleotides, trigger the activation of immune cells. Under normal conditions, the mucosal barrier shields against bacterial translocation. However, radiation, chemotherapy, and antibiotics, which are commonly received by patients with cancer, disrupt this protective barrier. This disruption may promote bacterial translocation, likely involving antibiotic-resistant pathogens, which calls for more antibiotics, leading to more severe diversity loss and dysbiosis.99 This triggers innate immune cell infiltration in the small intestine, potentially causing intestinal damage via reactive oxygen species, leading to aGVHD in allo-HCT recipients.100 GVHD can also be mediated by T-cell activation from antigen-presenting cells.99 Translocated bacteria can also directly activate and improve CD8+ TCR-T effectiveness through lipopolysaccharide/Toll-like receptor 4.92 Interestingly, flagellin reduces GVHD through the activation of Toll-like receptor 5 and the upregulation of regulatory T cells (Tregs).101 In addition, certain microbiota secrete cyclic dinucleotides, activating the stimulator of interferon genes pathway in hematopoietic and nonhematopoietic cells.102 This activation may influence HCT outcomes by promoting intestinal epithelial cell (IEC) repair103 and modulating alloreactivity.104 Moreover, stimulator of interferon genes activation enhances antitumor activity of T, NK, and dendritic cells.102
A noteworthy association has been discovered between peptidoglycan and CAR T-cell therapy. The biosynthesis of peptidoglycan is correlated with the CR rate at day 100 after CAR T-cell therapy.68 However, it is also associated with a diminished survival (<180 days) and a faster progression (<180 days).69 This discrepancy may be attributable to the exposure to different antibiotics. Indeed, in the latter cohort, when high-risk antibiotic exposure was excluded, the biosynthesis of peptidoglycan exhibited an association with prolonged survival (>180 days) and slower disease progression.69
Microbe-host signaling at a distance
The gut microbiota generates key metabolites, such as short-chain fatty acids (SCFAs), tryptophan catabolites, secondary bile acids (BAs), and polyamines (PAs), that fluctuate during cellular therapy.105 Here, we discuss their effects on cellular therapy outcomes.
SCFAs
The concentration of microbiota-derived SCFAs, especially butyrate, declines significantly in the intestinal tissue after allo-HCT.106 As class I histone deacetylase inhibitors, SCFAs increase histone acetylation in CD326+ IECs and T cells, especially in the Foxp3 promoter region.107,108 The decreased SCFA level, resulting in decreased IEC and Treg histone acetylation, compromises the integrity of the IEC junctions and FOXP3 expression in Tregs. This deterioration can potentially exacerbate the progression of GVHD. Additionally, the SCFA receptor G-protein–coupled receptor 43 (GPR43) located on the IEC serves a pivotal role in alleviating GVHD through initiating the extracellular signal-regulated kinase–nucleotide-binding oligomerization domain (NOD)-like receptor protein 3 (ERK-NLRP3) pathway.109 Moreover, SCFAs activate GPR43 on colonic Tregs, increase its population, and further ameliorate colonic inflammation.110
Another SCFA receptor, GPR109A, affects T-cell proliferation by enhancing mitochondrial oxidative phosphorylation while reducing T-cell apoptosis.86 Loss of butyrate also impairs liver-resident NK cell maturation through GPR109A by suppressing IL-18/IL-18 receptor signaling and mitochondrial activity.87
Recent studies on mice revealed that pentanoate and butyrate heighten the function of mammalian target of rapamycin (mTOR) and inhibit histone deacetylase activity in antigen-specific cytotoxic T lymphocytes (CTLs) and CAR T cells. This metabolic reprogramming leads to higher expression of CD25, increased production of effector molecules from CTLs and CAR T cells (including interferon gamma and tumor necrosis factor α), and further amplifies their antitumor activity.84,85
MTCs
Indole and indole derivatives, products of tryptophan metabolism driven by the gut microbiota, play a key role in mitigating GVHD and improving T-cell function. These microbial tryptophan catabolites (MTCs) help minimize gut epithelial damage and decrease bacterial translocation. They also effectively reduce inflammatory cytokine production, thereby mitigating GVHD-associated morbidity and mortality while preserving the GVL effect without hampering donor T-cell effectiveness.111
Furthermore, other MTCs like indole-3-propionic acid and indole-3-lactic acid, produced by Lactobacillus and Clostridium, enhance CD8+ T-cell function by altering their epigenetic landscape. Specifically, indole-3-propionic acid boosts the acetylation of H3K27 at the superenhancer of Tcf7. This acetylation not only fosters the stemness of T cells but also enhances the differentiation of CD8+ T cells into progenitor-exhausted CD8+ T cells.88 Similarly, indole-3-lactic acid enhances the binding of H3K27ac in the enhancer regions of IL12a,93 which can stimulate CD8+ T cells and potentiate the cytotoxicity of CAR T cells and CAR NK cells.94,95,112
BA
Microbiota-derived BA decreased significantly in patients with GVHD after allo-HCT. This reduction corresponds with heightened activation of T-cell farnesoid X receptor, resulting in an escalated effector T-cell response and increased GVHD-associated mortality.89 In contrast, deoxycholic acid mitigates the effector functionality of CD8+ T cells by targeting the plasma membrane Ca2+ adenosine triphosphatase, thereby inhibiting the signaling of the calcium-nuclear factor of activated T-cell pathway.113
Furthermore, BAs play a role in regulating CD4+ T cells. 3-oxolithocholic acid and isolithocholic acid inhibit T helper 17 cell differentiation by directly binding to the transcription factor retinoid-related orphan receptor-γt.114 Certain BAs, including isoallolithocholic acid, ω-muricholic acid, and isodeoxycholic acid, boost the generation of Tregs. Isoallolithocholic acid aids in the differentiation of Tregs through the induction of mitochondrial reactive oxygen species, subsequently leading to elevated expression of FOXP3.114 Specifically, isodeoxycholic acid promotes the induction of FOXP3 by countering farnesoid X receptor activity in antigen-presenting cells.115
Certain BAs also improve GVHD outcomes by protecting IECs. For example, tauroursodeoxycholic acid suppresses antigen-presenting functions of nonhematopoietic cells and prevents the apoptosis of the IECs without impairing T-cell GVL function.90
PA
Intestinal microbiota synthesizes a substantial quantity of PAs in the lower gastrointestinal tract.116 Intriguingly, increased plasma PAs were noted in allo-HCT recipients who did not develop gastrointestinal aGVHD or had no or mild oral mucositis.105,117 This effect may be partially explained by PAs promoting IEC renewal and maintaining IEC barrier integrity.118 PAs were lower in CD19 CAR T-cell therapy responders vs nonresponders.119 However, an in vitro study showed that spermidine, a key type of PA, enhanced CAR T-cell proliferation, memory phenotype, and cytotoxicity.96 This discrepancy may be partially because of the balance of spermidine and spermine, which is another major type of PA. They competitively bind to the mitochondrial trifunctional protein in CD8+ T cells, affecting fatty acid oxidation, energy production, and antitumor functionality.120
Other metabolites
Microbiota-derived urolithin A (UA) significantly enhances the differentiation of CAR T cells into memory stem T cells and central memory T cells, further bolstering the cytotoxicity of CAR T cells.91,97 UA facilitates the growth of CD8+ memory stem T cells through mitophagy dependent on Pink1. Concurrently, UA fosters mitochondrial health by keeping a balance between mitophagy and mitochondrial biogenesis, through the activation of the Wnt signaling pathway.97 Moreover, it strengthens the persistence and effector functions of CTLs and CAR T cells by activating extracellular signal–regulated kinase 1/2, triggering CTL autophagy, and fostering metabolic adaptation.91
Microbiota-derived inosine promotes T helper 1 cell activation and boosts T-cell cytotoxicity.121 Introducing inosine to CAR T cells during the manufacturing process can stimulate cellular stemness and augment their cytotoxicity.98
Furthermore, Erysipelotrichaceae- and Ruminococcaceae-derived riboflavin and 4-hydroxy-3-methylbut-2-enyl pyrophosphate (4-HMBPP) support the activation and cytotoxicity of mucosal-associated invariant T cells and Vδ2+ T cells in allo-HCT.29
Interventional applications of the microbiota in cellular therapy
FMT
Recent clinical trials in which fecal microbiota transplantation (FMT) is performed in allo-HCT recipients suggest improved outcomes for these patients. These improvements include the restoration of microbial diversity and composition,122,123 the treatment of multidrug-resistant infections,124-126 and lower GVHD.127-129
Although FMT provides various benefits to HCT recipients, the intricacy and uncertain nature of the fecal matter could potentially heighten the risk of infection. To offer a safer and more reliable treatment, research efforts are being directed toward identifying the crucial bacterial strains that can enhance therapeutic outcomes. In a preclinical model, investigators found that a combination of 17 Clostridial strains mitigate GVHD and improve survival.107 Another preclinical study demonstrated that a single strain of B fragilis can potentially reduce GVHD and extend survival.33
Prebiotic/dietary modification
Research findings indicate that dietary modifications enable recipients of cellular therapies to alter the composition of their microbiota. It has been demonstrated that adopting a lactose-free diet can reduce the level of Enterococcus, a bacteria observed to increase in certain patients with NHL and ALL,68 and that has ties with the onset of GVHD.40 As a result, a lactose-free diet extends the survival in a preclinical GVHD model.40 Additionally, high-salt diet increases the gut and intratumor abundance of Bifidobacterium, which enhances NK cell functions and tumor regression in a skin melanoma model.130 A recent phase 2 trial indicates that resistant potato starch increases fecal butyrate and microbial diversity in allo-HCT recipients, although its correlation with clinical outcome remains unclear.131
Iron-chelating agent
The oral administration of deferasirox, an iron-chelating agent, reduces oxygen levels in the gut. This decrease in gut oxygen levels facilitates an increase in microbial diversity in an allo-HCT model, consequently leading to longer survival and a reduction in GVHD.32
Medication usage
Nguyen et al introduced PARADIGM, a computational technique capable of elucidating the relationship between medication, microbiota composition, and the results of cellular therapy.132 Using PARADIGM with patient drug data, researchers can predict microbiota trajectories and allo-HCT outcomes, underlying its potential to guide optimal medication selection.
The studies linking antibiotic usage to the outcomes of cellular therapies have the potential to enhance antibiotic stewardship. Such information could assist physicians in making more informed decisions regarding the appropriate type, dosage, timing, and duration of antibiotics during cellular therapies. For instance, shifting from prophylactic ciprofloxacin/metronidazole to rifaximin or moving from permissive to restrictive use of antibiotics has shown positive effects on microbial diversity and allo-HCT outcomes.133,134
Gaps and future directions
Research on microbiota and cellular therapies reveals several intersecting findings across different therapies. First, microbial diversity decreases during the peritransplantation and cellular therapy infusion period; this reduction is shown to correlate with outcomes (Table 1).14,16,30,69 Second, poorer outcomes have been associated with antibiotic usage, particularly those targeting obligate anaerobes (Table 2).48,68 Finally, certain overlapping microbial taxa, such as B fragilis and Ruminococcus gnavus, exert parallel effects on CAR T-cell therapy and HCT.41,42,69 Specifically, these microbes are associated with no CR in CAR T-cell therapy,69 as well as decreased lymphocyte recovery,41 increased T-cell exhaustion,42 and increased relapse42 after HCT. However, our understanding is still incomplete regarding the mechanisms through which microbiota influence cellular therapy, a gap that currently hampers the translation of microbiota applications into clinical practice. Some outstanding questions include which microbial species play the most important role in affecting the therapeutic efficiency of effector cells? Are the insights disease dependent or treatment dependent? By what mechanisms do the microbial species affect effector cells, tumor cells, or cells within the tumor microenvironment? How do dietary modifications and medication usage change a patient's microbiota? How can we translate these findings into clinical practice?
There is a clear correlation between microbial diversity, the relative abundance of human gut microbiota, and the outcomes of cellular therapy.135 Yet these data prompt further inquiry into whether relative abundance accurately reflects the absolute abundance. The investigation and identification of microbiota depend heavily on DNA or RNA sequencing. Several methodologies have been developed to quantify the absolute abundance of microbiota, including microscopy, flow cytometry, 16S rRNA fluorescence in situ hybridization, spike-ins, and 16S rRNA digital or quantitative polymerase chain reaction.136 Upcoming studies may benefit from incorporating these methods to enrich the understanding of the role of the microbiota in cellular therapy.
Although the mechanisms are partly understood, evidence indicates that microbial metabolites may serve as a crucial link connecting basic research and clinical applications. A preclinical study showed that adding inosine during the CAR T-cell manufacturing process increases CAR T-cell stemness and antitumor function.98 Clinical studies demonstrate that SCFA supplementation can rebalance Tregs/T helper 17 cells, potentially reducing GVHD,137 and modulate T-cell function, thereby improving adoptive cell therapy outcomes.138 Furthermore, some trials are aiming to tune microbial metabolite levels through FMT139 or the administration of prebiotics.131 Therefore, careful monitoring of and adjusting microbial metabolite levels may present a viable approach for improving clinical outcomes.
Patients from the same region, medical center, or cultural background may have similar diets or comparable exposure to specific medications as influenced by treatment practice. These variables could potentially constrain the application of microbial findings from small, single-center cohorts. Additionally, the diverse methodologies used in microbial sample processing and analysis across different studies may further impede the broader application of findings. To gather a more holistic understanding of the relationship between microbial species and cellular therapy, multicenter studies are indispensable. Furthermore, harmonizing the procedures for microbial sample processing is crucial to ensure consistency and reproducibility of results. Finally, to establish causal relationships regarding the impact of the microbiota on cellular therapy, the use of single and multiple regression models as well as preclinical models is necessary.
Acknowledgments
M.S. receives research support from the NMDP (Amy Strelzer Manasevit Research Program), Damon Runyon Cancer Research Foundation (Clinical Investigator Award), the National Institutes of Health, National Heart, Lung, and Blood Institute (grant K08 HL156082-01A1), the American Society of Hematology (Scholar Award), and the Parker Institute for Cancer Immunotherapy.
Authorship
Contribution: J.X. and M.S. contributed to the content and writing of the manuscript, and all authors agreed to the contents of the manuscript before submission.
Conflict-of-interest disclosure: M.S. reports consultancy for CVS Caremark; an advisory role with A28 Therapeutics; and patents pertaining to the intestinal microbiome and chimeric antigen receptor T-cell therapy (US 17/229,184; US 63/303,461). J.X. declares no competing financial interests.
Correspondence: Melody Smith, Department of Medicine, Stanford University, CCSR Building, Room 2255, 269 W Campus Dr, Stanford, CA 94305-5170; email: melodysm@stanford.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal