In this issue of Blood, Blanquart and colleagues describe leveraging of different single-cell technologies and ex vivo studies to demonstrate how dysfunctional natural killer (NK) cells negatively impact survival of patients with multiple myeloma (MM).1
MM is a unique cancer model where characterizing immune surveillance vs escape is critical for understanding the pathogenesis of the disease. MM is preceded by 2 precursor conditions: monoclonal gammopathy of undetermined significance and smoldering MM, with up to 9% or 59%, respectively, of clonal and genetically altered plasma cells in the bone marrow.2 However, many patients never develop active disease despite decades of follow-up,2 which suggests that there is immune surveillance preventing further expansion. In addition, there is accumulating evidence that malignant transformation is partially associated with progressive immune dysfunction.3-5 Furthermore, there is unequivocal proof that different immunotherapies (eg, immunomodulatory drugs, monoclonal antibodies [mAbs], T-cell redirecting therapy) are effective and have sculptured the MM treatment landscape. Consequently, there is great interest in the identification of immune biomarkers predictive of response and resistance to immunotherapy.
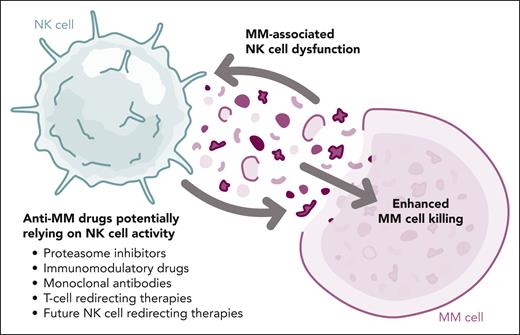
Unlike other immune players, such as T cells3-5 and myeloid-derived suppressor cells,6,7 there is limited knowledge about the phenotype and functional status of NK cells in MM. Having such data is important because NK cells play an important role in the mode of action of proteasome inhibitors, immunomodulatory drugs, and mAbs, and potentially of T-cell redirecting therapy after costimulation through cytokine secretion. In addition, there are specific NK cell therapies under development for MM.8,9 Thus, Blanquart et al provide novel and valuable data that may contribute to biomarker and drug development (see figure).
Interplay between NK cell functionality and treatment of patients with MM. MM, multiple myeloma; NK, natural killer. Professional illustration by Somersault18:24.
Interplay between NK cell functionality and treatment of patients with MM. MM, multiple myeloma; NK, natural killer. Professional illustration by Somersault18:24.
The authors also provide a transcriptional atlas of bone marrow and peripheral blood NK cells from 10 patients with MM and 10 age- and sex-matched healthy donors. The single-cell data reveal important differences in MM patients, such as the contraction of CD56dim cytotoxic and of adaptive-like subsets, and the expansion of late-stage “activated/inflammatory” CD56dim NK cells expressing NFκB and interferon-1 inflammatory signatures. Although these results in 10 newly diagnosed and relapsed patients are indicative of an altered NK cell compartment, studies are needed to understand the dynamics of the alterations during myelomagenesis, and how much treatment affects NK cell function.
Blanquart et al analyzed NK cell degranulation in ex vivo experiments. They found that, when compared with NK cells from healthy donors, those from newly diagnosed patients displayed lower killing and reduced ability to form conjugates with isatuximab-coated MM cell lines. Although these findings cannot be directly extrapolated to results observed in the current clinical trials, one may be tempted to speculate about a potential beneficial effect of autologous transplant on NK cell functionality, and how this may enhance antibody-dependent cellular cytotoxicity triggered by anti-CD38 mAbs during maintenance.10
The authors explored the independent prognostic value of frequency of CD16/CD226Lo NK cells in the bone marrow of transplant-eligible, newly diagnosed patients with MM enrolled in the IFM2009 clinical trial. The integration of single-cell RNA sequencing and spectral flow cytometry data plus the ex vivo experiments suggest that the CD16/CD226Lo phenotype enriched for NK cells with reduced effector functions in response to NK-receptor stimulation, lower polymerized actin content, and reduced motility. Accordingly, an above-median frequency of CD16/CD226Lo NK cells was associated with inferior survival. Other NK cell markers, such as CD28, TIGIT, PD-1, CD57, KLRG1, and CD38, were not prognostic. Interestingly, NK cell frequency did not correlate with CD16/CD226 expression and the combination of both variables into a single variable represented by the percentage of CD16/CD226Lo NK cells within bone marrow nucleated cells showed independent prognostic value for overall survival in a multivariate analysis with demographics, staging, cytogenetic risk, and treatment arm. Thus, the accumulation of altered NK cells in patients who were newly diagnosed with MM was associated with poorer outcomes in the context of bortezomib/lenalidomide/dexamethasone induction with or without upfront autologous transplant. Further analyses are needed to determine whether the CD16/CD226Lo NK cell phenotype is even more informative in patients treated with newer regimens that incorporate an anti-CD38 mAb.
This excellent study by Blanquart and colleagues addresses critical knowledge gaps and raises questions that still need to be answered. From the translational point of view, the optimal cutoffs of CD16/CD226Lo NK cells in different patient populations and treatment scenarios need to be defined. Likewise, are less invasive peripheral blood samples as informative as bone marrow aspirates? The authors did find similar cluster compositions of NK cells from blood and marrow of patients with MM. From the drug development point of view, how does the improved knowledge of MM-associated NK cell dysfunction influence the development of more efficient immunotherapies? After the recent success of T-cell redirecting therapy, the aid of naturally functional killer cells is poised to play a key role in improving survival outcomes for patients with MM.
Conflict-of-interest disclosure: B.P. reports honoraria for lectures, participating in advisory boards and/or consultancy with Adaptive, Amgen, Bristol Myers Squibb/Celgene, Gilead, GlaxoSmithKline (GSK), Janssen, Oncopeptides, Roche, Sanofi, and Takeda; and unrestricted grants from Beigene, Bristol Myers Squibb/Celgene, EngMab, GSK, Roche, Sanofi, and Takeda. J.A.M.-C. reports research funding from Roche-Genentech, Bristol Myers Squibb/Celgene, Janssen, Regeneron, Priothera, Palleon, AstraZeneca, and K36 Therapeutics; is inventor on a patent, “Genetically engineered animal models for multiple myeloma,” licensed to Modelling Immunotherapy in Oncology (MIMO) Biosciences; and is founder, has royalties in, and holds stock options in MIMO Biosciences.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal