In this issue of Blood, Mazziotta et al1 investigate CD8+ T-cell subpopulations as correlates with response to induction chemotherapy in acute myeloid leukemia (AML) and identify opposing roles of early memory vs terminally differentiated “senescence-like” cells.
The past years have seen ground-breaking progress in immune-based treatment approaches for lymphoid blood malignancies, including chimeric antigen receptor T cells and bispecific antibodies directed against B-cell–lineage markers in acute lymphoblastic leukemia and multiple myeloma, or therapeutic disruption of the PD-1/PD-L1 axis in Hodgkin lymphoma.2 On the other hand, myeloid malignancies and especially AML continue to be a much more challenging setting for developing novel immunotherapies, which often have only modest clinical activity in the form of short-lived, transient responses and are complicated by frequent dose-limiting toxicities. Recent setbacks are the discontinuation of clinicals trials investigating the CD47 antibody magrolimab due to insufficient activity and increased risk of death, or the largely disappointing activity of immune checkpoint blockade in AML/myelodysplastic syndrome, with the exception of rare responses, such as in patients with posttransplant leukemia cutis relapse.3,4
Although the experience with graft-versus-leukemia responses following allogeneic hematopoietic stem cell transplantation provides robust evidence that AML is sensitive to immunologic treatment strategies, the phenotypes and dynamics of effector cells that mediate leukemia-specific immunity in myeloid disease still remain incompletely understood. Transcriptomic studies have provided an opportunity to better define the bone marrow immune microenvironment, and they have yielded important insights, including the observations of inferior survival in AML with signatures of immune depletion or enrichment of senescent-like CD8+ T cells and associations of mutated TP53 status with increased infiltration with cytotoxic T cells.5-7 However, despite these studies, a more detailed dissection of the heterogeneity of T-cell subpopulations and a parsing of bystander from leukemia-specific T cells is still lacking. Certainly, an improved characterization of the cellular mediators of effective AML-directed immune responses promises a basis from which targeted immunomodulation and cellular therapies may be developed to ultimately unlock the full potential of this approach in AML.
Novel technologies, including single-cell RNA sequencing (scRNA-seq) or high-dimensional spectral flow cytometry, are refining immune phenotyping studies to unprecedented resolution. Integration with immunogenomics data such as T-cell receptor (TCR) sequences permit identification and monitoring of individual tumor-specific T-cell clones. This framework has profoundly advanced our understanding of T-cell responses in the setting of solid tumors, where numerous studies have converged on the paradigm of T-cell exhaustion as the phenotypic harbinger of tumor-specific immunity. Yet, in the bone marrow microenvironment, exhausted T cells are much more rare,8 which suggests fundamental differences in the underlying immunologic principles between solid and hematologic malignancies and prioritizes identification of therapeutically relevant T-cell populations in AML.
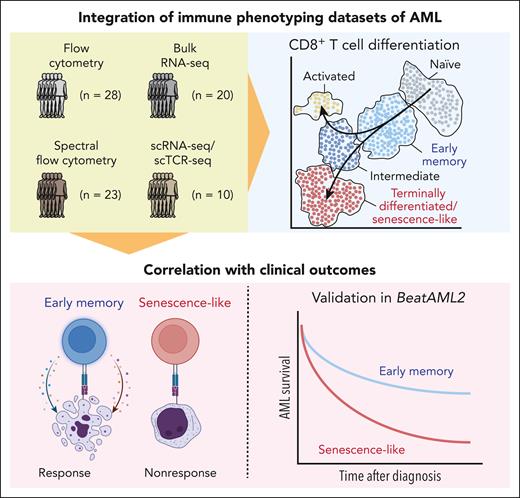
Mazziotta et al leverage these modern immune phenotyping tools to investigate CD8+ T-cell populations that associate with response to induction chemotherapy in AML by analyzing several complementary data sets, ranging from bulk transcriptomics to spectral flow cytometry and scRNA-seq in conjuncture with TCR sequencing (see figure). The authors develop a CD8+ T-cell classification system that follows the differentiation course from naïve and early memory to terminally differentiated senescence-like cells. In extensive validation analyses, including reanalysis of published AML scRNA-seq data and transcriptional profiles of flow-sorted T cells, the authors confirm this classification, which is further supported by RNA-based trajectory analyses and observations that T-cell clone sizes increase along this differentiation path. By applying this annotation system across several cohorts, the authors found that the ratio of early memory vs terminally differentiated CD8+ T cells was associated with response to AML induction chemotherapy and improved overall survival. In an elegant reanalysis of RNA-seq data from the BeatAML2 cohort,9 Mazziotta et al demonstrate that the association of an increased ratio of early memory/terminally differentiated T cells with improved overall survival also held up in an external validation cohort, even when considering European LeukemiaNet 2017 risk stratification or patient age.
Overall, this study confirms and extends prior analyses of CD8+ T-cell subpopulations, which have established associations with response to therapy or survival in AML, and thus motivates further investigations into immunotherapies for myeloid disease despite the recent setbacks in the field.6,7
As any relevant study, this work by Mazziotta et al also raises several new questions. Foremost, it would be important to understand the mechanism behind the reported clinical benefit of a higher early memory to terminally differentiated T-cell ratio. Does a higher number of early memory T cells indicate an immune system with more potential for generating novel leukemia-specific T-cell responses? Are more frequent senescent-like T cells a marker of a prior, sustained exposure to AML-specific antigens (eg, due to a process of immune selection that led to outgrowth of more resistant leukemia cells over time)? An important clue to these questions would be information on antigen specificity (eg, through the generation of functional data derived from coincubation of AML blasts with T cells, followed by TCR sequencing that could help to answer which of these subsets is enriched for leukemia-reactive T cells). But yet another question is the role of CD4+ T cells in AML-directed immunity and how this intersects with the insights into CD8+ T cells. Given the downregulation of HLA class II expression in posttransplant AML relapse,10 which implies that CD4+ T cells can drive AML-specific immune responses, it would be rewarding to investigate whether similar associations of CD4+ T-cell differentiation and treatment responses exist, or whether other mechanisms are at play.
In closing, the study by Mazziotta et al is a prime example of how careful curation of orthogonal immune phenotyping data sets and their thoughtful integrated analysis can strengthen and validate observations made from individual smaller cohorts. In light of the pace at which single-cell studies that investigate the bone marrow microenvironment in blood malignancies are being performed, this work can serve as a model for linking diverse data sets to arrive at unified insights and, by doing so, will help us to finally emerge from the dark age of immunotherapy in AML.
By analyzing several complementary immune phenotyping data sets, Mazziotta et al develop an annotation that follows the differentiation trajectory of CD8+ T cells from naïve via early memory to terminally differentiated “senescence-like” cells. Using this annotation, the authors find associations with response to induction chemotherapy and overall survival in AML across multiple cohorts, including a reanalysis of the BeatAML2 cohort. scTCR-seq, single cell TCR sequencing.
By analyzing several complementary immune phenotyping data sets, Mazziotta et al develop an annotation that follows the differentiation trajectory of CD8+ T cells from naïve via early memory to terminally differentiated “senescence-like” cells. Using this annotation, the authors find associations with response to induction chemotherapy and overall survival in AML across multiple cohorts, including a reanalysis of the BeatAML2 cohort. scTCR-seq, single cell TCR sequencing.
Conflict-of-interest disclosure: C.J.W. holds equity at BioNTech, Inc; has received research funding from Pharmacyclics, outside the submitted work; and is on the scientific advisory board of Repertoire, Inc, and Aethon Therapeutics. L.P. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal