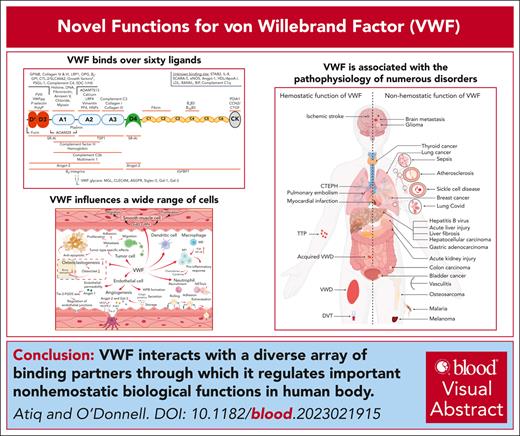
Visual Abstract
For many years, it has been known that von Willebrand factor (VWF) interacts with factor VIII, collagen, and platelets. In addition, the key roles played by VWF in regulating normal hemostasis have been well defined. However, accumulating recent evidence has shown that VWF can interact with a diverse array of other novel ligands. To date, over 60 different binding partners have been described, with interactions mapped to specific VWF domains in some cases. Although the biological significance of these VWF-binding interactions has not been fully elucidated, recent studies have identified some of these novel ligands as regulators of various aspects of VWF biology, including biosynthesis, proteolysis, and clearance. Conversely, VWF binding has been shown to directly affect the functional properties for some of its ligands. In keeping with those observations, exciting new roles for VWF in regulating a series of nonhemostatic biological functions have also emerged. These include inflammation, wound healing, angiogenesis, and bone metabolism. Finally, recent evidence supports the hypothesis that the nonhemostatic functions of VWF directly contribute to pathogenic mechanisms in a variety of diverse diseases including sepsis, malaria, sickle cell disease, and liver disease. In this manuscript, we review the accumulating data regarding novel ligand interactions for VWF and critically assess how these interactions may affect cellular biology. In addition, we consider the evidence that nonhemostatic VWF functions may contribute to the pathogenesis of human diseases beyond thrombosis and bleeding.
Introduction
von Willebrand factor (VWF) is a large multimeric plasma glycoprotein that plays key roles in normal hemostasis by binding to exposed collagen at sites of vascular injury and recruiting platelets to the site of injury.1 In addition, VWF also acts a carrier molecule for factor VIII (FVIII). Under normal conditions, ∼95% of plasma FVIII circulates in high affinity complex (dissociation constant [Kd], ∼0.2-0.5 nmol/L) with VWF.2 Quantitative or qualitative reductions in plasma VWF levels result in von Willebrand disease (VWD), which constitutes the commonest inherited bleeding disorder.3 Conversely, elevated plasma levels of the VWF-FVIII complex represent a dose-dependent risk factor for thrombosis.4,5 Recent studies have highlighted that VWF is able to interact with a diverse array of other proteins and reported novel biological functions extending beyond coagulation. In this article we review these data, together with accumulating evidence that nonhemostatic VWF functions may contribute to the pathogenesis of human diseases beyond thrombosis and bleeding.
Ligands that influence VWF life cycle, including biosynthesis, proteolysis, and clearance
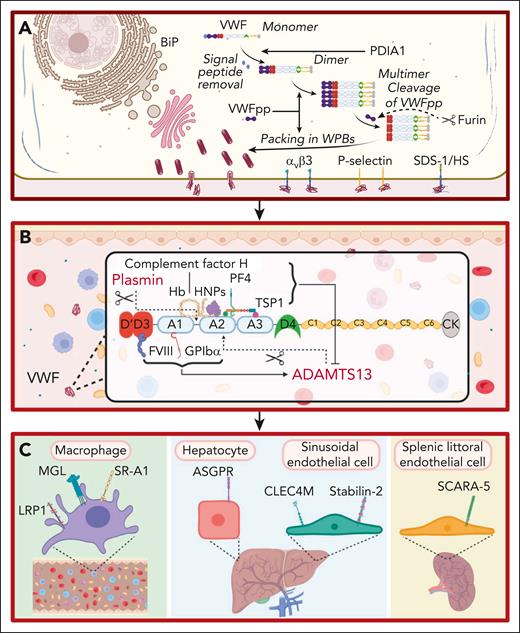
For many years, VWF was perceived to bind a limited number of ligands important in regulating its hemostatic efficacy. These included the following: (1) a FVIII binding site in the D’D3 region; (2) binding sites for collagen in the A1 and A3 domains; and (3) binding sites for platelet receptors glycoprotein Ibα (GPIbα) and αIIbβ3 located in the A1 domain and C4 domain, respectively.6-10 However, accumulating data have shown that additional binding partners play direct roles in the life cycle of VWF (Figure 1). For example, during posttranslational modification within endothelial cells (ECs), VWF interacts with a series of glycosyltransferases and glycosidases in the ER and Golgi, as well as chaperone binding proteins (eg, binding-immunoglobulin protein) and the protein disulfide isomerase A1 (Figure 1A).11 Importantly, the VWF propeptide (VWFpp) binds to the N-terminal D’D3 domains and regulates VWF multimerization and trafficking into Weibel-Palade bodies (WPBs; Figure 1A).12 VWFpp is cleaved from mature VWF by furin and stored within WPB (Figure 1A). After EC activation, VWFpp is secreted in equimolar amounts to VWF but has a much shorter plasma half-life (∼2-3 hours). Although the VWFpp has no defined extracellular function, some VWFpp dimers associate with the D’D3 domain of mature VWF in a noncovalent manner.13 Finally, after WPB exocytosis, elongated VWF strings can be tethered on the surface of activated ECs by a number of reported ligands including the integrin alpha-v beta-3 integrin (αvβ3), P-selectin, and syndecan-1-linked heparan sulfate (Figure 1A).14-16
Ligands involved in the life cycle of VWF. (A) Ligands involved in VWF biosynthesis and secretion. VWF trafficking through the endoplasmic reticulum is regulated by interaction with ligands including binding-immunoglobulin protein (BiP). VWFpp plays key roles in regulating VWF multimerization and packing in WPB. Furin cleaves VWFpp from mature VWF. VWF strings can be tethered on the surface of activated ECs by ligands including integrin αvβ3, P-selectin, and syndecan-1 (SDC-1)–linked heparan sulfate (HS). This figure includes many but not all ligands involved in VWF biosynthesis and secretion. (B) Ligands involved in VWF proteolysis. ADAMTS13 and plasmin independently proteolyze VWF. FVIII and GPIbα binding to VWF promote ADAMTS13-mediated proteolysis. Conversely, binding of PF4, complement factor H, hemoglobin, human neutrophile peptides, and thrombospondin 1 (TSP1) to VWF all attenuate VWF proteolysis by ADAMTS13. This figure includes many but not all ligands involved in VWF proteolysis. (C) Ligands involved in regulating VWF clearance. Macrophage receptors implicated in regulating VWF clearance include the low-density lipoprotein receptor–related protein 1 (LRP1), macrophage galactose lectin (MGL), and scavenger receptor class A member I (SR-A1). In addition, the asialoglycoprotein receptor (ASGPR) on hepatocytes, and C-type lectin domain family 4 member M (CLEC4M) and stabilin-2 (STAB2) on sinusoidal ECs also contribute to VWF clearance. Finally, scavenger receptor class A member 5 (SCARA5) on splenic littoral ECs has also been reported to interact with VWF.
Ligands involved in the life cycle of VWF. (A) Ligands involved in VWF biosynthesis and secretion. VWF trafficking through the endoplasmic reticulum is regulated by interaction with ligands including binding-immunoglobulin protein (BiP). VWFpp plays key roles in regulating VWF multimerization and packing in WPB. Furin cleaves VWFpp from mature VWF. VWF strings can be tethered on the surface of activated ECs by ligands including integrin αvβ3, P-selectin, and syndecan-1 (SDC-1)–linked heparan sulfate (HS). This figure includes many but not all ligands involved in VWF biosynthesis and secretion. (B) Ligands involved in VWF proteolysis. ADAMTS13 and plasmin independently proteolyze VWF. FVIII and GPIbα binding to VWF promote ADAMTS13-mediated proteolysis. Conversely, binding of PF4, complement factor H, hemoglobin, human neutrophile peptides, and thrombospondin 1 (TSP1) to VWF all attenuate VWF proteolysis by ADAMTS13. This figure includes many but not all ligands involved in VWF proteolysis. (C) Ligands involved in regulating VWF clearance. Macrophage receptors implicated in regulating VWF clearance include the low-density lipoprotein receptor–related protein 1 (LRP1), macrophage galactose lectin (MGL), and scavenger receptor class A member I (SR-A1). In addition, the asialoglycoprotein receptor (ASGPR) on hepatocytes, and C-type lectin domain family 4 member M (CLEC4M) and stabilin-2 (STAB2) on sinusoidal ECs also contribute to VWF clearance. Finally, scavenger receptor class A member 5 (SCARA5) on splenic littoral ECs has also been reported to interact with VWF.
After secretion from EC, VWF interacts with ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin type-1 repeats), which cleaves at Tyr1605-Met1606 within the A2 domain (Figure 1B).17 Recent crystal studies have provided insights into the mechanisms through which ADAMTS13 exosites interact with VWF to enable specific A2 domain cleavage in a shear-dependent manner.17 Several other VWF-binding ligands influence susceptibility to ADAMTS13 proteolysis (Figure 1B). FVIII binding to the D’D3 region and GPIbα binding to the A1 domain significantly enhance VWF A2 domain cleavage by ADAMTS13.17 Conversely, binding of platelet factor 4, human neutrophile peptide, complement factor H, hemoglobin, and thrombospondin 1 all attenuate VWF proteolysis by ADAMTS13 (Figure 1B).18-22 Finally, other VWF-binding ligands have been implicated in reducing multimers, including plasmin, which cleaves in the A1-A2 linker region (Figure 1B).23
Recent studies have identified cell surface receptors that bind to plasma VWF and regulate its clearance (Figure 1C). C-type lectin receptors shown to bind VWF glycans include asialoglycoprotein receptor expressed predominantly on hepatocytes, macrophage galactose lectin, and C-type lectin domain family 4 member M, which is expressed on liver sinusoidal ECs (Figure 1C).24-26 Scavenger receptors have also been reported to interact with VWF and contribute to its clearance. These include the low-density lipoprotein receptor–related protein 1, scavenger receptor class A member 1, stabilin-2, and scavenger receptor class A member 5 (Figure 1C).27-30 The specific VWF domains involved in modulating interaction with individual clearance receptors have not been fully defined (Figure 2). In addition, in contrast to the ability of VWF-binding partners to modulate susceptibility to ADAMTS13 proteolysis, it remains unclear whether any ligand binding interactions affect VWF clearance in vivo.
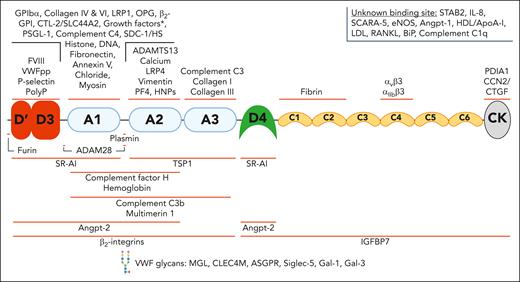
A broad spectrum of ligands interacts with VWF through binding sites across all its domains. VWF has been reported to interact with a wide variety of structurally diverse binding partners. The binding sites for some of these ligands has been localized to specific domains. This figure includes many but not all the reported VWF-ligands. The asterisk (∗) indicates growth factor interactions: VEGF-A165, PIGF-2, PDGF-AA/BB/CC/DD, FGF-2/7/18, TGF-β1, BMP-2, NGF-β, NT-3, and CXCL-12γ. ADAM28, A disintegrin and metalloproteinase 28; ApoA-I, apolipoprotein A-I; ASGPR, asialoglycoprotein receptor; β2-GPI, β2 glycoprotein I; BMP-2, bone morphogenetic protein 2; CCN2/CTGF, connective tissue growth factor; CTL-2, choline transporter-like protein 2; CXCL-12γ, C-X-C motif chemokine ligand 12 gamma; eNOS, endothelial nitric oxide synthase; HDL, high-density lipoprotein; HNPs, human neutrophil peptides; IGFBP7, insulin-like growth factor–binding protein 7; IL-8, interleukin-8; LDL, low-density lipoprotein; MGL, macrophage galactose lectin; NGF, nerve growth factor; NT-3, neurotrophin-3; PDGF, platelet-derived growth factor; PDIA1, protein disulfide isomerase A1; PF4, platelet factor 4; PIGF-2, placental growth factor-2; PolyP, polyphosphate; PSGL-1, P-selectin glycoprotein ligand-1; RANKL, RANK ligand; Siglec-5, sialic acid–binding immunoglobulin-like lectin 5; Slc44a2, solute carrier family 44 member 2; TGF, transforming growth factor.
A broad spectrum of ligands interacts with VWF through binding sites across all its domains. VWF has been reported to interact with a wide variety of structurally diverse binding partners. The binding sites for some of these ligands has been localized to specific domains. This figure includes many but not all the reported VWF-ligands. The asterisk (∗) indicates growth factor interactions: VEGF-A165, PIGF-2, PDGF-AA/BB/CC/DD, FGF-2/7/18, TGF-β1, BMP-2, NGF-β, NT-3, and CXCL-12γ. ADAM28, A disintegrin and metalloproteinase 28; ApoA-I, apolipoprotein A-I; ASGPR, asialoglycoprotein receptor; β2-GPI, β2 glycoprotein I; BMP-2, bone morphogenetic protein 2; CCN2/CTGF, connective tissue growth factor; CTL-2, choline transporter-like protein 2; CXCL-12γ, C-X-C motif chemokine ligand 12 gamma; eNOS, endothelial nitric oxide synthase; HDL, high-density lipoprotein; HNPs, human neutrophil peptides; IGFBP7, insulin-like growth factor–binding protein 7; IL-8, interleukin-8; LDL, low-density lipoprotein; MGL, macrophage galactose lectin; NGF, nerve growth factor; NT-3, neurotrophin-3; PDGF, platelet-derived growth factor; PDIA1, protein disulfide isomerase A1; PF4, platelet factor 4; PIGF-2, placental growth factor-2; PolyP, polyphosphate; PSGL-1, P-selectin glycoprotein ligand-1; RANKL, RANK ligand; Siglec-5, sialic acid–binding immunoglobulin-like lectin 5; Slc44a2, solute carrier family 44 member 2; TGF, transforming growth factor.
Novel ligands for VWF
Recent studies have described a diverse array of novel binding partners for VWF (Figure 2). Some of these ligands have been shown to bind during VWF biosynthesis within ECs. For example, the VWF A1 domain has been shown to mediate binding to angiopoietin-2 (Angpt-2) and osteoprotegerin (OPG).31-33 Both Angpt-2 and OPG are cotrafficked with VWF into WPB stores. P-selectin and FVIII both bind to the D’D3 domains of VWF and are also recruited into WPBs.6,34 More recently, it has been elucidated that complement factor H, insulin-like growth factor–binding protein 7, and interleukin-8 can also bind VWF and be recruited into WPBs.21,35,36 Although Angpt-2 and OPG first bind to VWF during its biosynthesis within ECs, these interactions remain intact after VWF secretion into the plasma. Mobayen et al recently reported that ∼70% of plasma Angpt-2 is circulating in high affinity complex with VWF.33 Importantly with respect to function, Angpt-2 binding to the VWF A1 domain did not alter platelet capture function.33 Similarly, OPG has been shown to remain attached to VWF strings after WPB exocytosis31 and circulate in complex with VWF in normal human plasma.37 In contrast to Angpt-2, OPG binding to the VWF A1 domain significantly attenuated platelet adhesion to VWF strings tethered on the surface of activated ECs.38
VWF is heavily glycosylated with 12 N-linked (NLG) and 10 O-linked glycans (OLGs) on each monomer.39 Previous studies have demonstrated that the NLGs of VWF interact with galectin-1 (Gal-1) and Gal-3 (Figure 2).40 VWF colocalized with galectins in ECs and remained attached after WPB exocytosis.40 Similar to OPG, VWF-platelet string formation was reduced in the presence of galectin binding. Furthermore, thrombus formation in a ferric chloride–induced injury model was significantly more rapid in Gal-1/Gal-3 double-deficient mice.40
Besides the ligands that engage with VWF within ECs, other ligands encounter VWF for the first time after it has been secreted into the plasma or extravascular vessel wall. An array of binding partners has been reported, including fibronectin, myosin, complement factors, histones, poly P, vimentin, thrombospondin 1, and multiple growth factors (Figure 2). It is important to emphasize that the strength of the biochemical evidence supporting the interaction of VWF with these individual binding partners varies considerably (supplemental Table 1, available on the Blood website). For some ligands, specific VWF domains have been implicated in binding (supplemental Table 1). Other ligands, notably putative clearance receptors, have been shown to interact with multiple discrete VWF domains (Figure 2). To date, the relative importance of these different VWF-binding sites in regulating clearance has not been elucidated. Nevertheless, specific VWF missense variants have been shown to promote enhanced clearance in vivo. Some VWF-binding partners have been investigated using both in vivo and in vitro studies (supplemental Table 1). Conversely, other partner interactions have only been assessed using in vitro studies, so it remains unclear whether these ligands will bind VWF in the presence of other abundant plasma glycoproteins such as albumin or immunoglobulin. In addition, binding affinities have been determined for only a minority of putative VWF ligands. Finally, some reported VWF-binding partners have yet to validated by studies performed in other independent laboratories (supplemental Table 1).
Interaction of VWF with structurally diverse binding ligands
Notwithstanding issues that need to be addressed in future studies, it is intriguing that VWF has the potential to interact with such a wide variety of structurally diverse binding partners. This is unusual compared with other coagulation factors but well established for other plasma glycoproteins such as albumin that displays marked ligand heterogeneity.41 Binding promiscuity may reflect the fact that VWF is a large adhesive molecule present in plasma at higher concentrations than most other coagulation proteins. Each VWF monomer is also highly glycosylated, with NLGs and OLGs together constituting ∼20% of the total monomeric mass. Mass spectroscopy studies have shown that many different VWF glycoforms may be present in a given individual at any specific time. Although the complex NLGs on VWF have been implicated in limiting some ligand interactions through steric hindrance, these carbohydrates can also mediate binding to ligands that possess carbohydrate-recognition domains.42
The unusual array of VWF-binding partners is at least in part attributable to the fact that it circulates in normal plasma as a series of heterogeneous multimers that may contain between 40 and 100 monomers. This unusual property facilitates clustering of ligand binding sites in multimeric VWF. The importance of VWF multimer size has been studied for a limited number of ligands but can influence some binding interactions (eg, GPIbα, collagen, and complement C3b). Finally, recent studies have demonstrated that VWF also has the potential to circulate in a range of different allosteric conformational variants determined by variability in intramolecular disulfide bond formation.43 This serves to further increase the number of VWF epitopes that may be available for potential ligand interactions.
Another intriguing observation is that so many VWF-binding partners appear to target the A1 domain (Figure 2). To date, it remains unclear whether there can be A1 occupancy with different ligand combinations or how these binding partners may compete for adjacent binding sites within A1. However, it has been shown that some A1 domain ligands (eg, galectins and OPG) can inhibit interaction with platelet GPIbα. Further studies will be required to define why the A1 domain is so important and to exclude the possibility that some of these reported interactions may be artifactual in nature due to the presence of ristocetin. Nevertheless, the A1 domain does have several important features. First, the domain is flanked by 2 OLG clusters, which have been shown to influence conformation of the A1 domain and regulate interaction with specific ligands (eg, GPIbα and macrophage galactose lectin). Second, the N- and C-terminal flanking regions of the A1 domain have been shown to form an autoinhibitory module. This means that A1 can adopt different active or inactive conformational states. Consequently, the precise A1 construct (particularly the N- and C-termini limits) used in binding studies has the potential to affect interactions.
VWF: novel biological functions beyond hemostasis
Together with the identification of diverse binding ligands, an array of novel biological functions for VWF beyond hemostasis have also been proposed (Figure 3). The evidence supporting roles for VWF in these processes is undoubtedly stronger for some than others. In addition, interpretation of results from experiments performed in VWF−/− mice needs to be considered carefully because these animals also lack WPB storage organelles within their ECs. Nonetheless, accumulating data suggest that at least some of these novel VWF functions have direct physiological or pathological significance, notably with respect to inflammation, angiogenesis, and wound healing.44-46
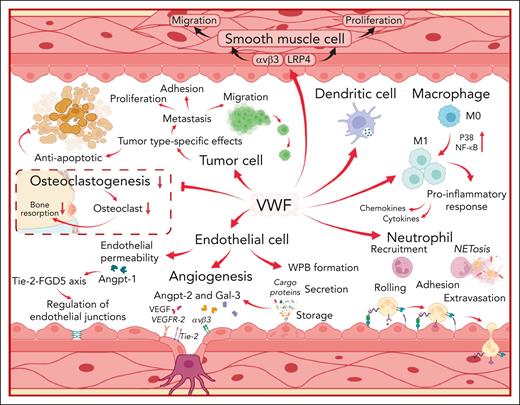
VWF interacts with a variety of different cell types to influence both physiological and pathological processes. The diverse biological roles of VWF are mediated through effects upon ECs, neutrophils, leucocytes, macrophages, dendritic cells, tumor cells, smooth muscle cells, and osteoclasts as illustrated. VWF may potentially effect other types of cells which are not included in this figure. LRP4, low-density lipoprotein receptor–related protein 4; M0, undifferentiated macrophage; M1, proinflammatory macrophage; NETosis, formation of neutrophil extracellular traps; P38, protein kinase 38; Tie-2, tyrosine kinase with immunoglobulin-like and epidermal growth factor-like domains 2; FGD5, FYVE, RhoGEF, and PH domain–containing protein 5; VEGFR-2, vascular endothelial growth factor receptor 2.
VWF interacts with a variety of different cell types to influence both physiological and pathological processes. The diverse biological roles of VWF are mediated through effects upon ECs, neutrophils, leucocytes, macrophages, dendritic cells, tumor cells, smooth muscle cells, and osteoclasts as illustrated. VWF may potentially effect other types of cells which are not included in this figure. LRP4, low-density lipoprotein receptor–related protein 4; M0, undifferentiated macrophage; M1, proinflammatory macrophage; NETosis, formation of neutrophil extracellular traps; P38, protein kinase 38; Tie-2, tyrosine kinase with immunoglobulin-like and epidermal growth factor-like domains 2; FGD5, FYVE, RhoGEF, and PH domain–containing protein 5; VEGFR-2, vascular endothelial growth factor receptor 2.
Effects of VWF on inflammation
It is well recognized that EC activation triggers exocytosis of VWF antigen (VWF:Ag) and VWFpp stores from WPBs.1 Moreover, plasma VWF:Ag and VWFpp levels have been used as biomarkers of clinical severity in patients with a variety of inflammatory and septic conditions.47-49 Rather than merely serving as a measure of EC activation and damage, more recent studies have demonstrated that VWF plays direct roles in regulating inflammatory responses. Pendu et al demonstrated that VWF binds to polymorphonuclear leukocytes (PMNs).50 Under shear, this VWF-leucocyte interaction consisted of initial transient rolling mediated by VWF-A1 domain binding to P-selectin glycoprotein ligand 1 on leucocytes. This was subsequently followed by more stable adhesion, mediated by the VWF-D’D3 and A1A3 domains interacting with β2-integrins on leucocytes (Figure 3).50 In addition, Slc44a2 on neutrophil surfaces has also been shown to interact with the VWF A1 domain.51 Functional polymorphisms at the SLC44A2 locus define expression of human neutrophil antigens 3A and 3B. Interestingly, recent studies have shown that VWF interaction is significantly attenuated for human neutrophil antigen 3b.51 In vivo studies have confirmed that VWF regulates vascular permeability and PMN extravasation into inflamed tissues (Figure 3). In VWF–/– mice or after VWF inhibition in wild-type mice, significantly reduced neutrophil recruitment has been observed in different inflammation models including (1) thioglycollate-induced peritonitis; (2) keratinocyte–derived chemokine–stimulated cremaster muscle; (3) immune-complex–mediated vasculitis; and (4) irritative contact dermatitis.52,53
In addition to binding to PMNs, VWF has also been shown to bind to macrophages but not to undifferentiated monocytes.54 Recent data have highlighted that VWF interaction with specific macrophage receptors (notably, low-density lipoprotein receptor–related protein 1) initiates significant downstream inflammatory signaling (including activation of MAPKinase protein kinase 38 and nuclear factor kappa κB) that ultimately triggers macrophages to adopt an M1 proinflammatory phenotype (Figure 3).54 Consequently, VWF binding leads to a significant increase in macrophage glycolysis, together with upregulated secretion of proinflammatory cytokines and chemokines.54 Altogether, these data define a novel biological role for VWF in linking primary hemostasis and innate immunity, which may be important at sites of vascular injury.
Beyond its effects on PMNs and macrophages, VWF can influence inflammatory responses in other ways. For example, VWF has also been reported to bind to dendritic cells (Figure 3).55 This raises the potential for VWF to also affect adaptive immune responses. In addition, VWF has also been implicated in the formation of neutrophil extracellular traps (NETs).56 In particular, the VWF A1 domain can bind directly to both histones and extracellular DNA (Figure 2).56 Furthermore, the inclusion of VWF in NETs was associated with significantly enhanced leucocyte recruitment.51 Finally, VWF has also been reported to influence complement activation (Figure 3). Complement factor H binds to the VWF A1 and A2 domains and has been reported to inhibit VWF cleavage by ADAMTS13.21,57 In addition, VWF also binds to complement C1q, C3, and C3b and serves as a cofactor for factor I–mediated cleavage of complement C3b.58,59
Effects of VWF on angiogenesis
A role for VWF in regulating angiogenesis is supported by various lines of evidence. First, angiodysplasia, particularly involving the GI tract, is a recognized complication in patients with both inherited and acquired VWD (eg, in Heyde syndrome with aortic stenosis).60 For reasons that remain poorly defined, angiodysplasia appears to be more marked in VWD subtypes characterized by loss of high molecular weight (HMW)-VWF multimers.60 Second, angiogenesis and vascular density are significantly increased in VWF–/– mice.61 Third, small interfering RNA (siRNA) inhibition of VWF expression in EC ex vivo is associated with enhanced proliferation, migration velocity, and angiogenesis.61 Fourth, abnormal angiogenesis has also been observed in studies of endothelial colony forming cells (ECFCs) derived from patients with VWD with specific VWF sequence variants and different types of VWD.61,62 Overall, these data demonstrate that VWF plays a predominantly inhibitory role in regulating angiogenesis through several distinct mechanisms. These include putative roles for partners Angpt-2 and Gal-3, which are both normally trafficked into WPB stores via interaction with VWF.63 Recent review manuscripts have considered in detail the mechanisms through which VWF may affect angiogenic regulation.44,64
Effects of VWF on wound healing
Recent studies suggest a role for VWF in promoting wound healing (Figure 3). Ishihara et al observed that healing of dermal skin wounds was significantly delayed in VWF–/– mice compared with wild-type controls.46 Furthermore, wounds in the VWF–/– mice contained reduced levels of vascular endothelial growth factor A (VEGF-A) and fibroblast growth factor 2 (FGF-2) and exhibited attenuated EC and smooth muscle proliferation.46 Subsequently, the heparin-binding domain in the VWF A1 domain (Tyr1328-Ala1350) was shown to bind to important growth factors, including members of the platelet-derived growth factor, VEGF, FGF, and transforming growth factor β families (Figure 2).46 Furthermore, VEGF-A2 colocalized with VWF in WPB stores and was cosecreted after EC activation. Finally, coimmunoprecipitation experiments showed that VEGF-A and FGF-2 in normal human plasma both circulate in complex with VWF.46 In contrast to the concept that VWF is predominantly an inhibitor of angiogenesis, there was impaired local angiogenesis at wound sites in VWF–/– mice, which may contribute to the impaired wound healing observed.46 Studies in several other animal models of ischemia have also reported proangiogenic and antiangiogenic roles for VWF, suggesting that these effects may vary under specific conditions and in different tissues.
Additional novel cellular interactions and biological functions for VWF
Although the evidence is less developed, studies have suggested additional biological roles for VWF. Similar to platelets and macrophages, VWF binding to vascular smooth muscle cells (VSMCs) has also been shown to trigger intracellular signaling (Figure 3).65,66 These cells regulate vasoconstriction, vasodilatation, and, thereby, blood pressure control. In addition, VSMCs also have an important role in atherosclerosis.67 Lagrange et al recently demonstrated that the low-density lipoprotein receptor–related protein 4 receptor on the surface of VSMCs binds to the VWF A2 domain.66 This binding triggers signaling through integrin αvβ3, leading to activation of protein kinase 38-MAPK and Src, followed by downstream phosphorylation of Akt and ERK1/2. Furthermore, this VWF signaling through the low-density lipoprotein receptor–related protein 4/αvβ3 complex led to VSMC proliferation, migration, and intimal hyperplasia.66 Interestingly, previous in vivo studies reported significant differences in atheroma development in VWF–/– mice compared with wild-type controls. Importantly, macrophage recruitment into the atheromatous plaques was attenuated in the VWF–/– mice.68 Cumulatively, these findings raise the intriguing possibility that VWF may contribute to atheroma progression by influencing both macrophage and VSMC biology.69
As already discussed, OPG binds to the VWF A1 domain within ECs, is stored within WPBs, and circulates in plasma in complex with VWF.31 OPG also has important intrinsic functions in regulating bone metabolism. In particular, OPG functions as a soluble decoy receptor for RANK ligand and thereby inhibits osteoclastogenesis (Figure 3).70 Interestingly, interaction of VWF-FVIII complex with OPG has been shown to increase the binding of OPG to RANK ligand, which in turn promoted downregulation of osteoclast differentiation.70,71 Additional studies will be required to determine whether the effect of VWF in regulating osteoclast biology has a clinically relevant effect on bone metabolism in vivo.
Pathogenic roles for VWF beyond hemostasis
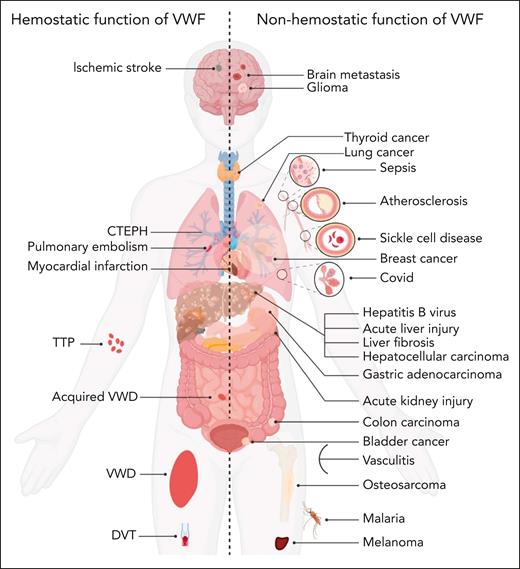
In addition to its established roles in the etiology of both bleeding and thrombotic disorders (including VWD, thromboembolic thrombocytopenic purpura, deep vein thrombosis, PE, MI, and ischemic stroke; Figure 4),4,5,72 additional pathogenic roles for VWF in a range of other nonhemostatic diseases has also been proposed (Figure 4; supplemental Table 2). These putative novel pathogenic roles for VWF are supported in some cases by significant data from in vivo studies involving a variety of different murine models. Nevertheless, the level of evidence regarding the involvement of VWF in these pathologies exhibits considerable variability, with certain disorders still in early stages of investigation.
VWF may contribute to disease pathobiology for a variety of both hemostatic and nonhemostatic disorders. Illustration of hemostatic disorders in which VWF has been implicated in playing a direct pathogenic role (left). Illustration of a series of nonhemostatic disorders in which putative pathogenic roles for VWF have been proposed (right). Some evidence suggests that VWF may potentially affect other types of diseases which are not included in this figure. CTEPH, chronic thromboembolic pulmonary hypertension; DVT, deep vein thrombosis; TTP, thromboembolic thrombocytopenic purpura.
VWF may contribute to disease pathobiology for a variety of both hemostatic and nonhemostatic disorders. Illustration of hemostatic disorders in which VWF has been implicated in playing a direct pathogenic role (left). Illustration of a series of nonhemostatic disorders in which putative pathogenic roles for VWF have been proposed (right). Some evidence suggests that VWF may potentially affect other types of diseases which are not included in this figure. CTEPH, chronic thromboembolic pulmonary hypertension; DVT, deep vein thrombosis; TTP, thromboembolic thrombocytopenic purpura.
VWF in SCD and malaria
Elevated plasma VWF levels, pathological ultra-large (UL) VWF multimers and mild decreases in plasma ADAMTS13 levels have been reported in patients with sickle cell disease (SCD).48,73 In addition, VWF has been implicated in the pathogenesis of SCD vaso-occlusive crises (VOCs) (supplemental Table 2).48,74 In particular, UL-VWF multimers secreted from ECs have been shown to bind and tether sickle erythrocytes under shear conditions. Recent studies in in a murine SCD model demonstrated that VWF–/– animals were protected against hemolysis, VOCs, and organ damage.74 Moreover, treatment with recombinant ADAMTS13 also significantly reduced hemolysis and organ damage in a humanized SCD murine model.74 Despite these exciting murine data, the role of the VWF-ADAMTS13 axis in contributing to VOC development in human patients with SCD has not yet been defined. For example, there is no clear evidence that ABO blood group (which affects plasma VWF:Ag levels and susceptibility to ADAMTS13 proteolysis) has any effect on SCD severity. In addition, it remains unclear whether the effect of VWF in the murine SCD model is entirely explained via VWF binding to sickle erythrocytes or whether VWF effects on inflammation may also be important.
Similar to SCD, markedly increased plasma VWF levels and abnormal UL-VWF multimers have also been postulated to contribute to disease pathogenesis in both malaria and acute COVID-19.47,75 Importantly, platelet decorated VWF strings have been shown to bind to Plasmodium falciparum–infected erythrocytes and thus may contribute to EC cytoadhesion. In particular, the A1 domain of VWF has also been shown to bind to P falciparum erythrocyte membrane protein 1 on the surface of malaria-infected erythrocytes.76 In addition, in vivo studies have shown that VWF–/– mice are significantly protected in a murine model of P berghei–induced experimental cerebral malaria.47 In contrast to SCD, ABO blood has been shown to significantly regulate malaria severity in human patients.77
VWF in liver disease
Acute and chronic liver disease are both associated with marked increases in plasma VWF:Ag levels (fivefold to 10-fold).78 In contrast to malaria, SCD, or COVID-19, HMW-VWF multimers are typically reduced in patients with liver disease, despite the fact that plasma ADAMTS13 levels are significantly reduced (threefold to fivefold).78 This paradox may be due to HMW-VWF multimer consumption in platelet-rich microthrombi within the liver. Alternatively, VWF proteolysis via ADAMTS-13–independent mechanisms may be occurring. Importantly, recent animal studies have demonstrated roles for VWF in contributing to acute and chronic liver pathobiology (Figure 4; supplemental Table 2).79,80 Notably, Groeneveld et al demonstrated that VWF-platelet aggregates formed in the liver of mice after acetaminophen overdose.79 In VWF–/– mice, these platelet aggregates were cleared more rapidly, which led to accelerated liver repair. Similarly, anti-VWF antibodies also reduced hepatic platelet accumulation in wild-type mice, leading to faster liver repair. A direct role for VWF in the pathogenesis of carbon tetrachloride–induced experimental liver fibrosis has also been reported.80 Once again, VWF–/– mice were shown to develop significantly reduced liver fibrosis compared with wild-type control animals.80 Further studies will be needed to elucidate the biological mechanism(s) through which VWF affects acute and chronic liver disease and its importance in human patients.
VWF and tumor cell biology
Increased plasma VWF levels have been associated with worse outcomes in multiple different types of cancer, including breast, thyroid, and gastric malignancies.45 Increased VWF-FVIII complex levels also likely contribute to cancer-associated thrombosis risk.45 Interestingly, more recent studies have suggested that VWF may also play roles in regulating specific aspects of tumor cell biology, notably with respect to metastasis, apoptosis, and tumor angiogenesis (supplemental Table 3).45,81 Importantly, however, previous in vivo studies performed in VWF–/– mice produced some unexpected results, with significantly enhanced metastasis in the absence of VWF. These findings may relate to the fact that VWF affects cancer biology differently depending upon the cancer type and stage. The putative mechanisms through which VWF may influence tumor cell biology have recently been reviewed in detail.82
Targeting novel functions of VWF as a therapeutic strategy
Recent studies suggest that targeting novel functions of VWF may offer an exciting novel therapeutic approach. For example, a novel A1 domain–targeted nanobody significantly reduced leukocyte recruitment and vascular permeability in 2 different murine models of inflammation (immune-complex–mediated vasculitis and irritant contact dermatitis, respectively).53 Collectively, these findings support the concept that anti-VWF targeted therapies may have clinical utilities beyond hemostasis.
It is interesting to consider the potential importance of nonhemostatic roles of VWF in patients with VWD. Angiodysplasia undoubtedly has direct significance with respect to increased risk for GI bleeding, particularly in patients with type 2A VWD and acquired VWD.60 Although there is limited evidence, it seems likely that nonhemostatic functions of VWF may also be important in the context of heavy menstrual bleeding. The menstrual cycle is characterized by monthly bleeding, followed by a period of local inflammation and wound repair in the uterus.83 In turn, this is followed by local angiogenesis that ultimately leads to rebleeding. Based on our current understanding regarding novel roles for VWF in regulating not only bleeding but also inflammation, wound repair, and angiogenesis, it is evident that VWF has the potential to affect multiple aspects of the menstrual cycle. This “perfect storm” may explain the enormous clinical burden associated with heavy menstrual bleeding, even in women with only mild-to-moderate reductions in plasma VWF levels in the low VWF range (30-50 IU/dL).84
Conclusions
The rapidly expanding list of binding ligands has led to the suggestion that VWF may be considered as a “shuttle bus” in vivo.85 Thus, some passengers board the VWF bus at its home terminus (ie, within ECs). Other passengers get onto the VWF bus as it travels along its route (ie, in the circulation). Passengers may disembark the VWF bus at times and may be replaced by other new passengers. At times, passengers may be involved in competition for seats on the VWF bus. Under specific physiological or pathological conditions, the number of VWF buses may be increased or decreased. Furthermore, the VWF bus may be more accessible for specific passenger types under certain conditions (eg, after A1 domain activation). Finally, the ultimate destination of VWF buses may vary. Under normal condition, most VWF will be cleared from the circulation by macrophages, liver sinusoidal ECs, and hepatocytes.86 Alternatively, at times of hemostatic challenge, VWF along with its passengers will instead be recruited to sites of vascular injury. In addition, VWF buses may be directed to additional sites under specific pathological conditions (eg, to leucocytes at sites of inflammation). Finally, in some circumstances, the VWF bus can be hijacked and used by nonhuman ligands. In particular, different microorganisms (including Staphylococcus aureus, Helicobacter pylori, and Schistosoma mansoni) have been shown to bind and use VWF to facilitate dissemination.87-89 Clearly, this analogy is a gross simplification but serves to integrate the plethora of novel binding partners for VWF proposed in recent studies. Validating these individual VWF interactions and elucidating the molecular mechanisms involved will require significant further research. Nevertheless, disentangling these additional roles of VWF extending beyond its classical prohemostatic functions may offer exciting opportunities to develop novel treatment approaches for important unmet clinical needs.
Acknowledgments
F.A. is supported by a Rubicon grant (452022310) from The Netherlands Organisation for Health Research and Development (ZonMw). J.S.O. is supported by funds from the National Institutes of Health, National Heart, Lung, and Blood Institute for the Zimmerman Program (HL081588) and a Science Foundation Ireland Principal Frontiers for the Future award (20/FFP-A/8952).
Authorship
Contribution: F.A. and J.S.O. contributed to literature review, interpretation of data, final draft writing, and critical revision; and both authors have participated sufficiently in this work, take public responsibility for the content, and have given consent to the final version of the article.
Conflict-of-interest disclosure: F.A. received research support from CSL Behring, Takeda, Octapharma, and Sobi; and received travel grants from Sobi. J.S.O. has served on the speaker’s bureau for Baxter, Bayer, Novo Nordisk, Sobi, Boehringer Ingelheim, Leo Pharma, Takeda, and Octapharma; has served on the advisory boards of Baxter, Sobi, Bayer, Octapharma, CSL Behring, Daiichi Sankyo, Boehringer Ingelheim, Takeda, and Pfizer; and received research grant funding awards from 3M, Baxter, Bayer, Pfizer, Shire, Takeda, 3M, and Novo Nordisk.
Correspondence: James S. O’Donnell, Irish Centre for Vascular Biology, Royal College of Surgeons in Ireland, Ardilaun House, 111 St Stephen’s Green, Dublin 2, D02 VN51, Ireland; email: jamesodonnell@rcsi.ie.
References
Author notes
All data are available on request from the corresponding author, James O’Donnell (jamesodonnell@rcsi.ie).
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal