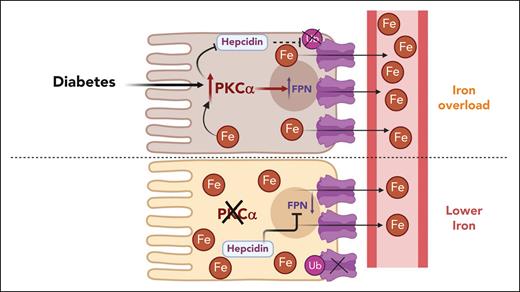
In this issue of Blood, Banerjee et al elucidate a novel mechanism underlying iron overload in diabetes.1 They demonstrate that protein kinase C alpha (PKCα) positively modulates the trafficking and localization of ferroportin (Fpn) in a hepcidin-independent manner, contributing to diabetic iron overload.
Given that Fpn plays a crucial role in systemic iron homeostasis and is negatively regulated by hepcidin, knowledge of other potential regulators under various physiological conditions and diseases remains limited. Multiple studies have shown significant increases in intestinal Fpn expression and body iron content in experimental models of diabetes.2 Furthermore, it has been shown that intestinal PKCα enhances the membrane expression of divalent metal transporter 1 in diabetes.3 PKC is a family of serine/threonine protein kinases that are activated under conditions of high blood glucose. This activation leads to accumulated reactive oxygen species and advanced glycation end products and redox stress. These effects lead to cell death and mediate a plethora of diabetic complications.4 Banerjee et al reveal that inhibition of PKCα reduces Fpn expression and iron overload in both physiological and pathological conditions, including diabetes and hereditary hemochromatosis. Thus, the research identifies PKCα as a novel regulator of Fpn, suggesting that targeting PKCα may help control systemic iron homeostasis.
Using murine models of streptozotocin-induced type 1 diabetes and type 2 diabetic db/db and KKAy mice, the study addresses body iron loading across different diabetic conditions, focusing on the upregulation of Fpn. The authors found that Fpn expression was significantly increased in the duodenum and spleen in all models. However, unlike streptozotocin-treated db/db mice, KKAy mice exhibited a larger increase in Fpn in the spleen. The authors further explored Fpn expression in humans and observed variations in the expression and localization of Fpn in enterocytes between human diabetic and control subjects. Diabetic groups showed elevated Fpn expression in epithelial cells and reduced intracellular iron content.
Banerjee et al described variable hepcidin expression in the 3 mouse models, indicating that hepcidin is not the primary mechanism for Fpn regulation in diabetes. To explore other mechanisms, the authors disrupted PKCα, which resulted in a significant decrease in serum iron content and markedly decreased Fpn expression in the duodenum and spleen. This highlights PKCα as an important positive regulator of Fpn under both physiological and diabetic conditions. Interestingly, in db/db;Pkcα−/− mice, Fpn expression was increased in the duodenum despite a significant decrease in the spleen, suggesting a more complex regulation of iron metabolism in these mice that requires further investigation.
The authors also discovered that PKCα decreases endocytic and increases exocytotic trafficking of Fpn, which was not through changes in Fpn ubiquitination, a critical step in hepcidin-induced internalization and degradation of Fpn.5 A significant decrease in Fpn protein and an increase in ferritin was observed in PKCα−/− bone marrow–derived macrophages compared with wild-type controls. Moreover, a PKCα inhibitor decreased total and membrane Fpn expression with a more pronounced decrease in the membrane pool. When using cells expressing Fpn tagged with green fluorescent protein in a doxycycline-inducible manner, the authors observed robust internalization of Fpn after PKCα inhibition, indicated by increased green fluorescent protein–positive puncta in the cytoplasm, independent of Fpn ubiquitination. Furthermore, PKCα regulates both endocytic and exocytic trafficking of Fpn. PKC inhibitors increase the internalization and degradation of Fpn via hepcidin, whereas PKCα activation increases the membrane pool of Fpn. However, the mechanisms underlying this need to be explored, including identifying PKCα substrates central for Fpn regulation. PKC is known to undergo cytosol-to-membrane translocation and regulates remodeling of the cytoskeleton filaments.6 The authors speculate that the activation of PKCα modulates the cytoskeleton dynamics that in turn control Fpn trafficking. This is a novel finding, and, if further validated, may lead to the discovery of other cytoskeletal modulators that can impact Fpn shuttling.
Banerjee and colleagues have demonstrated that PKCα is highly integrated into control of cellular iron levels. Iron excess activated PKCα in enterocytes and macrophages.7 Oral administration of FeSO4 increased PKCα phosphorylation in the duodenum, and there was also a time-dependent increase in phosphorylation of PKCα in bone marrow–derived macrophages treated with ferric ammonium citrate. Active PKCα reduced intracellular iron content under basal conditions and mitigated hepcidin-induced iron accumulation, without affecting baseline Fpn ubiquitination. Binding assays indicated that PKCα attenuated Fpn ubiquitination rather than altering hepcidin-Fpn interactions.
Collectively, these findings indicate that PKCα activation by excessive iron enhances Fpn-mediated iron efflux, thereby mitigating intracellular iron overload and highlighting the critical role of PKCα in regulating iron movement under conditions of iron excess.
Last, Banerjee et al evaluated whether targeting PKCα could alleviate iron overload associated with hereditary hemochromatosis. In hemochromatosis model Hfe−/− mice, Hfe−/;PKCα−/− mice displayed significantly lower serum iron content and Fpn protein expression. Treatment with PKC412, a PKC inhibitor,8 significantly decreased duodenal Fpn expression and serum and liver iron content in Hfe−/− mice, demonstrating that targeting PKCα could mitigate iron overload in hereditary hemochromatosis.
Taken together, these findings introduce PKCα as a novel regulator of Fpn, with PKCα-dependent upregulation of Fpn being the primary mechanism underlying diabetes-induced iron overload. Importantly, inhibition of PKCα may attenuate Fpn-dependent systemic iron overload (see figure). However, because PKCα regulates a spectrum of proteins and is involved in numerous physiological processes, future work is required to better understand the PKCα-Fpn regulatory axis and identify more specific targets for controlling iron homeostasis.
PKCα is a novel regulator of Fpn in diabetes. Diabetic hyperactivation of PKCα upregulates intestinal Fpn expression in a hepcidin-independent manner. PKCα decreases endocytic trafficking of Fpn and suppresses hepcidin-induced ubiquitination, and degradation of Fpn. Inhibition or genetic disruption PKCα alleviates iron overload. Fpn, ferroportin; PKCα, protein kinase C alpha.
PKCα is a novel regulator of Fpn in diabetes. Diabetic hyperactivation of PKCα upregulates intestinal Fpn expression in a hepcidin-independent manner. PKCα decreases endocytic trafficking of Fpn and suppresses hepcidin-induced ubiquitination, and degradation of Fpn. Inhibition or genetic disruption PKCα alleviates iron overload. Fpn, ferroportin; PKCα, protein kinase C alpha.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal