In this issue of Blood, Ducamp and colleagues1 provide important insights into the pathophysiology of erythroid heme synthesis disorders by establishing viable murine models of X-linked sideroblastic anemia (XLSA) and X-linked protoporphyria (XLPP), caused by loss-of-function and gain-of-function mutations, respectively, in the 5-aminolevulinic acid synthetase 2 (ALAS2) gene, which encodes the first enzyme in heme biosynthesis in erythroid cells.
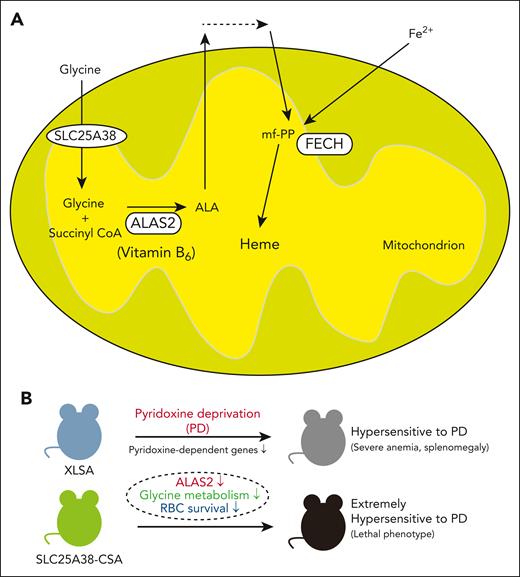
Heme is a prosthetic group that includes ferrous iron and protoporphyrin IX, which are essential for various biological processes such as oxygen transport, storage, and electron transfer. The mammalian heme biosynthetic pathway includes 8 enzymes; 5-aminolevulinate synthase (ALAS) catalyzes the first and rate-limiting step of this pathway, which converts glycine and succinyl-coenzyme A to 5-aminolevulinic acid (ALA) (see figure panel A). This process requires pyridoxal 5′-phosphate, the active form of pyridoxine, as a cofactor. There are 2 ALAS isozymes: ALAS1, which is encoded by a housekeeping gene located on chromosome 3p21.1, and ALAS2, which is encoded by an erythroid specific gene located on Xp11.21.2
Role of pyridoxine (vitamin B6) in erythroid cells. (A) A schematic representation of the heme biosynthetic pathway in erythroid cells. (B) Effect of pyridoxine deprivation on the phenotype of XLSA and SLC25A38-CSA models. FECH, ferrochelatase; mf-PP, metal-free protoporphyrin IX; PD; pyridoxine deprivation; RBC, red blood cell.
Role of pyridoxine (vitamin B6) in erythroid cells. (A) A schematic representation of the heme biosynthetic pathway in erythroid cells. (B) Effect of pyridoxine deprivation on the phenotype of XLSA and SLC25A38-CSA models. FECH, ferrochelatase; mf-PP, metal-free protoporphyrin IX; PD; pyridoxine deprivation; RBC, red blood cell.
XLSA and XLPP are diseases caused by ALAS2 mutations. XLSA is caused by loss of function of ALAS2, which is typically observed in males and presents with hypochromic, microcytic anemia, systemic iron overload, and ring sideroblasts (RSs) in the bone marrow (BM).2 In approximately two-thirds of patients with XLSA, anemia is partially or completely corrected by pyridoxine.3 On the other hand, gain-of-function mutations of ALAS2 cause XLPP, which is mainly characterized by cutaneous photosensitivity and liver failure. This condition resembles erythropoietic protoporphyria, which is caused by loss-of function mutations in ferrochelatase, the enzyme responsible for the final step of heme biosynthetic pathway that inserts ferrous iron into metal-free protoporphyrin IX (see figure panel A).2 Additionally, because of the high demand for glycine in ALA synthesis, autosomal recessive loss-of-function mutations in solute carrier family 25 member 38 (SLC25A38), the mitochondrial glycine importer (see figure panel A), cause congenital sideroblastic anemia (SLC25A38-CSA), which is similar to but more severe than XLSA. In most cases, SLC25A38-CSA cases do not respond to pyridoxine.3
Several attempts have been made previously to establish viable animal disease models of CSA. Murine models of XLSA, which involve disrupting the intronic enhancer region of ALAS2, were found to be embryonically lethal.4,5 In the current study, a series of viable ALAS2 knockin mice with either XLSA (including common mutation sites p.R170H, p.R411H, p.R452H) or XLPP (p.Q548X) were established via CRISPR-Cas9 gene editing. Furthermore, a conditional SLC25A38 null allele (Slc25a38fl) was derived.
When fed conventional rodent chow supplemented with 8.3 ppm of pyridoxine, phenotypical analyses revealed that male animals with XLSA exhibited microcytic, hypochromic anemia of various degrees, which depended on the mutation pattern. Animals with R411H presented with moderate anemia, and animals with R170H exhibited an almost normal phenotype. Although peripheral siderocytes were observed, there was no evidence of RSs in the BM. Conversely, mice with pan-hematopoietic deletion of Slc25a38 with VAV1-Cre exhibited moderately severe hypochromic, microcytic anemia with numerous peripheral siderocytes, splenomegaly, and systemic iron overload. Next, the effect of pyridoxine supplementation (10, 100, and 300 ppm) in these models was evaluated. Results showed that not only animals with XLSA but also animals with SLC25A38-CSA were responsive to pyridoxine.
Intriguingly, under pyridoxine restriction (0, 2 ppm), animals with XLSA, particularly those with R411H and R452H, exhibited reduced body weight, severe anemia, and splenomegaly only under absolute pyridoxine restriction (0 ppm). In contrast, SLC25A38-CSA animals experienced rapid anemia progression, resulting in a lethality under pyridoxine restriction. Animals with XLPP were relatively resistant to pyridoxine deficiency compared with animals with XLSA and SLC25A38-CSA.
The novel finding of the current study is the identification of severe and extreme hypersensitivity to pyridoxine deprivation, in mice models with XLSA and SLC25A38-CSA, respectively (see figure panel B). Furthermore, the potential efficacy of pyridoxine supplementation in SLC25A38-CSA animals is noteworthy. Pyridoxine is not only a cofactor of ALAS2 but a cofactor for multiple enzymes relevant to heme synthesis and other pathways important for erythroid proliferation and survival. For example, serine hydroxy methyltransferase converts serine to glycine in the mitochondria, which involves folate metabolism. These findings could explain the efficacy of folate and glycine supplementation in ameliorating zebra fish models of SLC25A38 deficiency.6 However, this treatment was reported to not have a clinical benefit in patients with SLC25A38-CSA.7 Therefore, further investigations should be pursued to better understand this discrepancy.
CSA murine models have provided significant insights into their human counterparts. However, the adult mice models did not have RS in the BM, which is a hallmark of the disease.2,3 The lack of systemic iron overload in these models may explain the absence of RS. Nevertheless, RS has been observed in human ex vivo XLSA models after the addition of exogenous iron in culture media, which mimics the iron overload state typically observed in patients with XLSA.5,8 Further, ferroptosis, a form of regulated cell death that is dependent on iron accumulation and lipid peroxidation, could account for the anemic phenotype in human ex vivo XLSA models and zebra fish models of ALAS2 deficiency.8,9 Patients with XLSA exhibited increased levels of erythroferrone, which is presumably a result of ineffective erythropoiesis.10 This increase in erythroferrone suppresses the production of hepcidin, a key regulator of iron homeostasis in the body, leading to systemic iron overload. Thus, investigating the erythroferrone-hepcidin axis in these CSA murine models could provide essential data.
In conclusion, pyridoxin is not just a cofactor of ALAS2. Its role in regulating erythroid metabolism, which can be used for the development of novel therapeutic approaches for XLSA and SLC25A38-CSA, should be validated.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal