Visual Abstract
Chronic graft-versus-host disease (cGVHD) is associated with morbidity, mortality, impaired quality of life, prolonged immunosuppressive therapy, and infection risk after allogeneic hematopoietic cell transplantation (HCT). Major strides have occurred in the understanding of cGVHD biology; National Institutes of Health Consensus meetings have refined rigorous approaches to diagnosis, staging, and response criteria; major interventional trials have established standard benchmarks for treatment outcome; and 3 agents to date have been US Food and Drug Administration approved for treating corticosteroid-refractory cGVHD. Promising results from several recent trials have led some, but not others, to conclude that the risk of developing cGVHD is sufficiently low to be considered a major post-HCT complication of the past. We propose that it is time to critically examine the results of contemporary graft-versus-host disease (GVHD) prophylaxis regimens and discuss the state of the science and associated controversies in the spectrum of conclusions reached as to the risk of cGVHD. With these data, the current cGVHD incidence can be most precisely determined, and the present and future burden of cGVHD-affected patients can be accurately modeled. Through review of existing evidence, we highlight unresolved needs and opportunities to refine best GVHD prophylaxis or preemptive therapy approaches and optimize established cGVHD therapy, and make the argument that support of preclinical and clinical research is critical in improving patient outcomes.
Introduction
Diverse opinions exist as to chronic graft-versus-host disease (cGVHD) incidence and severity. Some strongly favor the notion that cGVHD is a relatively infrequent complication of allogeneic hematopoietic cell transplantation (HCT). Conversely, others note that cGVHD rates continue to be too high and deserve to be tackled in preclinical and clinical settings to better understand cGVHD pathogenesis and biology as well as to develop new therapies. To assess the potential importance of cGVHD as a post-HCT complication worthy of expending resources to advance preclinical studies and perform clinical trials, taking stock of the current state of the science of cGVHD incidence is essential. Key considerations include the evolving landscape of cGVHD preventive and therapeutic approaches, predictive, diagnostic, and prognostic biomarkers, possibility of applying personalized cGVHD prevention and therapeutic approaches, and logistics of broadly exploiting these advances, including US average costs of cGVHD of >$300 000/year per patient.1 Here, we review available data to inform such opinions and offer our perspective as to the continued need to aggressively support research in this area. We acknowledge upfront that estimates of cGVHD incidence are influenced by risk factors both known and yet to be identified, graft-versus-host disease (GVHD) prophylaxis regimens, and year of publication that reflects standards of care and therapeutic options available at that time.
cGVHD: the clinical problem and current biologic understanding
cGVHD has been a long-standing barrier to the success of allogeneic HCT and a major contributor to mortality, with overall severity associated with greater risk of death.2 cGVHD features, including organ site involvement and severity, as well as other prognostic factors, influence mortality risk. Affected patients may be cured of their underlying hematologic malignancy or nonmalignant disorder, yet experience significant morbidity and mortality. Impairments in patient-reported quality of life rival those of other serious chronic medical and psychiatric illnesses.3 cGVHD, especially with fibrotic manifestations, such as cutaneous sclerosis, fasciitis, and bronchiolitis obliterans syndrome (BOS), can be disabling and require prolonged immunosuppressive (IS) therapy4: only one-third of subjects are able to successfully stop all IS therapy even 5 years after diagnosis and therapy.5
The National Institutes of Health Chronic GVHD Consensus Development Project has ushered in advances in rigorous approaches to cGVHD biology, pathology, diagnosis and staging, and response assessment. In 2020, the last Consensus Meeting articulated research priority areas in cGVHD prevention, early diagnosis and preemptive therapy, innovative treatment approaches, and therapy for highly morbid forms of cGVHD.6-10 Extensive preclinical work and human correlative studies have informed a greater biologic understanding of cGVHD pathogenesis conceptualized as 3 phases: tissue injury and acute inflammation, chronic inflammation and dysregulated immunity, and aberrant tissue repair and fibrosis.11,12 Major observational studies and interventional trials coordinated through a national Chronic GVHD Consortium have advanced the field, and trials conducted with industry partners have led to the US Food and Drug Administration (FDA) approval of 3 novel treatment agents in corticosteroid-refractory cGVHD.13
Consistent cGVHD incidence under conventional calcineurin inhibitor–based prophylaxis regimens
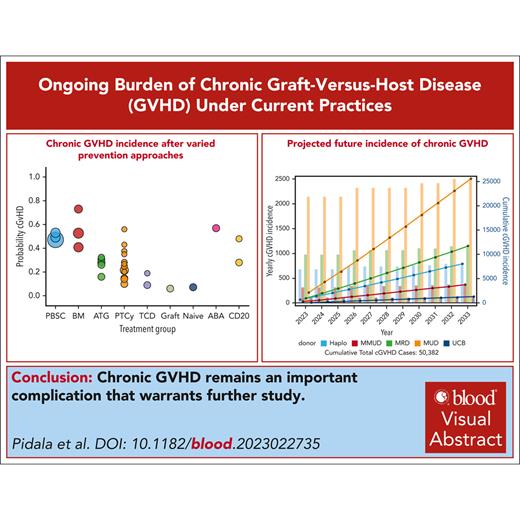
Historical benchmarks have suggested cGVHD incidence may be as high as 60% to 70%. Variation in cGVHD estimates after different prevention approaches are shown in Figure 1, with additional details presented in the supplemental Data (available on the Blood website).14-34 Given the diverse range of reported cGVHD outcomes following these approaches, we present them separately for those who appear to have lower cGVHD incidence, those with comparable cGVHD incidence to historical benchmarks, and those requiring additional evidence. Challenges in interpreting data from the various prophylaxis trials include varied interventions, study type, subject number, follow-up duration, cGVHD classification and reporting, and heterogeneity in patient-level factors.
Range of cGVHD incidence following different initial GVHD prevention strategies. The bubble plot provides summary data on reported cGVHD incidence following varied HCT/GVHD prevention strategies. Each bubble represents an individual study, and size of the bubble reflects number of subjects reported for each. The reported incidence for each trial reflects a 2-year point estimate, although in a few cases, the point estimate is at 1 year, 18 months, or 3 years, depending on what was available from the appropriate publication. ABA, calcineurin inhibitor (CNI)/methotrexate (MTX)/abatacept; ATG, use of anti-thymocyte globulin in GVHD prevention approach; BM, bone marrow graft with CNI/MTX prophylaxis; CD20, depletion of CD20+ B cells; Graft, graft manipulation, exemplified by defined ratio of graft regulatory T cells and conventional T cells; Naïve, naïve T-cell depletion; PBSC, peripheral blood mobilized stem cells with CNI/MTX GVHD prophylaxis; PTCy, posttransplant cyclophosphamide-based prophylaxis; TCD, ex vivo T-cell depletion/CD34+ selection.
Range of cGVHD incidence following different initial GVHD prevention strategies. The bubble plot provides summary data on reported cGVHD incidence following varied HCT/GVHD prevention strategies. Each bubble represents an individual study, and size of the bubble reflects number of subjects reported for each. The reported incidence for each trial reflects a 2-year point estimate, although in a few cases, the point estimate is at 1 year, 18 months, or 3 years, depending on what was available from the appropriate publication. ABA, calcineurin inhibitor (CNI)/methotrexate (MTX)/abatacept; ATG, use of anti-thymocyte globulin in GVHD prevention approach; BM, bone marrow graft with CNI/MTX prophylaxis; CD20, depletion of CD20+ B cells; Graft, graft manipulation, exemplified by defined ratio of graft regulatory T cells and conventional T cells; Naïve, naïve T-cell depletion; PBSC, peripheral blood mobilized stem cells with CNI/MTX GVHD prophylaxis; PTCy, posttransplant cyclophosphamide-based prophylaxis; TCD, ex vivo T-cell depletion/CD34+ selection.
To set the stage for comparisons with new approaches for GVHD prophylaxis, we first will review cGVHD incidence after conventional pharmacologic prophylaxis with unmanipulated mobilized peripheral blood stem cell (PBSC) grafts. In Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0201, the cGVHD incidence was 41% for unrelated donor (URD) bone marrow (BM) and 53% for URD PBSC graft recipients at 2 years after HCT.15 In BMT CTN 0402 for patients receiving sibling donor PBSC grafts, the cGVHD incidence was 45% using tacrolimus (TAC)/methotrexate (MTX) and 53% with TAC/sirolimus (SIR) GVHD prophylaxis.16 In a national Chronic GVHD Consortium longitudinal study of 911 subjects followed up closely from HCT onward (enrolled 2011-2014), the 2-year cumulative incidence of cGVHD was 47% (median onset of 7.4 months).14 Grafts consisted of PBSC grafts (81%) from matched sibling (33%), matched (44%) unrelated, or mismatched (20%) URD (2% were related haploidentical donors [haplo]). Calcineurin inhibitor (CNI)–based prophylaxis consisted of CNI/MTX (43%), CNI/SIR (11%), and CNI/mycophenolate mofetil (MMF) (7%); only 3% received antithymocyte globulin (ATG). In aggregate, these data are consistent with trials published over a decade earlier testing cyclosporine vs TAC (each with methotrexate), demonstrating a cGVHD incidence of 49% to 56% sibling and 70% to 76% for unrelated donor graft recipients and serving as a benchmark for evaluating novel approaches.17,18 In total, these studies indicate cGVHD is a common complication under conventional prophylaxis, and that this risk is clearly increased when using PBSC vs BM. Given predominant PBSC use in the current era, cGVHD reduction has required more potent GVHD prevention approaches, which are outlined in the next sections.
Approaches resulting in a lower cGVHD incidence than CNI-based GVHD prophylaxis historical benchmarks
In contrast to CNI/MTX-based regimens, over the past 25 years, new strategies have emerged that can prevent both acute GVHD and cGVHD.35,36 Published data support that recipients of grafts that were unmanipulated and ATG or posttransplant cyclophosphamide (PTCy) and grafts manipulated ex vivo to positively select CD34-expressing cells or negatively select naïve (CD45RA+) T cells have a reduced cGVHD incidence. We examine the details of several major prospective trials (Table 1) that have had a substantial influence on opinions in the field as to the importance or lack thereof of cGVHD as a continuing, key post-HCT complication. Randomized trials in the sibling and URD setting have clearly established ATG vs no ATG reduces cGVHD incidence (overall, ≈16%-32%; and moderate/severe, ≈6%-13%, both lower than no ATG randomized subjects).20-22,37 Notably, conclusions must be taken in the context of the nature of these trials that focused primarily on adults, myeloablative conditioning (MAC), PBSC grafts from matched related donor or matched unrelated donor (MUD), and CNI/MTX GVHD prophylaxis.
Summary of clinical trials for prevention approaches with low incidence of cGVHD
| Approach . | Trial . | No. . | Age, y . | BM/PBSC, % . | MAC/RIC, % . | MRD/MUD/MMUD/haplo, % . | Other IS therapy . | cGVHD incidence, % . |
|---|---|---|---|---|---|---|---|---|
| ATG | Kroger, 201621 | 83 | 39 (18-64) | 0/100 | 100/0 | 100/0/0/0 | CSA/MTX | 32.2 (2 y) |
| Chang, 202019 | 132 | 48 (40-61) | 4.5/95.5∗ | 100/0 | 100/0/0/0 | CSA/MTX/MMF | 27.9 (2 y) | |
| Finke, 200920 | 103 | 40 (18-60) | 20/80 | 100/0 | 0/80/20/0∗ | CSA/MTX | 30.8 (2 y) | |
| Soiffer, 201722 | 126 | 46 (18-64) | 18/75∗ | 100/0 | 0/100/0/0 | TAC/MTX | 16 (2 y) | |
| Walker, 201623 | 99 | 49 (40-57) | 11/89 | 67/33 | 0/84/16/0 | CNI/MTX (or MMF)∗ | 26 (2 y) | |
| CD34 selection | Devine, 201131 | 44 | 48.5 (21-59) | 0/100 | 100/0 | 100/0/0/0 | None | 19 (2 y) |
| Luznik, 202239 | 114 | 51 (22-66) | 6/94 | 100/0 | 38/62/0/0 | None∗ | 8.9 (2 y) | |
| Naïve T-cell depletion | Bleakley, 202232 | 138 | 37 (1-60) | 0/100 | 100/0∗ | 61/39/0/0 | TAC, TAC/MTX, TAC/MMF∗ | 7 (3 y) |
| PTCy | Bolanos-Meade, 201925 | 92 | 64 (56-72) | 0/100 | 0/100 | 32/54/10/0 | TAC/MMF | 28 (1 y) |
| Bolanos-Meade, 202324 | 214 | 64.2∗ | 0/100 | 0/100 | 28/68/3/0 | TAC/MMF | 21.9 (1 y) | |
| Shaw, 202329 | 80 | 51.5 (18-70) | 100/0 | 50/50 | 0/0/100/0 | SIR/MMF | 20 RIC, 37.5 MAC (3 y) | |
| Luznik, 202239 | 114 | 51 (20-66) | 90/10 | 100/0 | 38/62/0/0 | None | 27 (2 y) | |
| Luznik, 201026 | 117 | 50 (21-66) | 100/0 | 100/0 | 67/33/0/0∗ | None | 10 (2 y) | |
| Kanakry, 201430 | 92 | 49 (21-65) | 100/0 | 100/0 | 49/51/0/0∗ | None | 14 (2 y) | |
| Symons, 202040 | 96 | 42 (1-65) | 100/0 | 100/0 | 0/0/0/100 | TAC/MMF | 15 (1 y) | |
| Solomon, 201541 | 30 | 46 (24-60) | 0/100 | 100/0 | 0/0/0/100 | TAC/MMF | 56 (18 mo) | |
| Sanz, 202042 | 22 | 41 (18-55) | 0/100 | 100/0 | 0/0/0/100 | CSA/MMF | 43 (2 y) | |
| Sugita, 201943 | 50 | 36 (17-60) | 0/100 | 100/0 | 0/0/0/100 | TAC/MMF | 36 (2 y) | |
| Sugita, 201943 | 77 | 58 (22-65) | 0/100 | 0/100 | 0/0/0/100 | TAC/MMF | 27 (2 y) | |
| Al Malki, 202244 | 31 | 37 (21-58) | 0/100 | 0/100 | 0/0/0/100 | TAC/MMF | 35 (2 y) | |
| Fuchs, 202145 | 182 | 60 (20-70) | 100/0 | 0/100 | 0/0/0/100 | TAC/MMF | 26 (2 y) |
| Approach . | Trial . | No. . | Age, y . | BM/PBSC, % . | MAC/RIC, % . | MRD/MUD/MMUD/haplo, % . | Other IS therapy . | cGVHD incidence, % . |
|---|---|---|---|---|---|---|---|---|
| ATG | Kroger, 201621 | 83 | 39 (18-64) | 0/100 | 100/0 | 100/0/0/0 | CSA/MTX | 32.2 (2 y) |
| Chang, 202019 | 132 | 48 (40-61) | 4.5/95.5∗ | 100/0 | 100/0/0/0 | CSA/MTX/MMF | 27.9 (2 y) | |
| Finke, 200920 | 103 | 40 (18-60) | 20/80 | 100/0 | 0/80/20/0∗ | CSA/MTX | 30.8 (2 y) | |
| Soiffer, 201722 | 126 | 46 (18-64) | 18/75∗ | 100/0 | 0/100/0/0 | TAC/MTX | 16 (2 y) | |
| Walker, 201623 | 99 | 49 (40-57) | 11/89 | 67/33 | 0/84/16/0 | CNI/MTX (or MMF)∗ | 26 (2 y) | |
| CD34 selection | Devine, 201131 | 44 | 48.5 (21-59) | 0/100 | 100/0 | 100/0/0/0 | None | 19 (2 y) |
| Luznik, 202239 | 114 | 51 (22-66) | 6/94 | 100/0 | 38/62/0/0 | None∗ | 8.9 (2 y) | |
| Naïve T-cell depletion | Bleakley, 202232 | 138 | 37 (1-60) | 0/100 | 100/0∗ | 61/39/0/0 | TAC, TAC/MTX, TAC/MMF∗ | 7 (3 y) |
| PTCy | Bolanos-Meade, 201925 | 92 | 64 (56-72) | 0/100 | 0/100 | 32/54/10/0 | TAC/MMF | 28 (1 y) |
| Bolanos-Meade, 202324 | 214 | 64.2∗ | 0/100 | 0/100 | 28/68/3/0 | TAC/MMF | 21.9 (1 y) | |
| Shaw, 202329 | 80 | 51.5 (18-70) | 100/0 | 50/50 | 0/0/100/0 | SIR/MMF | 20 RIC, 37.5 MAC (3 y) | |
| Luznik, 202239 | 114 | 51 (20-66) | 90/10 | 100/0 | 38/62/0/0 | None | 27 (2 y) | |
| Luznik, 201026 | 117 | 50 (21-66) | 100/0 | 100/0 | 67/33/0/0∗ | None | 10 (2 y) | |
| Kanakry, 201430 | 92 | 49 (21-65) | 100/0 | 100/0 | 49/51/0/0∗ | None | 14 (2 y) | |
| Symons, 202040 | 96 | 42 (1-65) | 100/0 | 100/0 | 0/0/0/100 | TAC/MMF | 15 (1 y) | |
| Solomon, 201541 | 30 | 46 (24-60) | 0/100 | 100/0 | 0/0/0/100 | TAC/MMF | 56 (18 mo) | |
| Sanz, 202042 | 22 | 41 (18-55) | 0/100 | 100/0 | 0/0/0/100 | CSA/MMF | 43 (2 y) | |
| Sugita, 201943 | 50 | 36 (17-60) | 0/100 | 100/0 | 0/0/0/100 | TAC/MMF | 36 (2 y) | |
| Sugita, 201943 | 77 | 58 (22-65) | 0/100 | 0/100 | 0/0/0/100 | TAC/MMF | 27 (2 y) | |
| Al Malki, 202244 | 31 | 37 (21-58) | 0/100 | 0/100 | 0/0/0/100 | TAC/MMF | 35 (2 y) | |
| Fuchs, 202145 | 182 | 60 (20-70) | 100/0 | 0/100 | 0/0/0/100 | TAC/MMF | 26 (2 y) |
CSA, cyclosporine; MAC, myeloablative conditioning; MRD, matched related donor; MUD, matched unrelated donor; MMUD, mismatched unrelated donor; RIC, reduced-intensity conditioning.
Chang 202019: PBSC included PBSC N = 76 (57.6%) as well as bone marrow transplant (BMT) + PBSC N = 50 (37.9%). Finke 200920: N = 21 (20%) had human leukocyte antigen (HLA)-C mismatch. Soiffer 201722: N = 9 (7%) unknown graft source. Walker 2016: included CNI (either CSA or TAC) combined with MTX or MMF. Luznik 202239: reported approach/IS therapy per randomly assigned therapy, whereas N = 15 in the CD34 selection arm of the trial received TAC/MTX or other as treatment noncompliance, and N = 9 in the PTCy arm received noncompliant therapy of TAC/MTX or other. Bleakley 202232: had all myeloablative regimens, but 72% high intensity and 28% intermediate intensity; the combined report summarized different trials, which varied in the delivered pharmacologic immune suppression (included TAC alone, TAC/MTX, or TAC/MMF) alongside the naïve T-cell depletion approach. Bolanos-Meade25 2023: age reported as mean (64.2 years, ±8.5 years). Luznik 201026: 1 related donor was an HLA-identical parent. Kanakry 201430: 1 patient had a 10/10 HLA matched familial donor (grouped here with the matched sibling donor group).
In the BMT CTN 1301 phase 3 randomized study, 2-year rates of cGVHD, relapse-free survival in MAC recipients of human leukocyte antigen (HLA)–matched (matched related donor/MUD) BM grafts and either PTCy or TAC/MTX or ex vivo CD34-selected PBSCs were comparable. However, CD34 selection was associated with significantly lower moderate-to-severe cGVHD (<10%), between low cGVHD and these other outcome parameters.27 In a large nonrandomized trial,32 naïve T-cell depletion in MAC recipients receiving PBSC grafts demonstrated grade II to IV acute, grade III to IV acute, and 3-year cGVHD incidences of 71%, 4%, and 7%, respectively. GVHD prophylaxis mostly consisted of CNI/MTX (or CNI, or CNI/MMF). At 3 years, overall survival, relapse, and nonrelapse mortality (NRM) were 77%, 23%, and 8%, respectively, resulting in a cGVHD-free, relapse-free survival rate of 68%. A randomized phase 2 trial comparing naïve T-cell depletion with PTCy-based prophylaxis is planned.
Building on original studies from Johns Hopkins,38 PTCy-based prophylaxis has been tested in several major national BMT CTN trials. Although cGVHD incidence using PTCy-based regimens was variable based on graft source and specific PTCy prophylaxis (single agent vs additional CNI/MMF), when taken together, the overall (≈10%-38%) and moderate/severe (≈3%-30% across available publications) cGVHD incidence rates were reduced but not uniformly eliminated.24,25,27,28 Amidst these PTCy-based studies, risk factors for higher cGVHD incidence included both single-agent PTCy vs PTCy/other agent regimens and PBSC vs BM donor grafts.38-44 Studies also show significant differences between CNI/MTX and PTCy in reducing IS treatment.
In the aforementioned BMT CTN 1301, the moderate/severe cGVHD rates seen in patients with CNI-free GVHD prophylaxis of single-agent PTCy was comparable to standard-of-care TAC/MTX (control) at 27% vs 33.7%, respectively.39 In BMT CTN 1203, a 3-arm phase 2 randomized trial, reduced-intensity conditioned recipients of matched related or 7-8/8 URD grafts were randomized to TAC/MMF/PTCy vs TAC/MTX/bortezomib or maraviroc and compared with nonrandomized, contemporaneous TAC/MTX controls. The most promising intervention was TAC/MMF/PTCy,25 which led to a randomized phase 3 trial (BMT CTN 1703).
In the BMT CTN 1703 trial, patients receiving reduced-intensity conditioning or nonmyeloablative conditioning and sibling or 7-8/8 unrelated PBSC grafts were randomized to PTCy/TAC/MMF or TAC/MTX. The composite end point GVHD-free, relapse-free survival was improved in the PTCy/TAC/MMF arm, largely because of reduction in grade III to IV acute GVHD and cGVHD.24 The reported 1-year overall cGVHD incidence for PTCy/TAC/MMF vs TAC/MTX was 22% vs 35%, respectively, and 1-year IS therapy–free survival was 50% vs 40%, respectively. Among those with cGVHD, patients given PTCy/TAC/MMF or TAC/MTX had mild (67% vs 51%, respectively), moderate (26% vs 34%, respectively), or severe (7% vs 15%, respectively) cGVHD. In total, both the overall incidence and severity sufficient for therapy (moderate/severe) were lower but again neither were abolished by PTCy/TAC/MMF.
Altogether, PTCy-based prophylaxis did not eliminate but did reduce cGVHD incidence and severity compared with TAC/MTX-treated controls by 1-year post-HCT. Future studies will be needed to quantify cGVHD incidence and discern the evolution of presenting cGVHD features (including known late-onset phenotypes, such as BOS and cutaneous sclerosis) with longer follow-up. We also cite a series of representative haploidentical HCT prospective trials (Table 1).40-45 These largely recapitulate major themes cited above, namely the increased risk of cGVHD after PBSC (vs BM) and the ongoing presence of cGVHD despite PTCy-based prophylaxis.
Novel approaches with either comparable or insufficient data to date to fully evaluate cGVHD incidence compared with historical benchmarks
In the initial abatacept 2 trial of abatacept cytotoxic T-lymphocyte associated protein 4 (CTLA4-Ig), the only FDA-approved GVHD prophylaxis reagent, demonstrated abatacept/CNI/MTX improved grade III to IV acute GVHD and severe acute GVHD-free survival, yet the 1-year incidence of moderate/severe cGVHD of 45% in the 8/8 MUD cohort and 58% in the 7/8 MUD cohort failed to provide evidence of benefit in cGVHD prevention.34 A subsequent ongoing abatacept-based randomized trial aims to determine whether prolonged abatacept dosing (8 vs 4 total doses) will reduce cGVHD incidence.
Several other approaches designed with the goal of lowering cGVHD incidence and severity have been or are being explored. B-cell depletion through prophylactic rituximab demonstrated a 2-year incidence of any cGVHD and corticosteroid-requiring cGVHD of 48% and 31%, respectively.33 Results of a prospective trial of humanized anti-CD20 monoclonal antibody (mAb), obinutuzumab, for cGVHD prevention have been presented.46 Interim analyses of a single-center nonrandomized phase 2 as well as a randomized phase 2 trial of HLA-matched, ex vivo CD34-selected PBSCs with a defined ratio of regulatory T cells to T conventional cells transplanted into MAC patients have been previously reported to have low cGVHD incidence.47,48 Multicenter trials are underway in MAC patients receiving HLA-8/8 matched or unrelated donor grafts in a phase 3 randomized trial compared with standard of care. Final published results of these regulatory T-cell add-back trials will provide greater clarity to better enable outcome comparisons to the above-cited alternative prevention approaches. Additional considerations will include the higher cost of cell processing in contrast to pharmacologic therapies and requirement for technology access.
Impact of diverse GVHD prevention regimens on the risk of cGVHD and associated organ involvement
Taking into account the broad diversity and extent of the use of each by the HCT community at large as well as varied results, a careful review of available data is critical. Available Center for International Blood and Marrow Transplant Research data from a 2015 analysis suggest cGVHD incidence has actually increased: In an analysis of 26 563 patients transplanted over the 12-year period ending in 2007, incidence increased over each of 3 time intervals, 1995 to 1999, 2000 to 2003, and 2004 to 2007.49 Over that time period, the 1-year cGVHD incidence increased from 28% to 31% to 37%, respectively, as did severity, and no major improvements in survival after cGVHD diagnosis in the later time intervals were observed. Patients were heavily biased toward CNI/MTX prophylaxis, with only ≈20% to 30% treated with ATG or alemtuzumab (CAMPATH)-1 mAb and <5% receiving CD34-selected grafts per time period; <1% received PTCy. Thus, extrapolation of these data to current HCT practices has limitations. A similar study examining more recent time periods is warranted and of great priority for future agendas.
Compared with patients receiving MUD grafts and CNI/MTX or CNI/MMF, 1 major CIBMTR study,50 as well as other single-center analyses suggest differences in organ involvement after PTCy/haplo,51 lower disability, increased return to work, and greater likelihood and durability of stopping IS therapy.52,53 Another recent single-center analysis (N = 120, PTCy based [81% haplo] vs N = 194, CNI/MTX [all URD]) reported PTCy-based prophylaxis resulted in a decreased 1-year cGVHD incidence (24% vs 40%), moderate/severe cGVHD (12% vs 23%), and visceral cGVHD (15% vs 27%) for PTCy-based prophylaxis.54 Long-term results of an ATG randomized trial in URD graft recipients suggest that ATG reduces cGVHD incidence, and is associated with greater likelihood of IS therapy–free survival compared with CNI/MTX without ATG.55 Most ATG randomized trials have only described cGVHD incidence and IS therapy discontinuation, but have not provided description of cGVHD natural history. Similarly, limited analysis of cGVHD features has been done in other GVHD prevention trials with exceptions.53 Thus, there is limited evidence to support that, once established, cGVHD differs according to the preceding GVHD prophylactic regimen, although a few retrospective analyses support the finding that cGVHD after PTCy may differ in severity and organ involvement, largely derived from haplo/PTCy vs MUD/conventional CNI-based prophylaxis without ATG. Additional dedicated studies are needed to fully address this question in the modern era.
The goal of personalized cGVHD prevention
Widespread adoption of certain GVHD prevention strategies may prove to be overly aggressive for some patients, resulting in enhanced risks (serious infections, malignancy relapse). Although some risk enhancements are theoretical (increased relapse risk after PTCy), the predominance of evidence in randomized trials does not lend credence to this risk enhancement,24,39,56 whereas others suggest real threats (worsened survival after CD34 selection in CTN 1301). Although not extensively covered in this perspective, we also note the particular balance of concerns regarding GVHD risk and complications of HCT in the setting of nonmalignant disorders. Baseline risk factors for cGVHD development have been identified and verified in multiple studies. These include factors such as PBSC (vs BM), female donor/male recipient gender combination (vs others), female donor parity, URD (vs related donor), HLA mismatch (vs match), and older donor and patient ages. Known associations exist between conditioning therapy (eg, radiation and sclerotic GVHD, busulfan, and BOS) and cGVHD risk.6 The aforementioned factors may inform individual patient-level cGVHD risk, and thus could be considered in tailoring the initial prevention approach. However, current evidence does not definitively support applying 1 targeted prophylaxis approach vs another according to a certain single or combined series of known cGVHD clinical risk factors to optimize outcome. Although baseline cGVHD risk biomarkers (pre-HCT for prophylaxis, early post-HCT for preemptive therapy) could be considered, none is currently validated and qualified for clinical use.
The 2020 National Institutes of Health Consensus Development Project identified preemptive therapy as a strategy to tailor cGVHD preventive interventions to those with greater individual patient-level risk (subclinical cGVHD with risk assignment biomarkers) or early nondiagnostic cGVHD manifestations that could change cGVHD incidence and outcomes.8 Validated cGVHD risk assignment biomarkers that define patient eligibility, therapeutic strategies that mechanistically target cGVHD pathogenesis in an individual patient, and assessment of the balance of risk/benefit, safety, tolerability, cost, and ease of use across centers are needed. Several blood protein biomarkers (CXCL9, Dickkopf-3, CXCL10, and matrix metalloproteinase-3) measured earlier post-HCT (day 90-100) have been examined in large nationally representative cohorts with mixed results because of a low positive predictive value.57 Most biomarkers are of greater diagnostic than predictive utility.58,59 Results of a preemptive phase 2 double-blinded, randomized therapy trial with the Rho-associated coiled-coil containing protein kinase 2 (ROCK2) inhibitor, belumosudil, are eagerly awaited.
Current state of cGVHD therapy
Being a chronic disease, therapies are typically given over months to years. Each has associated adverse effects, some of which can be debilitating by themselves. The minority of patients with established cGVHD will have long-term complete resolution of disease with prednisone as the primary standard of care. Most reported outcome measurements are based on 1-mg/kg per day dosing as starting point with no standardized taper schedule (although real-world practices indicate ≈55% of patients with cGVHD initially are treated with prednisone alone).60 Per BMT CTN 0801, primary therapy results in a 50% success rate (complete or partial response without death/relapse/secondary therapy) by 6 months.61 Historically, trials have tested whether prednisone together with other systemic IS therapy could improve outcomes, but these trials were negative.62-66 Although combination primary therapy continues to be of interest, and newer trials are in development, studies to date have not shown significant improvements in treatment outcome in randomized clinical trials conducted to date. Under standard therapy, the expected failure-free survival (composite outcome including death, relapse, use of next-line systemic IS therapy) is 54% at 12 months, and 43% at 24 months.67 These data support that initial systemic therapy often fails, second-line therapy (and beyond) is commonly needed, and durable success is uncommon. New treatment approaches are of special interest, and interventions that can treat cGVHD while sparing or avoiding prednisone (and allied adverse effects), as discussed below, are a priority.9
Advances have expanded the range of therapeutic options for patients requiring second-line and subsequent lines of therapy. Three agents now have FDA approval for corticosteroid-refractory cGVHD (ibrutinib, ruxolitinib, and belumosudil). In a single-arm phase 2 trial, ibrutinib, a B-cell signaling inhibitor that had been an FDA-approved reagent to treat selected B-cell malignancies, was studied in 42 subjects with failure of 1 to 3 prior lines of therapy (and enrichment for skin erythema or mouth involvement).68 Best overall response was 67%, 71% of which were sustained response for >20 weeks and confirmed in a subsequent analysis.69 A pediatric study now has been completed, which demonstrated similar results.70 Ruxolitinib, a Janus kinase subtype 1 and 2 inhibitor that had been FDA approved to treat myelofibrosis and polycythemia vera, has been FDA approved as a second-line therapy for corticosteroid-resistant or corticosteroid-refractory cGVHD. Ruxolitinib was compared in a large randomized trial against a combined best alternative therapy group after failure of 1 to 2 prior lines of therapy, including subjects with corticosteroid-refractory and corticosteroid-dependent disease.71 Over 80% of enrolled subjects had received prior corticosteroid only or corticosteroid/CNI therapy. The 6-month overall response rate (ORR), best ORR, and failure-free survival were all significantly improved with ruxolitinib, yet ORR at 6 months was 49.7%. There is also increasing evidence for ruxolitinib efficacy in pediatrics,72 as well as use to treat patients with more advanced cGVHD.73 Belumosudil, a serine/threonine kinase inhibitor of Rho-associated coiled-coil kinase 2, has been studied in 2 phase 2 trials in patients with cGVHD with ORR of 76% for all subjects, consistent across prior cGVHD features and therapies. Subsequent reports have demonstrated a positive impact on patient-reported outcomes,74 and responses among those with lung involvement.75 Belumosudil is FDA approved for patients who have failed at least 2 prior lines of therapy. Other agents, including axatilimab, an mAb that blocks colony-stimulating factor (CSF) 1 receptor signaling by CSF1 (ie, macrophage CSF), are under an advanced stage of investigation.76
Challenges remain in how to apply these and other clinically available therapies to individual patients. Insightful reviews have outlined key considerations,77 although questions remain: The ideal agent to obtain an optimal treatment response for any given patient is unknown, and biomarkers to guide selection are lacking. Although trials have reported organ site responses, it is unclear whether any agent can be applied for organ-specific effects. The ideal sequencing of approved agents is also unknown. Strictly following the FDA indication for the 3 approved agents provides some guidance, yet largely follows the original trial eligibility rather than known best sequencing. Other commonly used, albeit not FDA-approved, treatments have a broad range of reported efficacy and varied inclusion of different cGVHD organ involvement and subgroups, toxicity levels, and logistical burden. Practice is heterogeneous, and most often driven by practical considerations, such as prior treatment history, tolerability, and logistical concerns. Among these concerns overall are cost; notably, previous work has shown differences in cost-effectiveness as measured by cost per treatment response.78 Common to these therapies is the infrequent nature of durable complete responses. Failure-free survival (FFS) in the second-line setting is expected to be 45% by 12 months, and 31% by 24 months based on data published in 2013 (where second-line agents included most commonly MMF, cyclosporine, TAC, SIR, MTX, and extracorporeal photopheresis).79 In the modern era, ruxolitinib appears to show improved FFS (after failure of 1-2 lines of therapy), with 2-year FFS near 60%. For belumosudil (after failure of 2-5 prior lines), FFS was 56% at 12 months, and 44% at 24 months.
Treatment of certain highly morbid cGVHD subtypes (eg, cutaneous sclerosis, BOS) remains especially challenging and points to the importance of retrospective analysis of cGVHD and cGVHD subtypes.10 Relatively few trials have been conducted exclusively in these cGVHD subgroups. For BOS, a multicenter phase 2 trial (36 subjects) has shown fluticasone, azithromycin, and montelukast therapy improved treatment failure (defined as ≥10% decline in forced expiratory volume in first second) over a historical benchmark of 40% (36% treatment failure rate by 6 months) to an observed 6% (95% confidence interval, 1%-19%) failure rate.80 Subsequent analysis suggests that chronic azithromycin exposure among BOS patients was not associated with increased risk for malignancy relapse, in contrast to azithromycin used as primary prophylaxis after HCT.81-83 Ruxolitinib has also been tested in a multicenter phase 2 trial in BOS with a best reported lung-specific ORR of 34% at 19 months median follow-up.84 For cutaneous sclerosis, imatinib, an FDA-approved tyrosine kinase inhibitor used to treat hematologic malignancies (N = 35), and rituximab, an FDA-approved anti-CD20 mAb (N = 37), were compared in a randomized phase 2 trial with similar results (26% and 27% of subjects, respectively, achieved significant clinical response by 6 months).85 Other trials have demonstrated activity of extracorporeal photopheresis in cutaneous cGVHD,86 and ruxolitinib in cutaneous sclerosis (47 subjects, mean of 3 prior lines of therapy, 49% partial response rate by 6 months).87
Overall, these data demonstrate that durable cure of cGVHD is uncommon whether in first-line or the more advanced therapy context. The therapeutic options have grown, yet treatment in most patients will fail, and require numerous lines of therapy. As a sobering overall success measure, after a median of 5.6 years after cGVHD, only 32% of subjects have successfully stopped all IS therapy.4 Clearly, for those patients who develop cGVHD, further efforts to discover and translate new therapies that can rapidly and successful induce complete responses are desperately needed.
Overall public health impact of cGVHD
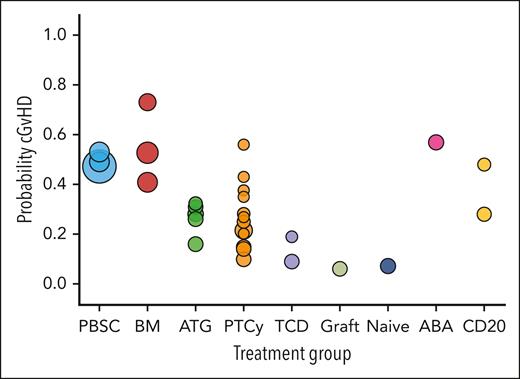
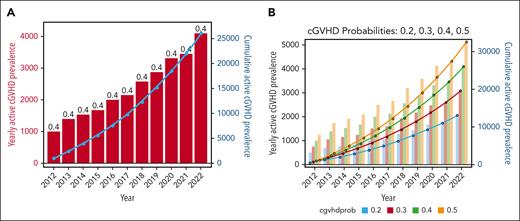
There is relatively little published evidence surrounding the current burden of cGVHD-affected patients. A previous study suggested a prevalence estimate of 14 017 people modeled in 2016.88 Considering HCT volumes (CIBMTR summary data), cGVHD incidence,49 and best assumptions for need for systemic therapy and death (supplemental Data and Methods),4 we estimated a current active cGVHD prevalence of ≈25 000 to 30 000 patients (Figure 2; supplemental Data and Methods). Using best estimates for annual HCT volumes (CIBMTR data), proportions of different HCT approaches (CIBMTR), and expected cGVHD incidence under each,15,16,19,20,24,29,34,45,50,89,90 we estimated an additional ≈50 000 people in the United States would develop cGVHD through the next 10 years (Figure 3; supplemental Data and Methods). Variations in assumptions for each are presented. Overall, these data support that a substantial number of patients are currently living with significant cGVHD, and that even major changes in HCT approaches will not eliminate a large number of future incident cases of cGVHD. However, these modeled outcomes will clearly need to be tracked and substantiated or adjusted as needed through future observational research.
Estimated current prevalence of active cGVHD. Estimated current prevalence of active cGVHD considering estimates through the 2012 to 2022 time frame (A) and variation in projections when considering different cGVHD risk (B). Red bars in panel A represent estimated yearly prevalence assuming an underlying overall cGVHD rate of 40%, with increasing percentages of being on immunosuppression by year (ranging from 40% in 2012 up to 90% in 2022) and increasing percentages of being alive by year (ranging from 70% in 2012 up to 95% in 2022). The left-hand y-axis applies to the yearly prevalence estimates. The blue line shows the cumulative active prevalence estimates, with the values at each year representing the sum of all years up to the listed year. The right-hand y-axis applies to the cumulative prevalence estimates. Panel B shows the same as panel A, but the underlying rate of cGVHD varies, with each bar within each year representing a particular assumed true prevalence, ranging from 20% to 50%. Modeling assumptions and variation explored are listed in the supplemental Methods. cgvhdprob, assumed cGVHD probability after transplant, varied through range of 20% to 50%.
Estimated current prevalence of active cGVHD. Estimated current prevalence of active cGVHD considering estimates through the 2012 to 2022 time frame (A) and variation in projections when considering different cGVHD risk (B). Red bars in panel A represent estimated yearly prevalence assuming an underlying overall cGVHD rate of 40%, with increasing percentages of being on immunosuppression by year (ranging from 40% in 2012 up to 90% in 2022) and increasing percentages of being alive by year (ranging from 70% in 2012 up to 95% in 2022). The left-hand y-axis applies to the yearly prevalence estimates. The blue line shows the cumulative active prevalence estimates, with the values at each year representing the sum of all years up to the listed year. The right-hand y-axis applies to the cumulative prevalence estimates. Panel B shows the same as panel A, but the underlying rate of cGVHD varies, with each bar within each year representing a particular assumed true prevalence, ranging from 20% to 50%. Modeling assumptions and variation explored are listed in the supplemental Methods. cgvhdprob, assumed cGVHD probability after transplant, varied through range of 20% to 50%.
Future incidence of chronic GVHD. Future incidence of cGVHD under base-case assumption considering most current available estimates (A), increased PTCy-based prophylaxis use (at 35% of matched related donor (MRD), 50% MUD, and 75% MMUD) (B), increased proportion of haploidentical donor (40% of HCT) with PTCy use (C), and increase in both haplo-HCT (40% of HCT) and marked PTCy use across other approaches (PTCy as 75% of MRD, MUD, and MMUD cases) (D). Bars in each figure represent the predicted yearly incidence of cGVHD under the appropriate assumptions, with the left-hand axis applying to these predictions; the lines in each figure represent the cumulative predicted incidence, with the values at each year representing the sum of all years up to the listed year. Modeling assumptions and variation explored are listed in the supplemental Methods. The projected number of cGVHD cases per donor type includes the following: (A) MRD, 11 688; MUD, 25 602; MMUD, 3760; haplo, 8074; UCB, 1258. (B) MRD, 10 790; MUD, 21 116; MMUD, 3627; haplo, 8074; UCB, 1258. (C) MRD, 11 688; MUD, 18 620; MMUD, 2686; haplo, 14 043; UCB, 315. (D) MRD, 8078; MUD, 12 950; MMUD, 2642; haplo, 14 043; UCB, 315. MMUD, mismatched unrelated donor; UCB, umbilical cord blood.
Future incidence of chronic GVHD. Future incidence of cGVHD under base-case assumption considering most current available estimates (A), increased PTCy-based prophylaxis use (at 35% of matched related donor (MRD), 50% MUD, and 75% MMUD) (B), increased proportion of haploidentical donor (40% of HCT) with PTCy use (C), and increase in both haplo-HCT (40% of HCT) and marked PTCy use across other approaches (PTCy as 75% of MRD, MUD, and MMUD cases) (D). Bars in each figure represent the predicted yearly incidence of cGVHD under the appropriate assumptions, with the left-hand axis applying to these predictions; the lines in each figure represent the cumulative predicted incidence, with the values at each year representing the sum of all years up to the listed year. Modeling assumptions and variation explored are listed in the supplemental Methods. The projected number of cGVHD cases per donor type includes the following: (A) MRD, 11 688; MUD, 25 602; MMUD, 3760; haplo, 8074; UCB, 1258. (B) MRD, 10 790; MUD, 21 116; MMUD, 3627; haplo, 8074; UCB, 1258. (C) MRD, 11 688; MUD, 18 620; MMUD, 2686; haplo, 14 043; UCB, 315. (D) MRD, 8078; MUD, 12 950; MMUD, 2642; haplo, 14 043; UCB, 315. MMUD, mismatched unrelated donor; UCB, umbilical cord blood.
Future directions
Despite strides outlined above, substantial additional progress is urgently needed in cGVHD research and clinical care. Needs include the following: (1) investments and buy-in by funding agencies, industry, and academia in cGVHD biology, prevention and therapeutic trials, and combinatorial and novel agent testing; (2) new preclinical murine models designed to better elucidate inciting factors and pathobiology of cGVHD that can better simulate clinical GVHD manifestations, including biology of varied organ manifestations; (3) testing of new therapies in distinct preclinical models that differ in their immunobiology and pathobiology; (4) correlative human preclinical and clinical studies in untreated and treated patients with cGVHD that may ultimately result in personalized therapies; (5) systematic and current investigation into how various GVHD prevention approaches affected overall cGVHD incidence, features, and outcomes following cGVHD onset and IS therapy withdrawal; (6) well-designed comparative trials to prospectively evaluate and compare modern GVHD prevention approaches; (7) novel trials testing risk-adapted prophylaxis approaches or preemptive therapy to optimize risk/benefit of interventions focused on preventing overall incidence of as well as morbidity and mortality associated with cGVHD; (8) innovative therapeutic trials (primary or secondary/advanced cGVHD treatment) with attention to trial comprehensive primary and secondary outcomes reflecting durable benefit and minimization of treatment complications, as well as efforts to improve the complete response rate, ORR, and durability of cGVHD therapies; (9) novel efforts focused on highly morbid cGVHD; (10) new strategies to optimize the quality of life and function of patients affected by cGVHD and emphasis on patient-reported outcomes in clinical trials and observational studies; (11) overcoming logistics that may preclude adoption into real-world practice, such as cost and cost-effectiveness of cGVHD prevention, acquisition and interpretation of biomarker data, access to required technology, and expertise and knowledge for application to a given patient population; and (12) dissemination of impactful and practice-changing results to enhance patients’ and providers’ awareness of current best practices.
Concluding perspective
Although progress in cGVHD prevention and therapy has been realized through the efforts of the scientific community in partnership with industry and funding agencies, there is compelling evidence based on incidence and prevalence data that cGVHD is not a complication of the past. Instead, cGVHD continues to be a significant cause of morbidity and mortality after allogeneic HCT that has limited the success and more widespread application of allogeneic HCT. Furthermore, there are strong indications that cGVHD will not soon disappear. From the vantage point of the authors, the overall current and projected future burden of cGVHD under current practices and the importance of these unresolved questions demand ongoing investigation to improve patient outcomes.
Acknowledgments
The authors thank Gerard Socie for helpful comments.
This work was supported in part by grants from National Institutes of Health (NIH), National Cancer Institute grants P01CA15396 and CA065493, NIH, National Heart, Lung, and Blood Institute grants P01HL158805, R01 HL118709, and HL155114, and NIH, National Institute of Allergy and Infectious Diseases grants P01AI056299 and R37AI34495.
Authorship
Contribution: J.A.P., L.L., and B.R.B. drafted the manuscript; T.A.G. designed and prepared the figures; and all authors conceptualized, critically revised, contributed substantially to the manuscript, and approved the manuscript.
Conflict-of-interest disclosure: J.A.P. reports consulting and advisory board membership for Syndax, CTI Biopharma, Amgen, Regeneron, and Incyte; and clinical trial support from Novartis, Amgen, Takeda, Janssen, Johnson and Johnson, Pharmacyclics, CTI Biopharma, and Bristol Myers Squibb. L.L. holds a patent with WindMiL therapeutics; receives grant/research/clinical trial support from Genentech; and is a consultant/advisory board member for Gilead Sciences, Rubius Therapeutics, Precision Biosciences, and Talaris Therapeutics. B.R.B. reports research funding from BlueRock Therapeutics and Carisma Therapeutics; and consulting fees from BlueRock Therapeutics, Editas Medicine, Janssen Oncology, Sandoz, Legend Biotech, GentiBio Inc, and Magenta Therapeutics. T.A.G. declares no competing financial interests.
Correspondence: Bruce R. Blazar, Division of Blood and Marrow Transplant and Cellular Therapy, Department of Pediatrics, University of Minnesota, Minneapolis, MN 55455; email: blaza001@umn.edu.
References
Author notes
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal