In this issue of Blood, Frigault et al1 describe the first-in-human trial of anti-CD37 chimeric antigen receptor (CAR) T cells in patients with refractory and/or relapsed (r/r) CD37-positive lymphoid neoplasms, an approach that may address some of our remaining unmet clinical needs (see figure).
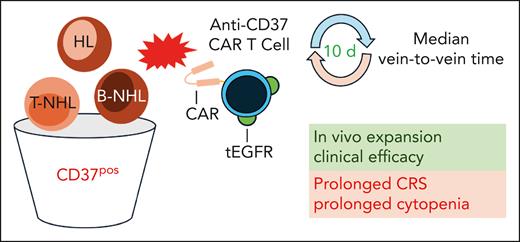
Patients with CD37-positive (CD37pos) HL and B- and T-cell non-Hodgkin lymphoma were treated with autologous anti-CD37 CAR T cells coexpressing a tEGFR. Median vein-to-vein time from apheresis to infusion of CAR T cells was 10 days. The CAR T cells expanded after infusion and showed clinical efficacy in heavily pretreated patients. A prolonged CRS and prolonged cytopenia were also observed. B-NHL, B-cell non-Hodgkin lymphoma; CAR, chimeric antigen receptor; CRS, cytokine release syndrome; HL, Hodgkin lymphoma; tEGFR, truncated epithelial growth factor receptor; T-NHL, T-cell non-Hodgkin lymphoma.
Patients with CD37-positive (CD37pos) HL and B- and T-cell non-Hodgkin lymphoma were treated with autologous anti-CD37 CAR T cells coexpressing a tEGFR. Median vein-to-vein time from apheresis to infusion of CAR T cells was 10 days. The CAR T cells expanded after infusion and showed clinical efficacy in heavily pretreated patients. A prolonged CRS and prolonged cytopenia were also observed. B-NHL, B-cell non-Hodgkin lymphoma; CAR, chimeric antigen receptor; CRS, cytokine release syndrome; HL, Hodgkin lymphoma; tEGFR, truncated epithelial growth factor receptor; T-NHL, T-cell non-Hodgkin lymphoma.
The introduction of CAR T-cell therapy has revolutionized the treatment of hematological (especially B-cell) neoplasms. Unfortunately, a substantial number of patients relapse after such treatment and, in these cases, a loss of the target antigen (eg, CD19) is often observed. Furthermore, there are still diseases that have a poor prognosis in the r/r situation and for which there is currently no effective cell-based therapy option, like T-cell lymphomas. This gap could potentially be filled by a CD37-targeted approach, as described by Frigault and colleagues.
CD37 is a member of the tetraspanin family, characterized by the presence of 4 hydrophobic transmembrane domains and found on the surface of lymphoid cells, particularly mature B cells. Tetraspanins are involved in numerous biological processes and are often referred to as “molecular facilitators.”2 CD37 interacts with multiple molecules, such as SYK, CD19, PI3Kγ/δ, and integrins, and has been shown to be involved among others in the survival, proliferation, and metabolism of both benign and malignant lymphocytes. Numerous studies have revealed that CD37 is also present in neoplasms of the T- and B-cell lineages (eg, diffuse large B-cell lymphoma, chronic lymphocytic leukemia, or peripheral T-cell lymphoma) and may represent a target for immune-based therapies.3
To date, antibody-based approaches, such as monoclonal antibodies (eg, otlertuzumab) and antibody-drug conjugates (eg, naratuximab emtansine), have been used in early clinical trials.4 Prior to this work, no clinical trials using anti-CD37 CAR T cells had been reported. The transformative nature of CAR T-cell therapy, particularly for B-cell neoplasms, is now well established. However, we continue to face unmet challenges where CD37-targeted CAR T cells may be viable option. A significant proportion of our patients treated with anti-CD19 CAR T cells relapse after loss of CD19. Relapse therapy targeting an alternative antigen would be beneficial. In addition, there are no approved CAR T-cell products for diseases such as r/r T-cell lymphoma, indicating a general need for appropriate CAR T cells. Preclinical studies, including one with the CAR construct used here, have demonstrated the efficacy and safety of anti-CD37 CAR T cells in cell lines and animal models of B- and T-cell lymphomas.3,5 In addition, these studies have demonstrated the efficacy of a combined anti-CD37 and anti-CD19 approach.
Frigault et al describe a phase 1 trial with anti-CD37 CAR T cells in patients with CD37+ T- and B-cell neoplasms who have undergone multiple prior treatments, including anti-CD19 CAR T-cell infusions and stem cell transplantation. The generated CAR T cells coexpress the anti-CD37 CAR and truncated epidermal growth factor receptor (EGFR) to primarily enumerate successfully transduced cells and feature a 4-1BB costimulatory domain. Despite patients having undergone several hematotoxic treatments, the CAR T cells could still be successfully manufactured, and the median vein-to-vein time was only 10 days, which is crucial for patients in such refractory and progressive disease states. In the final cell product, the predominant majority of CAR T cells were CD4+, which may have played a role in the later occurrence of cytokine-release syndrome (CRS), as they are considered the main mediators of this toxicity.6
The in vivo expansion of the CAR T cells was successful, and the efficacy data are cautiously optimistic. Four of the 5 patients had tumor responses, including 3 complete responses and 1 mixed response, indicating a high potential for anti–CAR-37 T cells in this group of heavily pretreated patients. Patients with relapse and progression after anti-CD37 CAR T-cell infusion showed a loss of CD37 expression after a relatively short time, possibly under selection pressure. Diffuse large B-cell lymphoma cases with mutation-induced loss of CD37 have been described and are associated with a poorer prognosis, which may also be attributed to the metabolic checkpoint function of CD37.7-9
However, Frigault et al also reveal therapy-related serious complications, with patients experiencing prolonged, mostly low-grade CRS and severe pancytopenia, requiring allogeneic hematopoietic stem cell transplantation in 2 cases. This outcome illustrates a critical challenge in CAR T-cell therapies: the balancing act between effectiveness and adverse effects. The authors demonstrate in various in vitro and in vivo experiments that the anti-CD37 CAR T cells are not directly cytotoxic to hematopoietic stem cells. The severe pancytopenia observed was associated with high levels of interleukin (IL)-18 in the patients, similar to observations in pancytopenic patients after treatment with anti-CD19 CAR T cells.10 IL-18 is derived primarily from epithelial and/or myeloid cells, and it remains to be determined whether IL-18 directly interferes with hematopoiesis or is a surrogate for general inflammatory activity that impairs bone marrow function. In addition, the association between IL-18 and cytopenias should not be overlooked in light of the many current strategies aimed at enhancing the release of IL-18 by CAR T cells to remodel the tumor microenvironment.
Frigault et al also discuss the use of tEGFR as part of the CAR construct as a safety switch. This feature was designed primarily to quantify CAR-expressing cells, but also to allow their ablation, if necessary, when using an anti-EGFR antibody such as cetuximab. However, they found that this mechanism did not work as expected under certain conditions, such as severe cytopenia, which could negatively impact antibody-dependent cytotoxicity, highlighting a critical area for future research and refinement.
In conclusion, this phase 1 trial of anti-CD37 CAR T cells opens a promising new avenue for treating r/r lymphoid malignancies. Although the results are promising in terms of efficacy, the adverse effects underscore the need for further research to optimize the safety profile of this innovative therapeutic approach. The journey of anti-CD37 from bench to bedside exemplifies the challenges and rewards of translating cutting-edge scientific research into potential clinical treatments. The insights gained from this trial are likely to inform future research directions, potentially leading to more refined and safer anti-CD37 CAR T-cell therapies.
Conflict-of-interest disclosure: D.M. has received speaker honoraria and consulting fees from AbbVie, Bristol Myers Squibb, BeiGene, Celgene, Galapagos, Gilead, Janssen, Miltenyi, and Novartis.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal